A Novel Multi-Dimensional Clinical Response Index Dedicated to Improving Global Assessment of Pain in Patients with Persistent Spinal Pain Syndrome after Spinal Surgery, Based on a Real-Life Prospective Multicentric Study (PREDIBACK) and Machine Learning Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Objectives
2.4. The Multidimensional Clinical Response Index (MCRI)
2.4.1. Input Data
2.4.2. Variable Reduction and Factor Analysis
2.4.3. Reduction of the Number of Items
2.4.4. Final Factor Analysis and Unidimensional Assessment
2.4.5. Multidimensional Assessment
2.4.6. Correlation between MCRI and NPRS, EQ-5D, ODI, HADS and PMI
2.4.7. Identifying the Patient Global Impression of Change (PGIC) Using the MCRI, NPRS, EQ-5D, ODI, HADS and PMI
3. Results
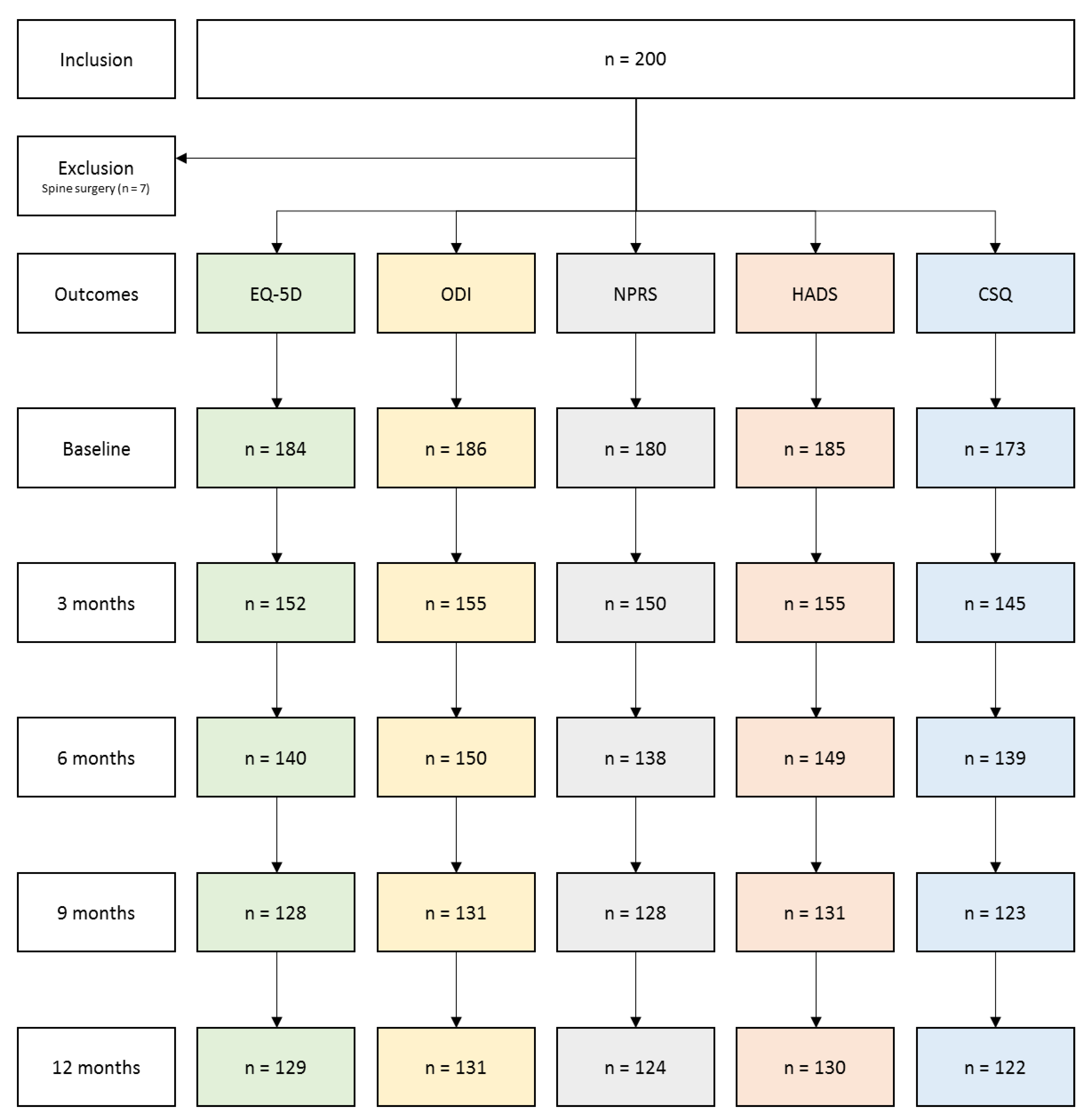
3.1. Follow-Up and Missing Data Description
3.2. Descriptive Statistics of Study Sample at Baseline
3.3. Variable Reduction and Factor Analysis
3.3.1. First Exploratory Analysis
3.3.2. Final Exploratory Analysis
3.3.3. Confirmatory Factor Analysis and Item Selection
3.3.4. Unidimensional Assessment
3.4. Multidimensional Assessment
3.5. The Correlation between MCRI and NPRS, EQ-5D, ODI, HADS and PMI
3.6. Identification of the Patient Global Impression of Change (PGIC) Using the MCRI, NPRS, EQ-5D, ODI, HADS and PMI
4. Discussion
4.1. The NRPS Score: A Gold-Standard Tool Designed to Assess Patient Pain and to Conduct Research on Pain, and Also a Uni-Dimensional Subjective Reflection of a Complex Puzzle. Past and Future Considerations
4.2. PSPS-T2 Patient Pathway
4.3. The Need for a Polaroid Picture of Pain “In Color”, Required to Design a Novel Multi-Dimensional Composite Pain Assessment Index
4.4. A Dynamic Multiplexed Vision of the Patient Pathway Focusing on Clinical Outcomes, Therapeutical Strategy Efficacy, Patient Profiling and AI-Based Outcome Predictions
4.5. Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macrae, W.A. Chronic Post-Surgical Pain: 10 Years On. Br. J. Anaesth. 2008, 101, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.; Peng, P. Failed Back Surgery Syndrome. Pain Med. 2011, 12, 577–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Law, J.D.; Lehman, R.A.; Kirsch, W.M. Reoperation after Lumbar Intervertebral Disc Surgery. J. Neurosurg. 1978, 48, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, T.R.; LaRocca, H.S. Repeat Lumbar Surgery. A Review of Patients with Failure from Previous Lumbar Surgery Treated by Spinal Canal Exploration and Lumbar Spinal Fusion. Spine 1981, 6, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.R.; Han, J.L.; Yang, S.; Hussaini, S.M.Q.; Elsamadicy, A.A.; Parente, B.; Xie, J.; Pagadala, P.; Lad, S.P. Prevalence of Specific Types of Pain Diagnoses in a Sample of United States Adults. Pain Physician 2017, 20, E257–E268. [Google Scholar]
- Christelis, N.; Simpson, B.; Russo, M.; Stanton-Hicks, M.; Barolat, G.; Thomson, S.; Schug, S.; Baron, R.; Buchser, E.; Carr, D.B.; et al. Persistent Spinal Pain Syndrome: A Proposal for Failed Back Surgery Syndrome and ICD-11. Pain Med. 2021, 22, 807–818. [Google Scholar] [CrossRef]
- Rigoard, P.; Ounajim, A.; Goudman, L.; Louis, P.-Y.; Yousri, S.; Roulaud, M.; Bouche, B.; Wood, C.; Page, P.; Lorgeoux, B.; et al. A Novel Multi-Dimensional Clinical Response Index Dedicated to Improve Pain Global Assessment in Patients with Persistent Spinal Pain Syndrome after Spinal Surgery, Based on a Real-Life Prospective Multicentric Study (PREDIBACK) and Machine Learning Techniques. J. Clin. Med. 2021, 10, 4764. [Google Scholar] [CrossRef]
- Schug, S.A.; Lavand’homme, P.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.-D. IASP Taskforce for the Classification of Chronic Pain The IASP Classification of Chronic Pain for ICD-11: Chronic Postsurgical or Posttraumatic Pain. Pain 2019, 160, 45–52. [Google Scholar] [CrossRef]
- Naiditch, N.; Billot, M.; Moens, M.; Goudman, L.; Cornet, P.; Le Breton, D.; Roulaud, M.; Ounajim, A.; Page, P.; Lorgeoux, B.; et al. Persistent Spinal Pain Syndrome Type 2 (PSPS-T2), a Social Pain? Advocacy for a Social Gradient of Health Approach to Chronic Pain. J. Clin. Med. 2021, 10, 2817. [Google Scholar] [CrossRef]
- Ounajim, A.; Billot, M.; Goudman, L.; Louis, P.-Y.; Yousri, S.; Roulaud, M.; Bouche, B.; Wood, C.; Page, P.; Lorgeoux, B.; et al. Finite Mixture Models Based on Pain Intensity, Functional Dis-Ability and Psychological Distress Assessment Allow to Identify Two Distinct Classes of Persistent Spinal Pain Syndrome Type 2 Patients Related to Their Quality of Life. J. Clin. Med. 2021, 10, 4676. [Google Scholar] [CrossRef]
- Breivik, H.; Eisenberg, E.; O’Brien, T. OPENMinds The Individual and Societal Burden of Chronic Pain in Europe: The Case for Strategic Prioritisation and Action to Improve Knowledge and Availability of Appropriate Care. BMC Public Health 2013, 13, 1229. [Google Scholar] [CrossRef] [Green Version]
- Al Kaisy, A.; Pang, D.; Desai, M.J.; Pries, P.; North, R.; Taylor, R.S.; Mc Cracken, L.; Rigoard, P. Failed Back Surgery Syndrome: Who Has Failed? Neurochirurgie 2015, 61 (Suppl. 1), S6–S14. [Google Scholar] [CrossRef]
- Assaker, R.; Zairi, F. Failed Back Surgery Syndrome: To Re-Operate or Not to Re-Operate? A Retrospective Review of Patient Selection and Failures. Neurochirurgie 2015, 61 (Suppl. 1), S77–S82. [Google Scholar] [CrossRef]
- Rigoard, P.; Desai, M.J.; Taylor, R.S. Failed Back Surgery Syndrome: What’s in a Name? A Proposal to Replace “FBSS” by “POPS”…. Neurochirurgie 2015, 61 (Suppl. 1), S16–S21. [Google Scholar] [CrossRef] [PubMed]
- Rigoard, P.; Slavin, K. Neurostimulation Options for Failed Back Surgery Syndrome: The Need for Rational and Objective Measurements. Proposal of an International Clinical Network Using an Integrated Database and Health Economic Analysis: The PROBACK Network. Neurochirurgie 2015, 61 (Suppl. 1), S131–S136. [Google Scholar] [CrossRef]
- Boonstra, A.M.; Schiphorst Preuper, H.R.; Balk, G.A.; Stewart, R.E. Cut-off Points for Mild, Moderate, and Severe Pain on the Visual Analogue Scale for Pain in Patients with Chronic Musculoskeletal Pain. Pain 2014, 155, 2545–2550. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; North, R.; Taylor, R.; Sculpher, M.; den Abeele, C.V.; Gehring, M.; Jacques, L.; Eldabe, S.; Meglio, M.; Molet, J.; et al. Spinal Cord Stimulation vs. Conventional Medical Management: A Prospective, Randomized, Controlled, Multicenter Study of Patients with Failed Back Surgery Syndrome (PROCESS Study). Neuromodulation Technol. Neural Interface 2005, 8, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Deer, T.R.; Mekhail, N.; Provenzano, D.; Pope, J.; Krames, E.; Leong, M.; Levy, R.M.; Abejon, D.; Buchser, E.; Burton, A.; et al. The Appropriate Use of Neurostimulation of the Spinal Cord and Peripheral Nervous System for the Treatment of Chronic Pain and Ischemic Diseases: The Neuromodulation Appropriateness Consensus Committee. Neuromodulation 2014, 17, 515–550, discussion 550. [Google Scholar] [CrossRef]
- Manca, A.; Eldabe, S.; Buchser, E.; Kumar, K.; Taylor, R.S. Relationship between Health-Related Quality of Life, Pain, and Functional Disability in Neuropathic Pain Patients with Failed Back Surgery Syndrome. Value Health 2010, 13, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Turner, J.A.; Franklin, G.; Heagerty, P.J.; Wu, R.; Egan, K.; Fulton-Kehoe, D.; Gluck, J.V.; Wickizer, T.M. The Association between Pain and Disability. Pain 2004, 112, 307–314. [Google Scholar] [CrossRef]
- Baber, Z.; Erdek, M.A. Failed Back Surgery Syndrome: Current Perspectives. J. Pain Res. 2016, 9, 979–987. [Google Scholar] [CrossRef] [Green Version]
- Schofferman, J.; Reynolds, J.; Herzog, R.; Covington, E.; Dreyfuss, P.; O’Neill, C. Failed Back Surgery: Etiology and Diagnostic Evaluation. Spine J. 2003, 3, 400–403. [Google Scholar] [CrossRef]
- Rigoard, P.; Blond, S.; David, R.; Mertens, P. Pathophysiological Characterisation of Back Pain Generators in Failed Back Surgery Syndrome (Part B). Neurochirurgie 2015, 61, S35–S44. [Google Scholar] [CrossRef]
- Waddell, G. The Back Pain Revolution; Elsevier Health Sciences: Amsterdam, The Netherlands, 2004; ISBN 978-0-7020-4325-3. [Google Scholar]
- Goudman, L.; De Smedt, A.; Eldabe, S.; Rigoard, P.; Linderoth, B.; De Jaeger, M.; Moens, M. Discover Consortium High-Dose Spinal Cord Stimulation for Patients with Failed Back Surgery Syndrome: A Multicenter Effectiveness and Prediction Study. Pain 2021, 162, 582–590. [Google Scholar] [CrossRef]
- Ferreira-Valente, M.A.; Pais-Ribeiro, J.L.; Jensen, M.P. Validity of Four Pain Intensity Rating Scales. Pain 2011, 152, 2399–2404. [Google Scholar] [CrossRef]
- Brooks, R. EuroQol: The Current State of Play. Health Policy 1996, 37, 53–72. [Google Scholar] [CrossRef]
- Fairbank, J.C.; Couper, J.; Davies, J.B.; O’Brien, J.P. The Oswestry Low Back Pain Disability Questionnaire. Physiotherapy 1980, 66, 271–273. [Google Scholar] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigoard, P.; Nivole, K.; Blouin, P.; Monlezun, O.; Roulaud, M.; Lorgeoux, B.; Bataille, B.; Guetarni, F. A Novel, Objective, Quantitative Method of Evaluation of the Back Pain Component Using Comparative Computerized Multi-Parametric Tactile Mapping before/after Spinal Cord Stimulation and Database Analysis: The “Neuro-Pain’t” Software. Neurochirurgie 2015, 61 (Suppl. 1), S99–S108. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Jahng, S. Determining the Number of Factors Using Parallel Analysis and Its Recent Variants. Psychol. Methods 2019, 24, 452–467. [Google Scholar] [CrossRef]
- Suh, Y. The Performance of Maximum Likelihood and Weighted Least Square Mean and Variance Adjusted Estimators in Testing Differential Item Functioning With Nonnormal Trait Distributions. Struct. Equ. Model. Multidiscip. J. 2015, 22, 568–580. [Google Scholar] [CrossRef]
- Estabrook, R.; Neale, M. A Comparison of Factor Score Estimation Methods in the Presence of Missing Data: Reliability and an Application to Nicotine Dependence. Multivar. Behav. Res. 2013, 48, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, L.; Scheman, J. Patient Global Impression of Change Scores within the Context of a Chronic Pain Rehabilitation Program. J. Pain 2009, 10, S73. [Google Scholar] [CrossRef]
- Freynhagen, R.; Baron, R.; Gockel, U.; Tölle, T.R. PainDETECT: A New Screening Questionnaire to Identify Neuropathic Components in Patients with Back Pain. Curr. Med. Res. Opin. 2006, 22, 1911–1920. [Google Scholar] [CrossRef]
- Nikaido, T.; Sumitani, M.; Sekiguchi, M.; Konno, S. The Spine PainDETECT Questionnaire: Development and Validation of a Screening Tool for Neuropathic Pain Caused by Spinal Disorders. PLoS ONE 2018, 13, e0193987. [Google Scholar] [CrossRef] [Green Version]
- Bouhassira, D.; Attal, N.; Alchaar, H.; Boureau, F.; Brochet, B.; Bruxelle, J.; Cunin, G.; Fermanian, J.; Ginies, P.; Grun-Overdyking, A.; et al. Comparison of Pain Syndromes Associated with Nervous or Somatic Lesions and Development of a New Neuropathic Pain Diagnostic Questionnaire (DN4). Pain 2005, 114, 29–36. [Google Scholar] [CrossRef]
- Udén, A.; Aström, M.; Bergenudd, H. Pain Drawings in Chronic Back Pain. Spine (Phila Pa 1976) 1988, 13, 389–392. [Google Scholar] [CrossRef]
- Dworkin, R.H.; Turk, D.C.; Wyrwich, K.W.; Beaton, D.; Cleeland, C.S.; Farrar, J.T.; Haythornthwaite, J.A.; Jensen, M.P.; Kerns, R.D.; Ader, D.N.; et al. Interpreting the Clinical Importance of Treatment Outcomes in Chronic Pain Clinical Trials: IMMPACT Recommendations. J. Pain 2008, 9, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Turk, D.C.; Dworkin, R.H.; Allen, R.R.; Bellamy, N.; Brandenburg, N.; Carr, D.B.; Cleeland, C.; Dionne, R.; Farrar, J.T.; Galer, B.S.; et al. Core Outcome Domains for Chronic Pain Clinical Trials: IMMPACT Recommendations. Pain 2003, 106, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, J.C.; Sullivan, M.D. Intensity of Chronic Pain--The Wrong Metric? N. Engl. J. Med. 2015, 373, 2098–2099. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.D.; Ballantyne, J.C. Must We Reduce Pain Intensity to Treat Chronic Pain? Pain 2016, 157, 65–69. [Google Scholar] [CrossRef]
- Kamper, S.J.; Apeldoorn, A.T.; Chiarotto, A.; Smeets, R.J.E.M.; Ostelo, R.W.J.G.; Guzman, J.; van Tulder, M.W. Multidisciplinary Biopsychosocial Rehabilitation for Chronic Low Back Pain. Cochrane Database Syst. Rev. 2014, CD000963. [Google Scholar] [CrossRef] [PubMed]
- Marris, D.; Theophanous, K.; Cabezon, P.; Dunlap, Z.; Donaldson, M. The Impact of Combining Pain Education Strategies with Physical Therapy Interventions for Patients with Chronic Pain: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Physiother. Theory Pract. 2021, 37, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Naiditch, N.; Billot, M.; Goudman, L.; Cornet, P.; Roulaud, M.; Ounajim, A.; Page, P.; Lorgeoux, B.; Nivole, K.; Pries, P.; et al. Professional Status of Persistant Spinal Pain Syndrome Patients after Spinal Surgery (PSPS-T2): What Does Really Matter? A pro-Spective Study Introducing the Concept of “Adapted Professional Activity” Infering from Clinical, Psychological and Social Influence. 2021. submitted. [Google Scholar]
- Rigoard, P.; Billot, M.; Ingrand, P.; Durand-Zaleski, I.; Roulaud, M.; Peruzzi, P.; Dam Hieu, P.; Voirin, J.; Raoul, S.; Page, P.; et al. How Should We Use Multicolumn Spinal Cord Stimulation to Optimize Back Pain Spatial Neural Targeting? A Prospective, Multicenter, Randomized, Double-Blind, Controlled Trial (ESTIMET Study). Neuromodulation 2021, 24, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Gewandter, J.S.; McDermott, M.P.; Evans, S.; Katz, N.P.; Markman, J.D.; Simon, L.S.; Turk, D.C.; Dworkin, R.H. Composite Outcomes for Pain Clinical Trials: Considerations for Design and Interpretation. Pain 2021, 162, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.V.; Allen, R.; Burke, L.; Farrar, J.T.; Gewandter, J.S.; Gilron, I.; Katz, N.P.; Markman, J.D.; Marshall, S.F.; Resnick, M.; et al. Evaluation of Composite Responder Outcomes of Pain Intensity and Physical Function in Neuropathic Pain Clinical Trials: An ACTTION Individual Patient Data Analysis. Pain 2018, 159, 2245–2254. [Google Scholar] [CrossRef]
- Pilitsis, J.G.; Fahey, M.; Custozzo, A.; Chakravarthy, K.; Capobianco, R. Composite Score Is a Better Reflection of Patient Response to Chronic Pain Therapy Compared With Pain Intensity Alone. Neuromodulation 2021, 24, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Rigoard, P.; Ounajim, A.; Goudman, L.; Roulaud, M.; Naiditch, N.; Bouche, B.; Page, P.; Lorgeoux, B.; Baron, S.; Adjali, N.; et al. Management of Persistent Spinal Pain Syndrome after Surgery: How to Change the Paradigm? 2021. submitted. [Google Scholar]


| Item | Functional Disability | Depression/Anxiety |
|---|---|---|
| EQ-5D_Mobility | 0.53 | 0.05 |
| EQ-5D_Self care | 0.48 | −0.07 |
| EQ-5D_Usual activities | 0.48 | 0.09 |
| ODI_Personal care | 0.46 | 0.03 |
| ODI_Lifting | 0.31 | −0.07 |
| ODI_Walking | 0.47 | −0.06 |
| ODI_Sitting | 0.54 | −0.12 |
| ODI_Standing | 0.59 | −0.12 |
| ODI_Sleeping | 0.32 | 0.11 |
| ODI_Sex life | 0.40 | 0.14 |
| ODI_Social life | 0.34 | 0.19 |
| ODI_Travelling | 0.47 | 0.05 |
| HAD_Feeling tense or ‘wound up’ | 0.11 | 0.31 |
| HAD_Enjoying things you used to enjoy | 0.16 | 0.43 |
| HAD_Feeling that something awful is about to happen | −0.10 | 0.52 |
| HAD_Laughing and seeing the good side of things | 0.04 | 0.50 |
| HAD_Worrying thoughts going through your mind | −0.03 | 0.51 |
| HAD_Feeling cheerful | 0.05 | 0.45 |
| HAD_Feeling slowed down | 0.30 | 0.29 |
| HAD_Frightened feeling like ‘butterflies’ in the stomach | −0.15 | 0.70 |
| HAD_Looking forward with enjoyment to things | 0.05 | 0.49 |
| HAD_Sudden feeling of panic | −0.06 | 0.56 |
| HAD_Enjoying a good book or radio/TV program | −0.09 | 0.40 |
| Functional Disability | - | 0.54 |
| Latent Variables | Standardized Coefficients | 95% CI | p-Value |
|---|---|---|---|
| Level 1: within-patients effects | |||
| Functional Disability | |||
| EQ5D_Mobility | 1 | - | - |
| EQ5D_Self care | 0.555 | [0.303,0.807] | <0.0001 |
| EQ5D_Usual activities | 0.971 | [0.741,1.201] | <0.0001 |
| ODI_Personal care | 0.901 | [0.494,1.307] | <0.0001 |
| ODI_Lifting | 0.663 | [0.338,0.988] | <0.0001 |
| ODI_Walking | 0.703 | [0.469,0.936] | <0.0001 |
| ODI_Sitting | 0.720 | [0.492,0.947] | <0.0001 |
| ODI_Standing | 0.842 | [0.582,1.102] | <0.0001 |
| ODI_Sleeping | 0.828 | [0.488,1.168] | <0.0001 |
| ODI_Sex life | 1.231 | [0.757,1.705] | <0.0001 |
| ODI_Social life | 1.354 | [0.761,1.947] | <0.0001 |
| ODI_Travelling | 0.951 | [0.607,1.295] | <0.0001 |
| HAD_Feeling slowed down | 0.944 | [0.632,1.257] | <0.0001 |
| Depression & anxiety | |||
| HAD_Feeling tense or ’wound up’ | 1 | - | - |
| HAD_Enjoying thing you used to enjoy | 1.914 | [1.231,2.597] | <0.0001 |
| HAD_Feeling that something awful is about to happen | 1.140 | [0.492,1.789] | 0.0006 |
| HAD_Laughing and seeing the good side of things | 1.560 | [0.960,2.160] | <0.0001 |
| HAD_Worrying thoughts going through your mind | 1.094 | [0.680,1.508] | <0.0001 |
| HAD_Feeling cheerful | 1.183 | [0.802,1.563] | <0.0001 |
| HAD_Frightened feeling like ’butterflies’ in the stomach | 1.279 | [0.765,1.793] | <0.0001 |
| HAD_Looking forward with enjoyment to things | 1.609 | [1.108,2.110] | <0.0001 |
| HAD_Sudden feeling of panic | 1.003 | [0.549,1.455] | <0.0001 |
| HAD_Enjoying a good book or radio/TV program | 0.822 | [0.454,1.191] | <0.0001 |
| Level 2: between-patients effects | |||
| Functional Disability | |||
| EQ5D_Mobility | 1 | - | - |
| EQ5D_Self care | 0.928 | [0.673,1.183] | <0.0001 |
| EQ5D_Usual activities | 0.840 | [0.678,1.002] | <0.0001 |
| ODI_Personal care | 0.987 | [0.683,1.291] | <0.0001 |
| ODI_Lifting | 0.878 | [0.607,1.148] | <0.0001 |
| ODI_Walking | 1.041 | [0.840,1.242] | <0.0001 |
| ODI_Sitting | 0.642 | [0.373,0.911] | <0.0001 |
| ODI_Standing | 0.829 | [0.607,1.051] | <0.0001 |
| ODI_Sleeping | 0.501 | [0.288,0.713] | <0.0001 |
| ODI_Sex life | 0.865 | [0.427,1.304] | 0.00011 |
| ODI_Social life | 0.901 | [0.534,1.267] | <0.0001 |
| ODI_Travelling | 0.748 | [0.555,0.941] | <0.0001 |
| HAD_Feeling slowed down | 0.434 | [0.227,0.641] | <0.0001 |
| Depression & anxiety | |||
| HAD_Feeling tense or ’wound up’ | 1 | - | - |
| HAD_Enjoying thing you used to enjoy | 0.857 | [0.216,1.496] | 0.0088 |
| HAD_Feeling that something awful is about to happen | 1.740 | [1.160,2.320] | <0.0001 |
| HAD_Laughing and seeing the good side of things | 1.078 | [0.572,1.583] | <0.0001 |
| HAD_Worrying thoughts going through your mind | 1.482 | [1.130,1.834] | <0.0001 |
| HAD_Feeling cheerful | 0.820 | [0.412,1.228] | <0.0001 |
| HAD_Frightened feeling like ’butterflies’ in the stomach | 1.393 | [0.860,1.925] | <0.0001 |
| HAD_Looking forward with enjoyment to things | 0.819 | [0.219,1.419] | 0.0075 |
| HAD_Sudden feeling of panic | 1.392 | [0.784,2.001] | <0.0001 |
| HAD_Enjoying a good book or radio/TV program | 0.339 | [−0.009,0.687] | 0.057 |
| Goodness of Fit Measures | Two Factor CFA Model | Functional Disability | Depression & Anxiety |
|---|---|---|---|
| Chi-squared test * | Chi2 = 940.66; p < 0.0001 | Chi2 = 333.84; p < 0.0001 | Chi2 = 260.00; p < 0.0001 |
| RMSAE | 0.046; 90% CI = [0.042,0.050] | 0.056; 90% CI = [0.048,0.063] | 0.064; 90% CI = [0.056,0.072] |
| Robust CFI | 0.848 | 0.876 | 0.886 |
| ODI Score | EQ-5D Index | NPRS | HADS Total Score | Mapping Intensity | |
|---|---|---|---|---|---|
| Baseline | |||||
| Functional Disability | 0.92 95% CI = [0.89,0.94] | −0.81 95% CI = [−0.86,−0.74] | 0.40 95% CI = [0.24,0.53] | 0.35 95% CI = [0.20,0.49] | 0.30 95% CI = [0.14,0.44] |
| Depression/Anxiety | 0.38 95% CI = [0.23,0.52] | −0.49 95% CI = [−0.60,−0.35] | 0.35 95% CI = [0.20,0.49] | 0.96 95% CI = [0.94,0.97] | −0.02 95% CI = [−0.19,0.15] |
| M3 | |||||
| Functional Disability | 0.95 95% CI = [0.93,0.97] | −0.83 95% CI = [−0.88,−0.75] | 0.52 95% CI = [0.36,0.65] | 0.48 95% CI = [0.32,0.61] | 0.30 95% CI = [0.12,0.47] |
| Depression/Anxiety | 0.51 95% CI = [0.34,0.64] | −0.53 95% CI = [−0.65,−0.37] | 0.36 95% CI = [0.18,0.52] | 0.97 95% CI = [0.96,0.98] | 0.04 95% CI = [−0.15,0.23] |
| M6 | |||||
| Functional Disability | 0.97 95% CI = [0.96,0.98] | −0.87 95% CI = [−0.91,−0.81] | 0.68 95% CI = [0.55,0.78] | 0.53 95% CI = [0.37,0.66] | 0.26 95% CI = [0.07,0.43] |
| Depression/Anxiety | 0.56 95% CI = [0.41,0.68] | −0.62 95% CI = [−0.73,−0.48] | 0.48 95% CI = [0.31,0.62] | 0.98 95% CI = [0.96,0.98] | 0.11 95% CI = [−0.09,0.30] |
| M9 | |||||
| Functional Disability | 0.96 95% CI = [0.95,0.98] | −0.87 95% CI = [−0.91,−0.80] | 0.60 95% CI = [0.44,0.72] | 0.48 95% CI = [0.30,0.63] | 0.36 95% CI = [0.16,0.53] |
| Depression/Anxiety | 0.55 95% CI = [0.39,0.68] | −0.60 95% CI = [−0.71,−0.44] | 0.41 95% CI = [0.21,0.57] | 0.97 95% CI = [0.96,0.98] | 0.24 95% CI = [0.03,0.43] |
| M12 | |||||
| Functional Disability | 0.96 95% CI = [0.95,0.98] | −0.86 95% CI = [−0.91,−0.79] | 0.59 95% CI = [0.43,0.71] | 0.57 95% CI = [0.40,0.70] | 0.38 95% CI = [0.18,0.55] |
| Depression/Anxiety | 0.54 95% CI = [0.38,0.68] | −0.52 95% CI = [−0.66,−0.35] | 0.40 95% CI = [0.20,0.56] | 0.92 95% CI = [0.88,0.95] | 0.07 95% CI = [−0.15,0.28] |
| Variables | 1st Principal Component: 49.99% of the Total Variance |
|---|---|
| Eigenvalue | 1.99 |
| Functional Disability score | −0.811 |
| NPRS score | −0.771 |
| Depression & anxiety score | −0.684 |
| Mapping intensity score | −0.529 |
| Variables | ODI | EQ-5D | NPRS | Total HADS | Mapping Intensity |
|---|---|---|---|---|---|
| Correlations at baseline | |||||
| MCRI | −0.677 *** | 0.690 *** | −0.677 *** | −0.622 *** | −0.423 *** |
| ODI | 1 | −0.631 *** | 0.415 *** | 0.279 *** | 0.272 *** |
| EQ-5D | - | 1 | −0.346 *** | −0.434 *** | −0.250 *** |
| NPRS | - | - | 1 | 0.289 *** | 0.235 ** |
| Total HADS | - | - | - | 1 | 0.131 |
| Mapping intensity | - | - | - | - | 1 |
| Correlations at 3 months | |||||
| MCRI | −0.771 *** | 0.714 *** | −0.761 *** | −0.616 *** | −0.527 *** |
| ODI | 1 | −0.728 *** | 0.567 *** | 0.472 *** | 0.261 *** |
| EQ-5D | - | 1 | −0.521 *** | −0.492 *** | −0.244 ** |
| NPRS | - | - | 1 | 0.406 *** | 0.322 *** |
| Total HADS | - | - | - | 1 | 0.050 |
| Mapping intensity | - | - | - | - | 1 |
| Correlations at 6 months | |||||
| MCRI | −0.762 *** | 0.788 *** | −0.828 *** | −0.631 *** | −0.537 *** |
| ODI | 1 | −0.776 *** | 0.689 *** | 0.542 *** | 0.262 *** |
| EQ-5D | - | 1 | −0.659 *** | −0.586 *** | −0.310 *** |
| NPRS | - | - | 1 | 0.442 *** | 0.455 *** |
| Total HADS | - | - | - | 1 | 0.106 |
| Mapping intensity | - | - | - | - | 1 |
| Correlations at 9 months | |||||
| MCRI | −0.775 *** | 0.803 *** | −0.774 *** | −0.655 *** | −0.637 *** |
| ODI | 1 | −0.784 *** | 0.608 *** | 0.611 *** | 0.314 *** |
| EQ-5D | - | 1 | −0.645 *** | −0.581 *** | −0.350 *** |
| NPRS | - | - | 1 | 0.483 *** | 0.424 *** |
| Total HADS | - | - | - | 1 | 0.244 ** |
| Mapping intensity | - | - | - | - | 1 |
| Correlations at 12 months | |||||
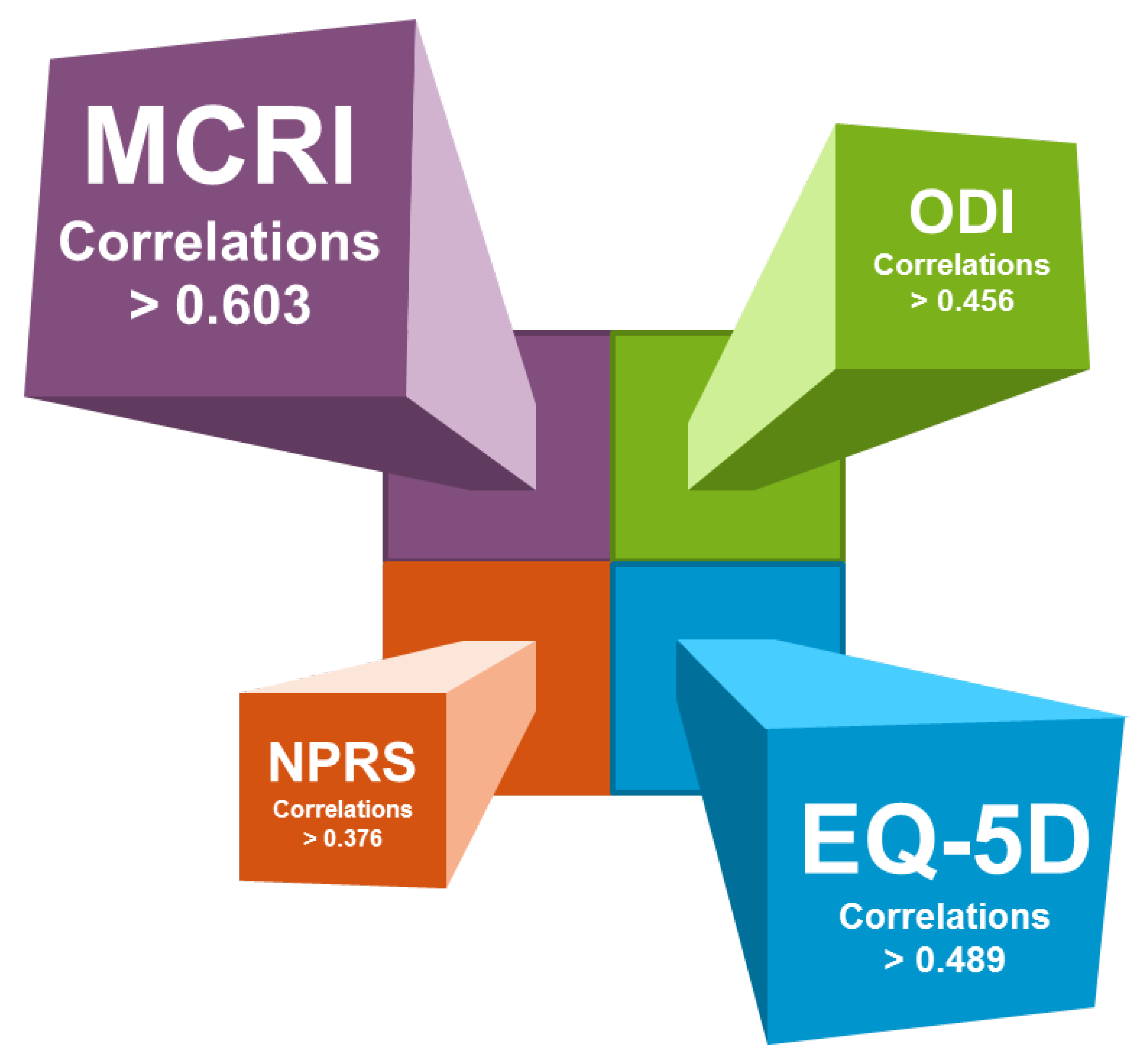
| MCRI | −0.758 *** | 0.730 *** | −0.806 *** | −0.603 *** | −0.638 *** |
| ODI | 1 | −0.749 *** | 0.514 *** | 0.476 *** | 0.456 *** |
| EQ-5D | - | 1 | −0.544 *** | −0.520 *** | −0.489 *** |
| NPRS | - | - | 1 | 0.376 *** | 0.553 *** |
| Total HADS | - | - | - | 1 | 0.291 *** |
| Mapping intensity | - | - | - | - | 1 |
| Satisfactory Self-Perceived Clinical Improvement | Unsatisfactory Self-Perceived Clinical Improvement | |
|---|---|---|
| Change between M0–M3 | ||
| MCRI ≥ 1.05 < 1.05 | 16 (sensitivity = 64.0%) 9 | 29 95 (specificity = 76.6%) |
| ODI ≥ 6.7 < 6.7 | 14 (sensitivity = 56.0%) 11 | 27 93 (specificity = 77.5%) |
| EQ-5D ≥ 0.13 < 0.13 | 12 (sensitivity = 50.0%) 12 | 38 79 (specificity = 67.5%) |
| NPRS ≥ 2 < 2 | 13 (sensitivity = 52.0%) 12 | 25 88 (specificity = 77.9%) |
| HADS ≥ 5 < 5 | 14 (sensitivity = 56.0%) 11 | 23 97 (specificity = 80.8%) |
| Mapping intensity≥ 468 < 468 | 15 (sensitivity = 60.0%) 10 | 44 77 (specificity = 63.6%) |
| Change between M0–M6 | ||
| MCRI ≥ 1.05 < 1.05 | 19 (sensitivity = 65.5%) 10 | 25 90 (specificity = 78.3%) |
| ODI ≥ 6.7 < 6.7 | 21 (sensitivity = 72.4%) 8 | 33 78 (specificity = 70.3%) |
| EQ-5D ≥ 0.13 < 0.13 | 21 (sensitivity = 75.0%) 7 | 31 72 (specificity = 69.9%) |
| NPRS ≥ 2 < 2 | 19 (sensitivity = 70.4%) 8 | 28 72 (specificity = 72.0%) |
| HADS ≥ 5 < 5 | 17 (sensitivity = 58.6%) 12 | 21 90 (specificity = 81.1%) |
| Mapping intensity ≥ 468 < 468 | 19 (sensitivity = 65.5%) 10 | 51 61 (specificity = 54.5%) |
| Change between M0–M9 | ||
| MCRI ≥ 1.05 < 1.05 | 22 (sensitivity = 75.9%) 7 | 22 77 (specificity = 77.8%) |
| ODI ≥ 6.7 < 6.7 | 19 (sensitivity = 65.5%) 10 | 27 71 (specificity = 72.4%) |
| EQ-5D ≥ 0.13 < 0.13 | 16 (sensitivity = 57.1%)12 | 30 65 (specificity = 68.4%) |
| NPRS ≥ 2 < 2 | 20 (sensitivity = 69.0%)9 | 33 60 (specificity = 64.5%) |
| HADS ≥ 5 < 5 | 17 (sensitivity = 58.6%) 12 | 20 78 (specificity = 79.6%) |
| Mapping intensity ≥ 468 < 468 | 12 (sensitivity = 55.6%) 15 | 37 57 (specificity = 60.6%) |
| Change between M0–M12 | ||
| MCRI ≥ 1.05 < 1.05 | 24 (sensitivity = 77.4%) 7 | 19 75 (specificity = 79.8%) |
| ODI ≥ 6.7 < 6.7 | 20 (sensitivity = 64.5%) 11 | 27 66 (specificity = 71.0%) |
| EQ-5D ≥ 0.13 < 0.13 | 20 (sensitivity = 69.0%) 9 | 29 62 (specificity = 68.1%) |
| NPRS ≥ 2 < 2 | 22 (sensitivity = 71.0%) 9 | 27 59 (specificity = 68.6%) |
| HADS ≥ 5 < 5 | 20 (sensitivity = 64.5%) 11 | 22 71 (specificity = 76.3%) |
| Mapping intensity≥ 468 < 468 | 19 (sensitivity = 65.5%) 10 | 31 59 (specificity = 65.6%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigoard, P.; Ounajim, A.; Goudman, L.; Louis, P.-Y.; Slaoui, Y.; Roulaud, M.; Naiditch, N.; Bouche, B.; Page, P.; Lorgeoux, B.; et al. A Novel Multi-Dimensional Clinical Response Index Dedicated to Improving Global Assessment of Pain in Patients with Persistent Spinal Pain Syndrome after Spinal Surgery, Based on a Real-Life Prospective Multicentric Study (PREDIBACK) and Machine Learning Techniques. J. Clin. Med. 2021, 10, 4910. https://doi.org/10.3390/jcm10214910
Rigoard P, Ounajim A, Goudman L, Louis P-Y, Slaoui Y, Roulaud M, Naiditch N, Bouche B, Page P, Lorgeoux B, et al. A Novel Multi-Dimensional Clinical Response Index Dedicated to Improving Global Assessment of Pain in Patients with Persistent Spinal Pain Syndrome after Spinal Surgery, Based on a Real-Life Prospective Multicentric Study (PREDIBACK) and Machine Learning Techniques. Journal of Clinical Medicine. 2021; 10(21):4910. https://doi.org/10.3390/jcm10214910
Chicago/Turabian StyleRigoard, Philippe, Amine Ounajim, Lisa Goudman, Pierre-Yves Louis, Yousri Slaoui, Manuel Roulaud, Nicolas Naiditch, Bénédicte Bouche, Philippe Page, Bertille Lorgeoux, and et al. 2021. "A Novel Multi-Dimensional Clinical Response Index Dedicated to Improving Global Assessment of Pain in Patients with Persistent Spinal Pain Syndrome after Spinal Surgery, Based on a Real-Life Prospective Multicentric Study (PREDIBACK) and Machine Learning Techniques" Journal of Clinical Medicine 10, no. 21: 4910. https://doi.org/10.3390/jcm10214910
APA StyleRigoard, P., Ounajim, A., Goudman, L., Louis, P.-Y., Slaoui, Y., Roulaud, M., Naiditch, N., Bouche, B., Page, P., Lorgeoux, B., Baron, S., Charrier, E., Poupin, L., Rannou, D., de Montgazon, G. B., Roy-Moreau, B., Grimaud, N., Adjali, N., Nivole, K., ... Billot, M. (2021). A Novel Multi-Dimensional Clinical Response Index Dedicated to Improving Global Assessment of Pain in Patients with Persistent Spinal Pain Syndrome after Spinal Surgery, Based on a Real-Life Prospective Multicentric Study (PREDIBACK) and Machine Learning Techniques. Journal of Clinical Medicine, 10(21), 4910. https://doi.org/10.3390/jcm10214910










