Effects of Robotic-Assisted Gait Training in Children and Adolescents with Cerebral Palsy: A Network Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection Criteria
2.2. Information Sources
2.3. Search Strategy
2.4. Study Selection and Data Extraction
2.5. Assessment of Risk of Bias
2.6. Data Synthesis and Analysis
3. Results
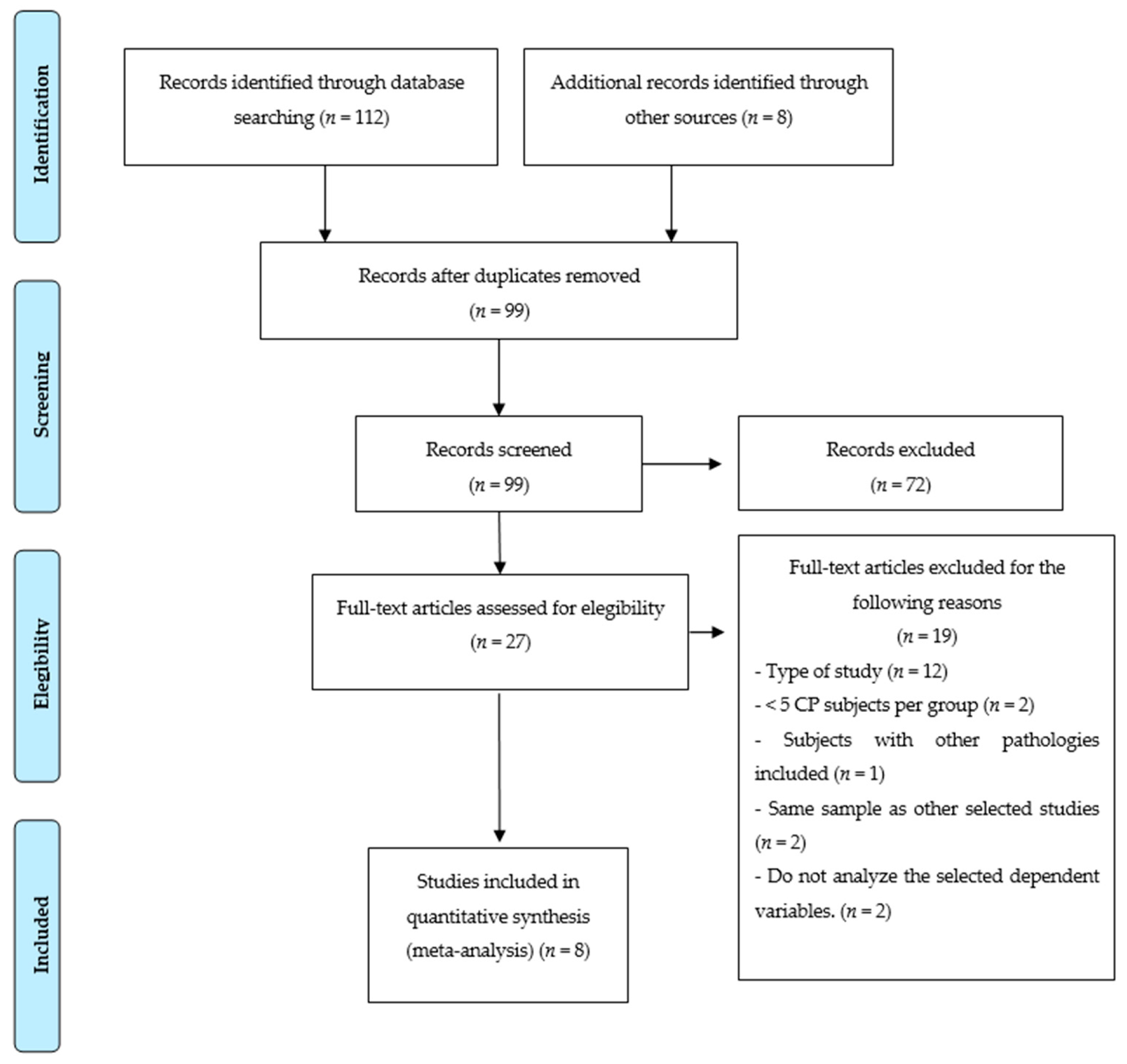
3.1. Study Selection
3.2. Trial Characteristics
3.3. Assessment of Risk of Bias
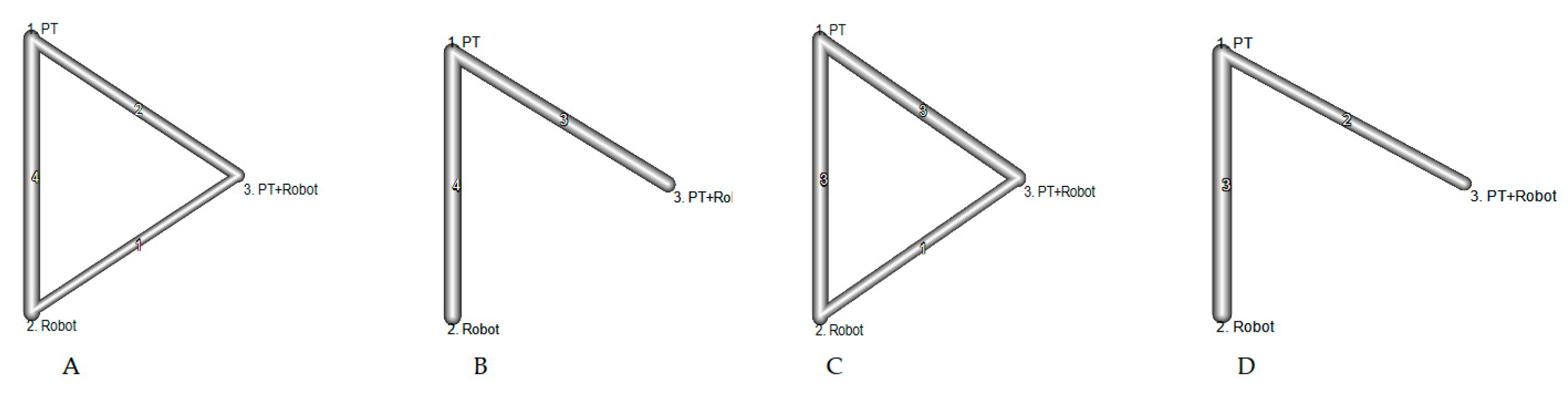
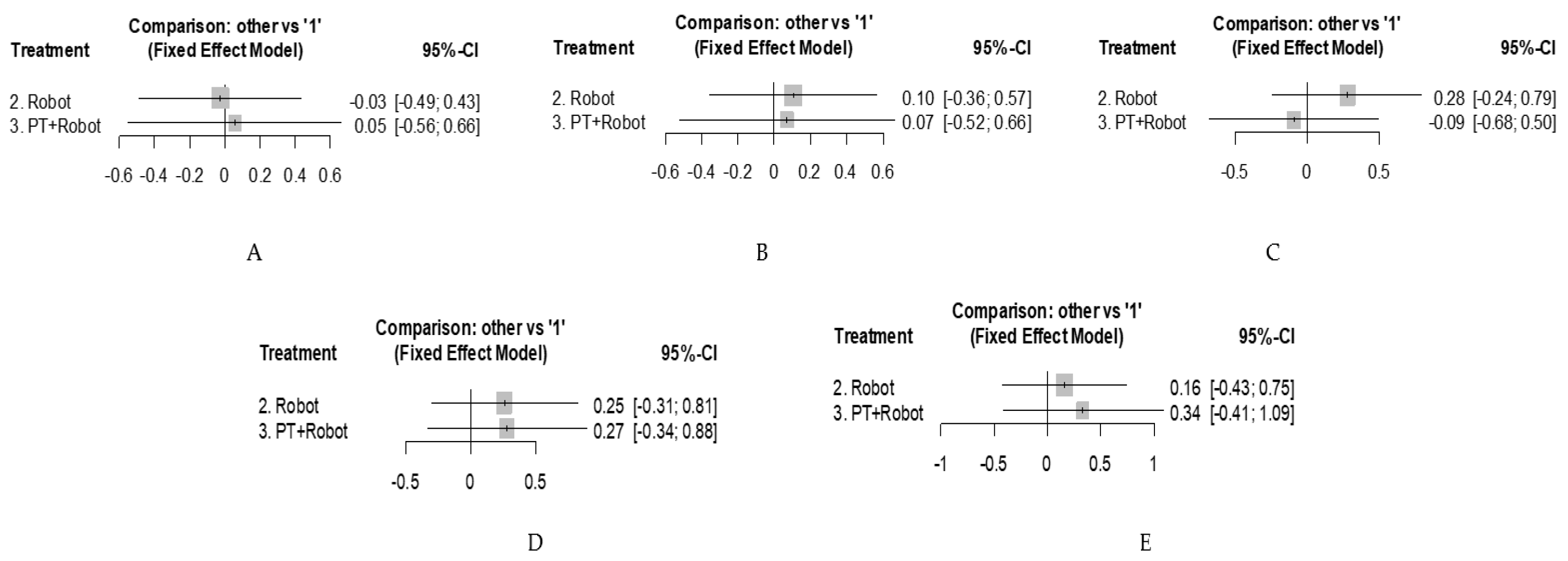
3.4. Network Meta-Analysis
4. Discussion
4.1. Limitations
4.2. Implications for Practice and Research
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Data Sources | Search Strategy | Records Identified | Selected Studies |
|---|---|---|---|
| PubMed | ((((((“cerebral palsy”) AND “robotic assisted gait training”) OR “robotic-assisted locomotor training”) OR “robotic-assisted therapy”) OR “lokomat”) OR “walkbot”) OR “robotic assisted treadmill” | 37 | 16 |
| PEDro | (1) Abstract and title: robotic-assisted Topic: cerebral palsy Method: clinical trial (2) Abstract and title: Robotic training Topic: cerebral palsy Method: clinical trial | 8 | 1 |
| Web of Science | “cerebral palsy” AND (“robotic assisted gait training” OR “robotic-assisted locomotor training” OR “robotic-assisted therapy” OR “lokomat” OR “walkbot” OR “robotic assisted treadmill”) | 36 | 1 |
| Cochrane | “cerebral palsy” AND (“robotic assisted gait training” OR “robotic-assisted locomotor training” OR “robotic-assisted therapy” OR “lokomat” OR “walkbot” OR “robotic assisted treadmill”) | 22 | 0 |
| Psycinfo | “cerebral palsy” AND (“robotic assisted gait training” OR “robotic-assisted locomotor training” OR “robotic-assisted therapy” OR “lokomat” OR “walkbot” OR “robotic assisted treadmill”) | 9 | 1 |
| Ibecs | “cerebral palsy” AND (“robotic assisted gait training” OR “lokomat” OR “walkbot” OR “robotic assisted treadmill”) | 0 | 0 |
| Lilacs | “cerebral palsy” AND (“robotic assisted gait training” OR “lokomat” OR “walkbot” OR “robotic assisted treadmill”) | 0 | 0 |
Appendix B
| Study | Sequence | Allocation | Blinding1 | Blinding2 | Outcome1 | Outcome2 | Other |
|---|---|---|---|---|---|---|---|
| Smania et al., 2011 [28] | + | + | ? | ? | + | + | + |
| Arellano-Martínez et al., 2013 [29] | + | ? | ? | ? | ? | ? | + |
| Druzbicki et al., 2013 [30] | + | ? | ? | + | + | ? | + |
| Peri et al., 2017 [31] | - | ? | ? | ? | + | + | + |
| Wu et al., 2017 [32] | + | + | - | - | + | + | + |
| Wallard et al., 2018 [33] | + | + | ? | ? | + | ? | + |
| Aras et al., 2019 [34] | + | + | ? | ? | + | + | + |
| Yazici et al., 2019 [35] | - | - | - | - | ? | + | + |
References
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M.; Damiano, D.; Dan, B.; Jacobsson, B. A Report: The Definition and Classification of Cerebral Palsy April 2006. Dev. Med. Child Neurol. Suppl. 2007, 109, 8–14. [Google Scholar] [PubMed]
- European Commission; Joint Research Centre. Surveillance of Cerebral Palsy in Europe: Development of the JRC SCPE Central Database and Public Health Indicators; Publications Office of the European Union: Luxembourg, 2017. [Google Scholar] [CrossRef]
- te Velde, A.; Morgan, C.; Novak, I.; Tantsis, E.; Badawi, N. Early Diagnosis and Classification of Cerebral Palsy: An Historical Perspective and Barriers to an Early Diagnosis. J. Clin. Med. 2019, 8, 1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottos, M.; Gericke, C. Ambulatory Capacity in Cerebral Palsy: Prognostic Criteria and Consequences for Intervention. Dev. Med. Child Neurol. 2003, 45, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Novak, I.; Morgan, C.; Adde, L.; Blackman, J.; Boyd, R.N.; Brunstrom-Hernandez, J.; Cioni, G.; Damiano, D.; Darrah, J.; Eliasson, A.-C.; et al. Early, Accurate Diagnosis and Early Intervention in Cerebral Palsy: Advances in Diagnosis and Treatment. JAMA Pediatr. 2017, 171, 897. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, P. Cerebral Palsy: What Parents and Doctors Want to Know. BMJ 2003, 326, 970–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanger, T.D.; Delgado, M.R.; Gaebler-Spira, D.; Hallett, M.; Mink, J.W. Classification and Definition of Disorders Causing Hypertonia in Childhood. Pediatrics 2003, 111, e89–e97. [Google Scholar] [CrossRef] [Green Version]
- Damiano, D.L.; DeJong, S.L. A Systematic Review of the Effectiveness of Treadmill Training and Body Weight Support in Pediatric Rehabilitation. J. Neurol. Phys. Ther. 2009, 33, 27–44. [Google Scholar] [CrossRef] [Green Version]
- Ketelaar, M.; Vermeer, A.; Hart, H.; van Petegem-van Beek, E.; Helders, P.J. Effects of a Functional Therapy Program on Motor Abilities of Children with Cerebral Palsy. Phys. Ther. 2001, 81, 1534–1545. [Google Scholar] [CrossRef]
- Meyer-Heim, A.; van Hedel, H.J.A. Robot-Assisted and Computer-Enhanced Therapies for Children with Cerebral Palsy: Current State and Clinical Implementation. Semin. Pediatr. Neurol. 2013, 20, 139–145. [Google Scholar] [CrossRef]
- Schmidt, H.; Werner, C.; Bernhardt, R.; Hesse, S.; Krüger, J. Gait Rehabilitation Machines Based on Programmable Footplates. J. Neuroeng. Rehabil. 2007, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Mehrholz, J.; Thomas, S.; Kugler, J.; Pohl, M.; Elsner, B. Electromechanical-Assisted Training for Walking after Stroke. Cochrane Database Syst. Rev. 2020, 10, CD006185. [Google Scholar] [CrossRef] [PubMed]
- Borggraefe, I.; Meyer-Heim, A.; Kumar, A.; Schaefer, J.S.; Berweck, S.; Heinen, F. Improved Gait Parameters after Robotic-Assisted Locomotor Treadmill Therapy in a 6-Year-Old Child with Cerebral Palsy. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.; Pinto, S.M.; das Virgens Chagas, D.; Dos Santos, J.L.P.; de Sousa Oliveira, T.; Batista, L.A. Robotic Gait Training for Individuals With Cerebral Palsy: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2017, 98, 2332–2344. [Google Scholar] [CrossRef]
- Lefmann, S.; Russo, R.; Hillier, S. The Effectiveness of Robotic-Assisted Gait Training for Paediatric Gait Disorders: Systematic Review. J. Neuroeng. Rehabil. 2017, 14, 1. [Google Scholar] [CrossRef] [Green Version]
- Rouse, B.; Chaimani, A.; Li, T. Network Meta-Analysis: An Introduction for Clinicians. Intern. Emerg. Med. 2017, 12, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 2015, 162, 777. [Google Scholar] [CrossRef] [Green Version]
- McKenzie, J.E.; Brennan, S.E.; Ryan, R.E.; Thomson, H.J.; Johnston, R.V.; Thomas, J. Chapter 3: Defining the Criteria for Including Studies and How They Will Be Grouped for the Synthesis. In Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021); The Cochrane Collaboration; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; 2021; Available online: https://training.cochrane.org/handbook/current/chapter-03 (accessed on 20 August 2021).
- Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, B. Development and Reliability of a System to Classify Gross Motor Function in Children with Cerebral Palsy. Dev. Med. Child Neurol. 1997, 39, 214–223. [Google Scholar] [CrossRef]
- Palisano, R.J.; Rosenbaum, P.; Bartlett, D.; Livingston, M.H. Content Validity of the Expanded and Revised Gross Motor Function Classification System. Dev. Med. Child Neurol. 2008, 50, 744–750. [Google Scholar] [CrossRef]
- World Health Organization (Ed.) International Classification of Functioning, Disability and Health: ICF; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orwin, R.G.; Vevea, J.L. Evaluating Coding Decisions. Handb. Res. Synth. Meta-Anal. 2009, 2, 177–203. [Google Scholar]
- Russell, D.J.; Rosenbaum, P.L.; Cadman, D.T.; Gowland, C.; Hardy, S.; Jarvis, S. The Gross Motor Function Measure: A means to evaluate the effects of physical therapy. Dev. Med. Child Neurol. 2008, 31, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.; Beath, T.; Bell, J.; Jacobson, G.; Phair, T.; Salbach, N.M.; Wright, F.V. Test-Retest Reliability of the 10-Metre Fast Walk Test and 6-Minute Walk Test in Ambulatory School-Aged Children with Cerebral Palsy. Dev. Med. Child Neurol. 2008, 50, 370–376. [Google Scholar] [CrossRef]
- Rubio-Aparicio, M.; Marín-Martínez, F.; Sánchez-Meca, J.; López-López, J.A. A Methodological Review of Meta-Analyses of the Effectiveness of Clinical Psychology Treatments. Behav. Res. Methods 2018, 50, 2057–2073. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Jackson, D.; Barrett, J.K.; Lu, G.; Ades, A.E.; White, I.R. Consistency and Inconsistency in Network Meta-analysis: Concepts and Models for Multi-arm Studies. Res. Synth. Methods 2012, 3, 98–110. [Google Scholar] [CrossRef] [Green Version]
- Smania, N.; Bonetti, P.; Gandolfi, M.; Cosentino, A.; Waldner, A.; Hesse, S.; Werner, C.; Bisoffi, G.; Geroin, C.; Munari, D. Improved Gait after Repetitive Locomotor Training in Children with Cerebral Palsy. Am. J. Phys. Med. Rehabil. 2011, 90, 137–149. [Google Scholar] [CrossRef]
- Arellano-Martínez, I.T.; Rodríguez-Reyes, G.; Quiñones-Uriostegui, I.; Arellano-Saldaña, M.E. Spatial-temporal analysis and clinical findings of gait: Comparison of two modalities of treatment in children with cerebral palsy-spastic hemiplegia. Preliminary report. Cir. Cir. 2013, 81, 14–20. [Google Scholar]
- Drużbicki, M.; Rusek, W.; Snela, S.; Dudek, J.; Szczepanik, M.; Zak, E.; Durmala, J.; Czernuszenko, A.; Bonikowski, M.; Sobota, G. Functional Effects of Robotic-Assisted Locomotor Treadmill Thearapy in Children with Cerebral Palsy. J. Rehabil. Med. 2013, 45, 358–363. [Google Scholar] [CrossRef] [Green Version]
- Peri, E.; Turconi, A.C.; Biffi, E.; Maghini, C.; Panzeri, D.; Morganti, R.; Pedrocchi, A.; Gagliardi, C. Effects of Dose and Duration of Robot-Assisted Gait Training on Walking Ability of Children Affected by Cerebral Palsy. Technol. Health Care Off. J. Eur. Soc. Eng. Med. 2017, 25, 671–681. [Google Scholar] [CrossRef]
- Wu, M.; Kim, J.; Arora, P.; Gaebler-Spira, D.J.; Zhang, Y. Effects of the Integration of Dynamic Weight Shifting Training Into Treadmill Training on Walking Function of Children with Cerebral Palsy: A Randomized Controlled Study. Am. J. Phys. Med. Rehabil. 2017, 96, 765–772. [Google Scholar] [CrossRef]
- Wallard, L.; Dietrich, G.; Kerlirzin, Y.; Bredin, J. Effect of Robotic-Assisted Gait Rehabilitation on Dynamic Equilibrium Control in the Gait of Children with Cerebral Palsy. Gait Posture 2018, 60, 55–60. [Google Scholar] [CrossRef]
- Aras, B.; Yaşar, E.; Kesikburun, S.; Türker, D.; Tok, F.; Yılmaz, B. Comparison of the Effectiveness of Partial Body Weight-Supported Treadmill Exercises, Robotic-Assisted Treadmill Exercises, and Anti-Gravity Treadmill Exercises in Spastic Cerebral Palsy. Turk. J. Phys. Med. Rehabil. 2019, 65, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Yazıcı, M.; Livanelioğlu, A.; Gücüyener, K.; Tekin, L.; Sümer, E.; Yakut, Y. Effects of Robotic Rehabilitation on Walking and Balance in Pediatric Patients with Hemiparetic Cerebral Palsy. Gait Posture 2019, 70, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: New York, NY, USA, 1977. [Google Scholar]
- Bayon, C.; Raya, R. Robotic Therapies for Children with Cerebral Palsy: A Systematic Review. Transl. Biomed. 2016, 7, 44. [Google Scholar] [CrossRef] [Green Version]
- Fahr, A.; Keller, J.W.; van Hedel, H.J.A. A Systematic Review of Training Methods That May Improve Selective Voluntary Motor Control in Children with Spastic Cerebral Palsy. Front. Neurol. 2020, 11, 572038. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Heim, A.; Borggraefe, I.; Ammann-Reiffer, C.; Berweck, S.; Sennhauser, F.H.; Colombo, G.; Knecht, B.; Heinen, F. Feasibility of Robotic-Assisted Locomotor Training in Children with Central Gait Impairment. Dev. Med. Child Neurol. 2007, 49, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Heim, A.; Ammann-Reiffer, C.; Schmartz, A.; Schafer, J.; Sennhauser, F.H.; Heinen, F.; Knecht, B.; Dabrowski, E.; Borggraefe, I. Improvement of Walking Abilities after Robotic-Assisted Locomotion Training in Children with Cerebral Palsy. Arch. Dis. Child. 2009, 94, 615–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patritti, B.; Romaguera, F.; Deming, L.; Mirelman, A.; Pelliccio, M.; Nimec, D.; Bonato, P. Enhancement and Retention of Locomotor Function in Children with Cerebral Palsy after Robotic Gait Training. Gait Posture 2009, 30, S9–S10. [Google Scholar] [CrossRef]
- Borggraefe, I.; Kiwull, L.; Schaefer, J.S.; Koerte, I.; Blaschek, A.; Meyer-Heim, A.; Heinen, F. Sustainability of Motor Performance after Robotic-Assisted Treadmill Therapy in Children: An Open, Non-Randomized Baseline-Treatment Study. Eur. J. Phys. Rehabil. Med. 2010, 46, 125–131. [Google Scholar]
- Borggraefe, I.; Schaefer, J.S.; Klaiber, M.; Dabrowski, E.; Ammann-Reiffer, C.; Knecht, B.; Berweck, S.; Heinen, F.; Meyer-Heim, A. Robotic-Assisted Treadmill Therapy Improves Walking and Standing Performance in Children and Adolescents with Cerebral Palsy. Eur. J. Paediatr. Neurol. 2010, 14, 496–502. [Google Scholar] [CrossRef]
- Schroeder, A.S.; Homburg, M.; Warken, B.; Auffermann, H.; Koerte, I.; Berweck, S.; Jahn, K.; Heinen, F.; Borggraefe, I. Prospective Controlled Cohort Study to Evaluate Changes of Function, Activity and Participation in Patients with Bilateral Spastic Cerebral Palsy after Robot-Enhanced Repetitive Treadmill Therapy. Eur. J. Paediatr. Neurol. 2014, 18, 502–510. [Google Scholar] [CrossRef]
- Jin, L.H.; Yang, S.; Choi, J.Y.; Sohn, M.K. The Effect of Robot-Assisted Gait Training on Locomotor Function and Functional Capability for Daily Activities in Children with Cerebral Palsy: A Single-Blinded, Randomized Cross-Over Trial. Brain Sci. 2020, 10, 801. [Google Scholar] [CrossRef] [PubMed]
| Study | N | Country | Mean Age | % Male | CP Type | GMFCS Level | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EG | CG | EG | CG | EG | CG | EG | CG | EG | CG | ||
| Smania et al., 2011 [28] | 9 | 9 | Italy | 13.88 | 12.79 | 44.44 | 66.67 | Spastic bilateral | I, II, IV | I, III, IV | |
| Arellano-Martínez et al., 2013 [29] | 8 | 6 | Mexico | 7.5 | 6.83 | 25 | 83.33 | Spastic unilateral | II | ||
| Druzbicki et al., 2013 [30] | 26 | 9 | Poland | 10.1 | 11 | 54 | 54 | Spastic bilateral | II, III | II | |
| Peri et al., 2017 [31] | 12/10/12 1 | 10 | Italy | 8/6.8/10.8 1 | 9.3 | 50/40/58.33 1 | 50 | Spastic bilateral | I, II, III | ||
| Wu et al., 2017 [32] | 11 | 12 | USA | 11.3 | 10.5 | 54.55 | 66.67 | Spastic bilateral | I, II, III, IV | ||
| Wallard et al., 2018 [33] | 14 | 16 | France | 8.3 | 9.6 | 57.14 | 43.75 | Spastic bilateral | II | ||
| Aras et al., 2019 [34] | 10 | 10/9 2 | Turkey | 9.3 | 9.3/9.3 2 | 60 | 60/66.7 2 | Spastic bilateral, Spastic unilateral | II, III | ||
| Yazici et al., 2019 [35] | 12 | 12 | Turkey | 8 | 9 | 50 | 50 | Spastic unilateral | I, II | ||
| Study | N | Robot | Number of Sessions | Session Time (min) | EG Includes Physiotherapy | CG Includes Physiotherapy | Type of Physiotherapy Intervention | |||
|---|---|---|---|---|---|---|---|---|---|---|
| EG | CG | EG | CG | EG | CG | |||||
| Smania et al., 2011 [28] | 9 | 9 | GaitTrainer | 10 | 10 | 30 + 10 | 40 | Yes | Yes | Stretching, joint mobilizations, strength exercises, balance, and gait exercises |
| Arellano-Martínez et al., 2013 [29] | 8 | 6 | Lokomat | 10 | 10 | 30 | 30 | No | Yes | Gait in hydrotherapy tank |
| Druzbicki et al., 2013 [30] | 26 | 9 | Lokomat | 20 | 20 | 45 | - | Yes | Yes | Motor control, increasing stability in the sitting and upright positions, developing walking skills |
| Peri et al., 2017 [31] | 12/10/12 1 | 10 | Lokomat | 40/20 + 20/20 + 20 1 | 40 | 30/30/30 1 | - | No/Yes/Yes 1 | Yes | Gait training, balance, functional skills, strength, stretching |
| Wu et al., 2017 [32] | 11 | 12 | 3DcaLT | 18 | 18 | 30–40 | 30–40 | No | Yes | Gait treadmill training |
| Wallard et al., 2018 [33] | 14 | 16 | Lokomat | 20 | 20 | 40 | - | No | Yes | Unspecified physiotherapy |
| Aras et al., 2019 [34] | 10 | 10/9 2 | Lokomat | 20 | 20/20 2 | 45 | 45/45 2 | No | Yes | - PBWSTE - ATE |
| Yazici et al., 2019 [35] | 12 | 12 | Innowalk-Pro | 36 | - | 30 | - | Yes | Yes | Active functional strength exercises, stretching, squats, stair climbing, functional reach, balance board, single leg balance |
| Study | GMFM-D | GMFM-E | Speed | Endurance | Step Length | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| EG | CG | EG | CG | EG | CG | EG | CG | EG | CG | |
| Smania et al., 2011 [28] | 0.268 (−0.557; 1.093) | −0.108 (−0.88; 0.664) | 0.506 (−0.46; 1.472) | 0.022 (−0.74; 0.784) | 0.602 (−0.439; 1.643) | −0.602 (−1.643; 0.439) | ||||
| Arellano-Martínez et al., 2013 [29] | −0.055 (−0.884; 0.774) | −0.178 (−1.246; 0.89) | ||||||||
| Druzbicki et al., 2013 [30] | 0.139 (−0.275; 0.553) | 0.258 (−0.561; 1.077) | 0.121 (−0.291; 0.533) | 0.09 (−0.678; 0.858) | ||||||
| Peri et al., 2017 [31] | 0.069 (−0.568; 0.706)/0.168 (−0.565; 0.901)/ 0.136 (−0.511; 0.783) 1 | 0.11 (−0.611; 0.831) | 0.075 (−0.562; 0.712)/0.04 (−0.671; 0.751)/0 (−0.633; 0.633) 1 | 0.062 (−0.651; 0.775) | 0.095 (−0.544; 0.734)/0.081 (−0.634; 0.796)/−0.092 (−0.731; 0.547) 1 | 0.014 (−0.697; 0.725) | ||||
| Wu et al., 2017 [32] | 0.05 (−0.62; 0.72) | 0.332 (−0.379; 1.043) | 0.057 (−0.613; 0.727) | 0.061 (−0.574; 0.696) | 0.14 (−0.544; 0.824) | −0.057 (−0.692; 0.578) | 0.529 (−0.333; 1.391) | −0.017 (−0.65; 0.616) | 0.369 (−0.401; 1.139) | 0.186 (−0.473; 0.845) |
| Wallard et al., 2018 [33] | 0.393 (−0.281; 1.067) | 0.125 (−0.416; 0.666) | 0.555 (−0.205; 1.315) | 0.08 (−0.455; 0.615) | 0.664 (−0.163; 1.491) | 0.095 (−0.442; 0.632) | 0.627 (−0.177; 1.431) | 0 (−0.531; 0.531) | ||
| Aras et al., 2019 [34] | 0.191 (−0.55; 0.932) | 0.259 (−0.505; 1.023)/0.422 (−0.487; 1.331) 2 | 0.13 (−0.595; 0.855) | 0.171 (−0.564; 0.906)/0.389 (−0.499; 1.277) 2 | 0.305 (−0.481; 1.091) | 0.305 (−0.481; 1.091)/0 (−0.762; 0.762) 2 | 0.273 (−0.497; 1.043) | 0.227 (−0.526; 0.98)/0.38 (−0.504; 1.264) 2 | 0 (−0.71; 0.71) | 0 (−0.71; 0.71)/0.903 (−0.404; 2.21) 2 |
| Yazici et al., 2019 [35] | 0.344 (−0.373; 1.061) | 0.281 (−0.409; 0.971) | 0.303 (−0.397; 1.003) | 0.122 (−0.521; 0.765) | −0.563 (−1.402; 0.276) | −0.1 (−0.741; 0.541) | 1.243 (−0.129; 2.615) | 0.375 (−0.356; 1.106) | ||
| OUTCOME | PT d (CI) | ROBOT d (CI) | t2 | I2 |
|---|---|---|---|---|
| GMFM D | 0.082 [−0.573; 0.738] | 0.054 [−0.557; 0.664] | 0 | 0% [0.0%; 0.0%] |
| GMFM E | −0.033 [−0.677; 0.610] | 0.071 [−0.523; 0.665] | 0 | 0% [0.0%; 20.2%] |
| Speed | −0.368 [−1.153; 0.417] | −0.091 [−0.682; 0.499] | 0 | 0% [0.0%; 21.3%] |
| Endurance | 0.020 [−0.659; 0.700] | 0.273 [−0.336; 0.883] | 0 | 0% [0.0%; 50.7%] |
| Step Length | 0.174 [−0.778; 1.127] | 0.336 [−0.414; 1.086] | 0.094 | 23.8% [0.0%; 88.3%] |
| Outcome | QBD | df | p |
|---|---|---|---|
| GMFM D | 0.00 | 2 | 0.999 |
| GMFM E | 0.12 | 2 | 0.943 |
| Speed | 0 | 0 | - |
| Endurance | 0.95 | 2 | 0.622 |
| Step Length | 0 | 0 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olmos-Gómez, R.; Gómez-Conesa, A.; Calvo-Muñoz, I.; López-López, J.A. Effects of Robotic-Assisted Gait Training in Children and Adolescents with Cerebral Palsy: A Network Meta-Analysis. J. Clin. Med. 2021, 10, 4908. https://doi.org/10.3390/jcm10214908
Olmos-Gómez R, Gómez-Conesa A, Calvo-Muñoz I, López-López JA. Effects of Robotic-Assisted Gait Training in Children and Adolescents with Cerebral Palsy: A Network Meta-Analysis. Journal of Clinical Medicine. 2021; 10(21):4908. https://doi.org/10.3390/jcm10214908
Chicago/Turabian StyleOlmos-Gómez, Raquel, Antonia Gómez-Conesa, Inmaculada Calvo-Muñoz, and José A. López-López. 2021. "Effects of Robotic-Assisted Gait Training in Children and Adolescents with Cerebral Palsy: A Network Meta-Analysis" Journal of Clinical Medicine 10, no. 21: 4908. https://doi.org/10.3390/jcm10214908
APA StyleOlmos-Gómez, R., Gómez-Conesa, A., Calvo-Muñoz, I., & López-López, J. A. (2021). Effects of Robotic-Assisted Gait Training in Children and Adolescents with Cerebral Palsy: A Network Meta-Analysis. Journal of Clinical Medicine, 10(21), 4908. https://doi.org/10.3390/jcm10214908






