Right Ventricular Structure and Function in Young Adults Born Preterm at Very Low Birth Weight
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Visits
2.3. Transthoracic Echocardiography
2.4. Assessment of Right Ventricular Strain
2.5. Lung Function Testing
2.6. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Echocardiographic Outcomes
3.2.1. Structure
3.2.2. Function
3.3. Impact of Perinatal Risk Factors
3.3.1. Gestational Age
3.3.2. Birth Weight
3.3.3. Bronchopulmonary Dysplasia
3.3.4. Antenatal Steroid Exposure
3.4. Lung Function in Adulthood
3.5. Relationship to Left Ventricular Indices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saigal, S.; Doyle, L.; Saigal, S.; Doyle, L.W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008, 371, 261–269. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.I.; Bell, E.; Walsh, M.C.; Carlo, W.A.; Shankaran, S.; Laptook, A.R.; Sánchez, P.J.; Van Meurs, K.P.; Wyckoff, M.H.; et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA 2015, 314, 1039–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darlow, B.A.; Cust, A.E.; Donoghue, D.A. Improved outcomes for very low birthweight infants: Evidence from New Zealand national population based data. Arch. Dis. Child.—Fetal Neonatal Ed. 2003, 88, F23–F28. [Google Scholar] [CrossRef]
- Chow, S.S.W.; Creighton, P.; Chambers, G.M.; Lui, K. Report of the Australian and New Zealand Neonatal Network 2017; Australian and New Zealand Neonatal Network (ANZNN): Sydney, Australia, 2019. [Google Scholar]
- Fanaroff, A.A.; Stoll, B.J.; Wright, L.L.; Carlo, W.A.; Ehrenkranz, R.A.; Stark, A.R.; Bauer, C.R.; Donovan, E.F.; Korones, S.B.; Laptook, A.R.; et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am. J. Obstet. Gynecol. 2007, 196, 147.e1–147.e8. [Google Scholar] [CrossRef] [PubMed]
- Raju, T.N.K.; Buist, A.S.; Blaisdell, C.J.; Moxey-Mims, M.; Saigal, S. Adults born preterm: A review of general health and system-specific outcomes. Acta Paediatr. 2017, 106, 1409–1437. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.W.; Anderson, P.J. Adult outcome of extremely preterm infants. Pediatrics 2010, 126, 342–351. [Google Scholar] [CrossRef] [Green Version]
- Hack, M.; Flannery, D.J.; Schluchter, M.; Cartar, L.; Borawski, E.; Klein, N. Outcomes in Young Adulthood for Very-Low-Birth-Weight Infants. N. Engl. J. Med. 2002, 346, 149–157. [Google Scholar] [CrossRef]
- Crump, C.; Sundquist, J.; Winkleby, M.A.; Sundquist, K. Gestational age at birth and mortality from infancy into mid-adulthood: A national cohort study. Lancet Child Adolesc. Health 2019, 3, 408–417. [Google Scholar] [CrossRef]
- Bensley, J.G.; Moore, L.; De Matteo, R.; Harding, R.; Black, M.J. Impact of preterm birth on the developing myocardium of the neonate. Pediatr. Res. 2018, 83, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Bensley, J.G.; Stacy, V.K.; De Matteo, R.; Harding, R.; Black, M.J. Cardiac remodelling as a result of pre-term birth: Implications for future cardiovascular disease. Eur. Heart J. 2010, 31, 2058–2066. [Google Scholar] [CrossRef]
- Aye, C.Y.L.; Lewandowski, A.J.; Lamata, P.; Upton, R.; Davis, E.; Ohuma, E.O.; Kenworthy, Y.; Boardman, H.; Wopperer, S.; Packham, A.; et al. Disproportionate cardiac hypertrophy during early postnatal development in infants born preterm. Pediatr. Res. 2017, 82, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, A.J.; Augustine, D.; Lamata, P.; Davis, E.F.; Lazdam, M.; Francis, J.; McCormick, K.; Wilkinson, A.R.; Singhal, A.; Lucas, A.; et al. Preterm heart in adult life: Cardiovascular magnetic resonance reveals distinct differences in left ventricular mass, geometry, and function. Circulation 2013, 127, 197–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, S.L.; Bray, H.; Troughton, R.; Elliott, J.; Frampton, C.; Horwood, J.; Darlow, B.A. Cardiovascular Outcomes in Young Adulthood in a Population-Based Very Low Birth Weight Cohort. J. Pediatr. 2020, 225, 74–79.e3. [Google Scholar] [CrossRef] [PubMed]
- Goss, K.N.; Haraldsdottir, K.; Beshish, A.G.; Barton, G.P.; Watson, A.M.; Palta, M.; Chesler, N.C.; Francois, C.J.; Wieben, O.; Eldridge, M.W. Association Between Preterm Birth and Arrested Cardiac Growth in Adolescents and Young Adults. JAMA Cardiol. 2020, 5, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, R.R.; Beare, R.; Doyle, L.W.; Smolich, J.J.; Cheung, M.M.H.; Callanan, C.; Davis, N.; De Luca, C.R.; Duff, J.; Hutchinson, E.; et al. Elevated Blood Pressure with Reduced Left Ventricular and Aortic Dimensions in Adolescents Born Extremely Preterm. J. Pediatr. 2016, 172, 75–80.e2. [Google Scholar] [CrossRef]
- Cox, D.J.; Bai, W.; Price, A.N.; Edwards, A.D.; Rueckert, D.; Groves, A.M. Ventricular remodeling in preterm infants: Computational cardiac magnetic resonance atlasing shows significant early remodeling of the left ventricle. Pediatr. Res. 2019, 85, 807–815. [Google Scholar] [CrossRef]
- Huckstep, O.J.; Williamson, W.; Telles, F.; Burchert, H.; Bertagnolli, M.; Herdman, C.; Arnold, L.; Smillie, R.; Mohamed, A.; Boardman, H.; et al. Physiological Stress Elicits Impaired Left Ventricular Function in Preterm-Born Adults. J. Am. Coll. Cardiol. 2018, 71, 1347–1356. [Google Scholar] [CrossRef]
- Goss, K.N.; Beshish, A.G.; Barton, G.P.; Haraldsdottir, K.; Levin, T.S.; Tetri, L.H.; Battiola, T.J.; Mulchrone, A.M.; Pegelow, D.F.; Palta, M.; et al. Early Pulmonary Vascular Disease in Young Adults Born Preterm. Am. J. Respir. Crit. Care Med. 2018, 198, 1549–1558. [Google Scholar] [CrossRef]
- Carr, H.; Cnattingius, S.; Granath, F.; Ludvigsson, J.F.; Edstedt Bonamy, A.K. Preterm Birth and Risk of Heart Failure Up to Early Adulthood. J. Am. Coll. Cardiol. 2017, 69, 2634–2642. [Google Scholar] [CrossRef]
- Crump, C.; Groves, A.; Sundquist, J.; Sundquist, K. Association of Preterm Birth With Long-term Risk of Heart Failure Into Adulthood. JAMA Pediatr. 2021, 175, 689–697. [Google Scholar] [CrossRef]
- Crump, C.; Howell, E.A.; Stroustrup, A.; McLaughlin, M.A.; Sundquist, J.; Sundquist, K. Association of Preterm Birth With Risk of Ischemic Heart Disease in Adulthood. JAMA Pediatr. 2019, 173, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, J.R.; Hyde, M.J.; Gale, C.; Santhakumaran, S.; Modi, N. Preterm birth and the metabolic syndrome in adult life: A systematic review and meta-analysis. Pediatrics 2013, 131, e1240–e1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darlow, B.A.; Horwood, L.J.; Woodward, L.J.; Elliott, J.M.; Troughton, R.W.; Elder, M.J.; Epton, M.J.; Stanton, J.D.; Swanney, M.P.; Keenan, R.; et al. The New Zealand 1986 very low birth weight cohort as young adults: Mapping the road ahead. BMC Pediatr. 2015, 15, 90. [Google Scholar] [CrossRef] [Green Version]
- Darlow, B.A. Incidence of retinopathy of prematurity in New Zealand. Arch. Dis. Child. 1988, 63, 1083–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [Green Version]
- Mosteller, R.D. Simplified calculation of body-surface area. N. Engl. J. Med. 1987, 317, 1098. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kingsford, R.A.; Horwood, J.; Epton, M.J.; Swanney, M.P.; Stanton, J.; Darlow, B.A. Lung Function of Adults Born at Very Low Birth Weight. Pediatrics 2020, 145, e20192359. [Google Scholar] [CrossRef]
- Blencowe, H.; Cousens, S.; Chou, D.; Oestergaard, M.; Say, L.; Moller, A.B.; Kinney, M.; Lawn, J. Born too soon: The global epidemiology of 15 million preterm births. Reprod. Health 2013, 10 (Suppl. 1), S2. [Google Scholar] [CrossRef] [Green Version]
- Phad, N.S.; de Waal, K.; Holder, C.; Oldmeadow, C. Dilated hypertrophy: A distinct pattern of cardiac remodeling in preterm infants. Pediatr. Res. 2020, 87, 146–152. [Google Scholar] [CrossRef]
- Bensley, J.G.; De Matteo, R.; Harding, R.; Black, M.J. The effects of preterm birth and its antecedents on the cardiovascular system. Acta Obstet. Gynecol. Scand. 2016, 95, 652–663. [Google Scholar] [CrossRef] [Green Version]
- Telles, F.; McNamara, N.; Nanayakkara, S.; Doyle, M.P.; Williams, M.; Yaeger, L.; Marwick, T.H.; Leeson, P.; Levy, P.T.; Lewandowski, A.J. Changes in the Preterm Heart From Birth to Young Adulthood: A Meta-analysis. Pediatrics 2020, 146, e20200146. [Google Scholar] [CrossRef]
- Carluccio, E.; Biagioli, P.; Lauciello, R.; Zuchi, C.; Mengoni, A.; Bardelli, G.; Alunni, G.; Gronda, E.G.; Ambrosio, G. Superior Prognostic Value of Right Ventricular Free Wall Compared to Global Longitudinal Strain in Patients with Heart Failure. J. Am. Soc. Echocardiogr. 2019, 32, 836–844.e1. [Google Scholar] [CrossRef] [PubMed]
- Fine, N.M.; Chen, L.; Bastiansen, P.M.; Frantz, R.P.; Pellikka, P.A.; Oh, J.K.; Kane, G.C. Outcome prediction by quantitative right ventricular function assessment in 575 subjects evaluated for pulmonary hypertension. Circ. Cardiovasc. Imaging 2013, 6, 711–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Negishi, K.; Kwon, D.H.; Popovic, Z.B.; Grimm, R.A.; Marwick, T.H. Validation of global longitudinal strain and strain rate as reliable markers of right ventricular dysfunction: Comparison with cardiac magnetic resonance and outcome. J. Cardiovasc. Ultrasound 2014, 22, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Burchert, H.; Lewandowski, A.J. Preterm Birth Is a Novel, Independent Risk Factor for Altered Cardiac Remodeling and Early Heart Failure: Is it Time for a New Cardiomyopathy? Curr. Treat. Opt. Cardiovasc. Med. 2019, 21, 8. [Google Scholar] [CrossRef] [Green Version]
- Ghio, S.; Guazzi, M.; Scardovi, A.B.; Klersy, C.; Clemenza, F.; Carluccio, E.; Temporelli, P.L.; Rossi, A.; Faggiano, P.; Traversi, E.; et al. Different correlates but similar prognostic implications for right ventricular dysfunction in heart failure patients with reduced or preserved ejection fraction. Eur. J. Heart Fail. 2017, 19, 873–879. [Google Scholar] [CrossRef] [Green Version]
- Bosch, L.; Lam, C.S.P.; Gong, L.; Chan, S.P.; Sim, D.; Yeo, D.; Jaufeerally, F.; Leong, K.T.G.; Ong, H.Y.; Ng, T.P.; et al. Right ventricular dysfunction in left-sided heart failure with preserved versus reduced ejection fraction. Eur. J. Heart Fail. 2017, 19, 1664–1671. [Google Scholar] [CrossRef] [Green Version]
- Lewandowski, A.J.; Bradlow, W.M.; Augustine, D.; Davis, E.F.; Francis, J.; Singhal, A.; Lucas, A.; Neubauer, S.; McCormick, K.; Leeson, P. Right ventricular systolic dysfunction in young adults born preterm. Circulation 2013, 128, 713–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, A.; Lamata, P.; Williamson, W.; Alsharqi, M.; Tan, C.M.J.; Burchert, H.; Huckstep, O.J.; Suriano, K.; Francis, J.M.; Pelado, J.L.; et al. Multimodality Imaging Demonstrates Reduced Right-Ventricular Function Independent of Pulmonary Physiology in Moderately Preterm-Born Adults. JACC Cardiovasc. Imaging 2020, 13, 2046–2048. [Google Scholar] [CrossRef] [PubMed]
- Abman, S.H.; Hansmann, G.; Archer, S.L.; Ivy, D.D.; Adatia, I.; Chung, W.K.; Hanna, B.D.; Rosenzweig, E.B.; Raj, J.U.; Cornfield, D.; et al. Pediatric Pulmonary Hypertension. Circulation 2015, 132, 2037–2099. [Google Scholar] [CrossRef] [PubMed]
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.-B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health 2019, 7, e37–e46. [Google Scholar] [CrossRef] [Green Version]
| PT/VLBW n = 229 | Controls n = 100 | p | |
|---|---|---|---|
| Demographics | |||
| Female n (%) | 127 (55.5) | 63 (63) | 0.249 |
| Ethnicity n (%) | |||
| Māori/Pacific Island | 72 (31.5) | 24 (24) | 0.24 |
| Asian | 3 (1.3) | 2 (2) | |
| European | 154 (67.2) | 74 (74) | |
| Age at assessment (years) | 28.4 ± 1.1 | 28.2 ± 0.9 | 0.114 |
| Height cm | 168 ± 9 | 172 ± 9 | <0.001 |
| Weight kg | 75 ± 19 | 81 ± 18 | 0.005 |
| BMI kg/m2 | 27.4 ± 6.3 | 26.3 ± 6.2 | 0.14 |
| BSA m2 | 1.85 ± 0.3 | 1.96 ± 0.2 | <0.001 |
| Smokers n (%) | 71 (31) | 21 (21) | 0.06 |
| Antihypertensive treatment n (%) | 5 (2.2) | 0 | 0.33 |
| Perinatal Characteristics | |||
| Birth weight g | 1135 ± 234 | 3372 ± 565 | <0.001 |
| Birth weight <1 kg n (%) | 63 (27.6) | NA | - |
| Gestation weeks | 29.3 ± 2.5 | NA | - |
| BPD n (%) | 46 (20) | NA | - |
| ANS n (%) | 129 (56.3) | NA | - |
| Small for gestational age (SGA) | 72 (31.4%) | NA | - |
| PT/VLBW n = 229 | Control n = 100 | p | |
|---|---|---|---|
| Structural | |||
| End diastolic area indexed to BSA (cm2/m2) | 11.00 (0.17) | 11.7 (0.24) | 0.028 |
| End systolic area indexed to BSA (cm2/m2) | 6.37 (0.11) | 6.53 (0.16) | 0.424 |
| RV basal diameter (cm) | 3.07 (0.03) | 3.28 (0.05) | <0.001 |
| RV mid-cavity diameter (cm) | 3.24 (0.03) | 3.36 (0.05) | 0.062 |
| RV length (cm) | 7.25 (0.05) | 7.5 (0.08) | 0.009 |
| Right atrial volume indexed to BSA (cm3/m2) | 25.3 (0.46) | 28.5 (0.7) | <0.001 |
| Right atrial area indexed to BSA (cm2/m2) | 7.37 (0.1) | 7.82 (0.15) | 0.01 |
| Right ventricular wall thickness (mm) | 3.8 (0.1) | 3.8 (0.1) | 0.85 |
| Functional | |||
| 2D TAPSE (cm) | 2.23 (0.02) | 2.2 (0.03) | 0.36 |
| Tissue doppler TAPSV (cm/sec) | 12.6 (0.12) | 12.9 (0.18) | 0.085 |
| Fractional area change % | 42.5 (0.52) | 43.8 (0.76) | 0.187 |
| RV index of myocardial performance | 0.45 (0.01) | 0.43 (0.01) | 0.27 |
| RV TDI e’/a’ | 1.38 (0.03) | 1.37 (0.05) | 0.87 |
| Strain | n = 116 | n = 60 | |
| Longitudinal freewall strain % | −26.7 (0.27) | −27.9 (0.38) | 0.01 |
| Global longitudinal strain % | −23.6 (0.27) | −24.9 (0.37) | 0.003 |
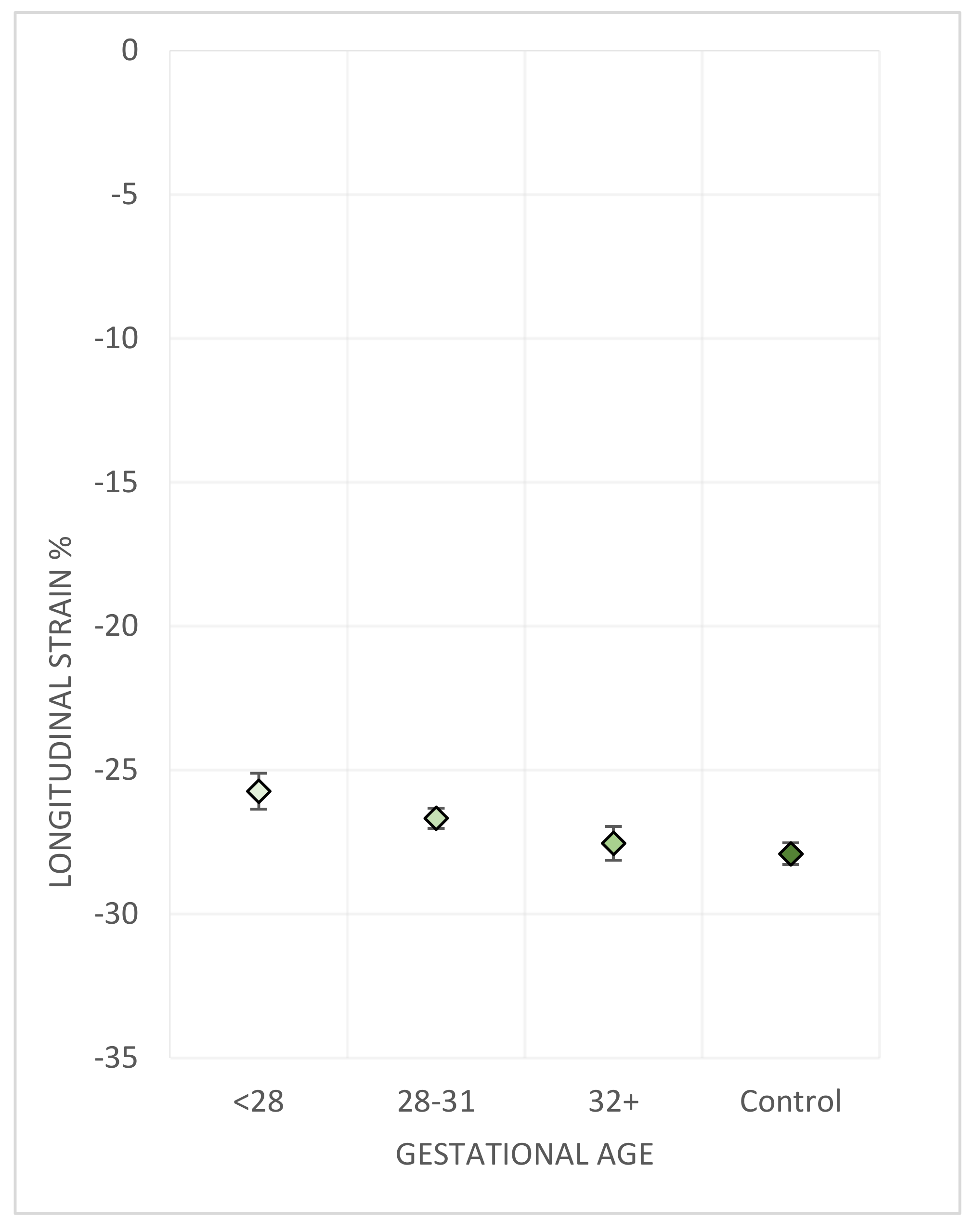
| Gestational Age | p | ||||
|---|---|---|---|---|---|
| <28 | 28–31 | 32+ | Control | ||
| n = 55 | n = 131 | n = 41 | n = 100 | ||
| Right Ventricular Structure | |||||
| End diastolic area indexed to BSA (cm2/m2) | 10.8 (0.4) | 11.0 (0.2) | 11.3 (0.4) | 11.7 (0.2) | 0.13 |
| End systolic area indexed to BSA (cm2/m2) | 6.2 (0.3) | 6.4 (0.2) | 6.6 (0.3) | 6.5 (0.2) | 0.55 |
| RV basal diameter (cm) | 3.0 (0.06) | 3.1 (0.04) | 3.2 (0.07) | 3.3 (0.05) | 0.001 |
| RV mid cavity diameter (cm) | 3.2 (0.07) | 3.3 (0.05) | 3.4 (0.08) | 3.4 (0.05) | 0.06 |
| RV Length (cm) | 7.3 (0.1) | 7.2 (0.07) | 7.4 (0.12) | 7.5 (0.08) | 0.05 |
| RA volume indexed to BSA (cm3/m2) | 24.1 (1.0) | 25.3 (0.6) | 26.9 (1.1) | 28.8 (0.7) | <0.001 |
| RA area indexed to BSA (cm2/m2) | 7.2 (0.2) | 7.4 (0.12) | 7.8 (0.22) | 7.8 (0.15) | 0.01 |
| Right ventricular wall thickness (cm) | 3.9 (0.1) | 3.8 (0.1) | 3.7 (0.1) | 3.8 (0.1) | 0.83 |
| Right Ventricular Systolic Strain | |||||
| Longitudinal RV freewall strain % | −25.7 (0.6) | −26.7 (0.4) | −27.6 (0.6) | −27.9 (0.4) | 0.01 |
| Global longitudinal RV strain % | −22.3 (0.6) | −23.8 (0.3) | −23.9 (0.6) | −24.9 (0.4) | 0.004 |
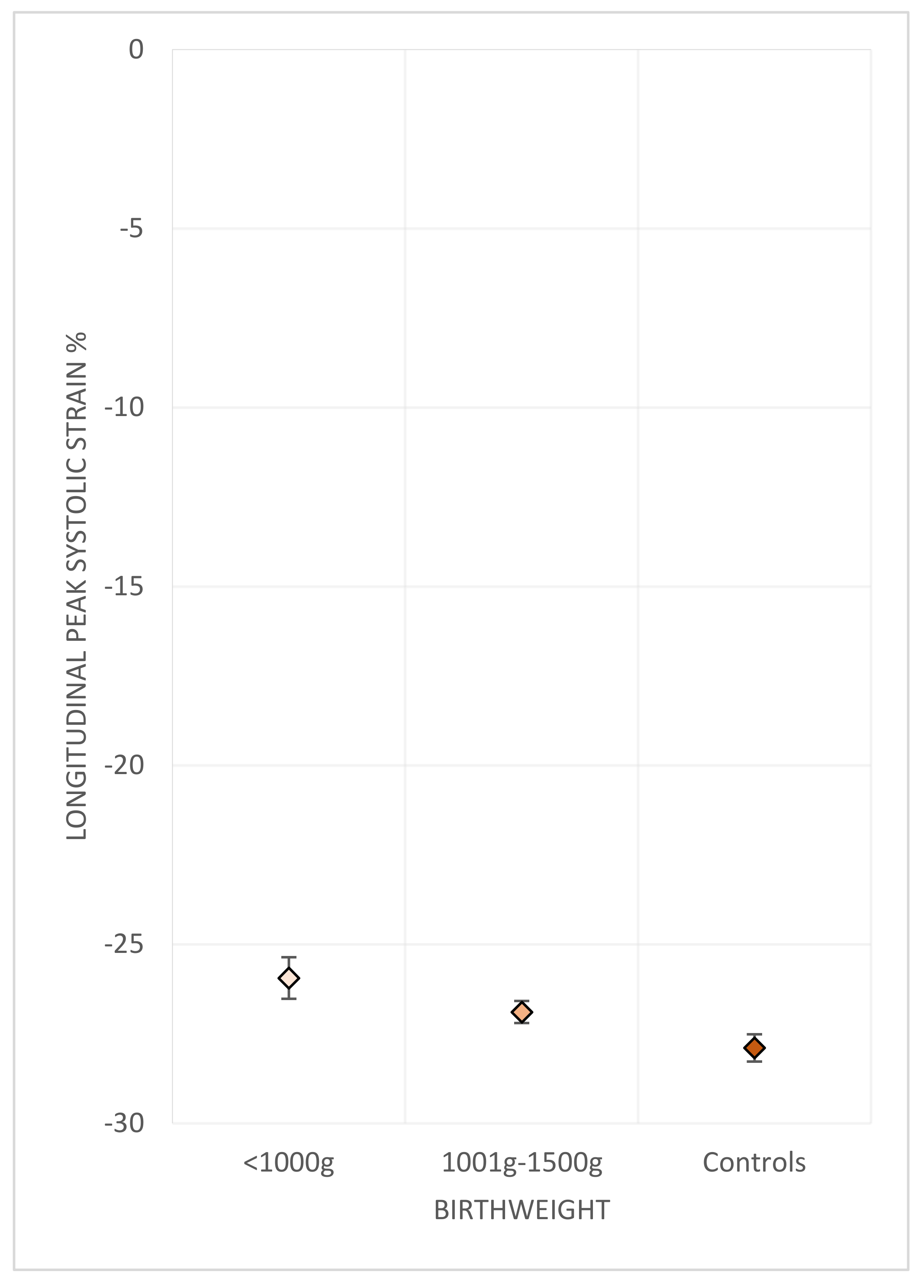
| Birth Weight | ||||
|---|---|---|---|---|
| ≤1000 g n = 63 | 1001–1500 g n = 165 | Control n = 100 | p | |
| Right Ventricular Structure | ||||
| End diastolic area indexed to BSA (cm2/m2) | 10.4 (0.3) | 11.2 (0.2) | 11.6 (0.2) | 0.012 |
| End systolic area indexed to BSA (cm2/m2) | 6.0 (0.2) | 6.5 (0.1) | 6.5 (0.2) | 0.12 |
| RV basal diameter (cm) | 3.0 (0.6) | 3.1 (0.4) | 3.3 (0.5) | <0.001 |
| RV mid cavity diameter (cm) | 3.0 (0.06) | 3.3 (0.04) | 3.4 (0.05) | <0.001 |
| RV Length (cm) | 7.1 (0.1) | 7.3 (0.1) | 7.5 (0.1) | 0.004 |
| Right atrial volume indexed to BSA (cm3/m2) | 23 (0.9) | 26.2 (0.5) | 28.8 (0.7) | <0.001 |
| Right atrial area indexed to BSA (cm2/m2) | 7.0 (0.2) | 7.5 (0.1) | 7.8 (0.1) | 0.001 |
| Right ventricular wall thickness (mm) | 3.8 (0.1) | 3.8 (0.1) | 3.8 (0.1) | 0.988 |
| Right Ventricular Systolic Strain | ||||
| Longitudinal freewall strain % | −25.9 (0.6) | −26.9 (0.3) | −27.9 (0.4) | 0.012 |
| Global longitudinal strain % | −22.2 (0.6) | −23.9 (0.3) | −24.9 (0.4) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greer, C.; Harris, S.L.; Troughton, R.; Adamson, P.D.; Horwood, J.; Frampton, C.; Darlow, B.A. Right Ventricular Structure and Function in Young Adults Born Preterm at Very Low Birth Weight. J. Clin. Med. 2021, 10, 4864. https://doi.org/10.3390/jcm10214864
Greer C, Harris SL, Troughton R, Adamson PD, Horwood J, Frampton C, Darlow BA. Right Ventricular Structure and Function in Young Adults Born Preterm at Very Low Birth Weight. Journal of Clinical Medicine. 2021; 10(21):4864. https://doi.org/10.3390/jcm10214864
Chicago/Turabian StyleGreer, Charlotte, Sarah L. Harris, Richard Troughton, Philip D. Adamson, John Horwood, Chris Frampton, and Brian A. Darlow. 2021. "Right Ventricular Structure and Function in Young Adults Born Preterm at Very Low Birth Weight" Journal of Clinical Medicine 10, no. 21: 4864. https://doi.org/10.3390/jcm10214864
APA StyleGreer, C., Harris, S. L., Troughton, R., Adamson, P. D., Horwood, J., Frampton, C., & Darlow, B. A. (2021). Right Ventricular Structure and Function in Young Adults Born Preterm at Very Low Birth Weight. Journal of Clinical Medicine, 10(21), 4864. https://doi.org/10.3390/jcm10214864






