More than 50% of Persistent Myocardial Scarring at One Year in “Infarct-like” Acute Myocarditis Evaluated by CMR
Abstract
:1. Introduction
- (1)
- The evolution of CMR parameters between the acute phase and 12 months thereafter.
- (2)
- The predictors of persistent myocardial scarring at one year and the long-term prognosis of the infarct-like form.
2. Methods
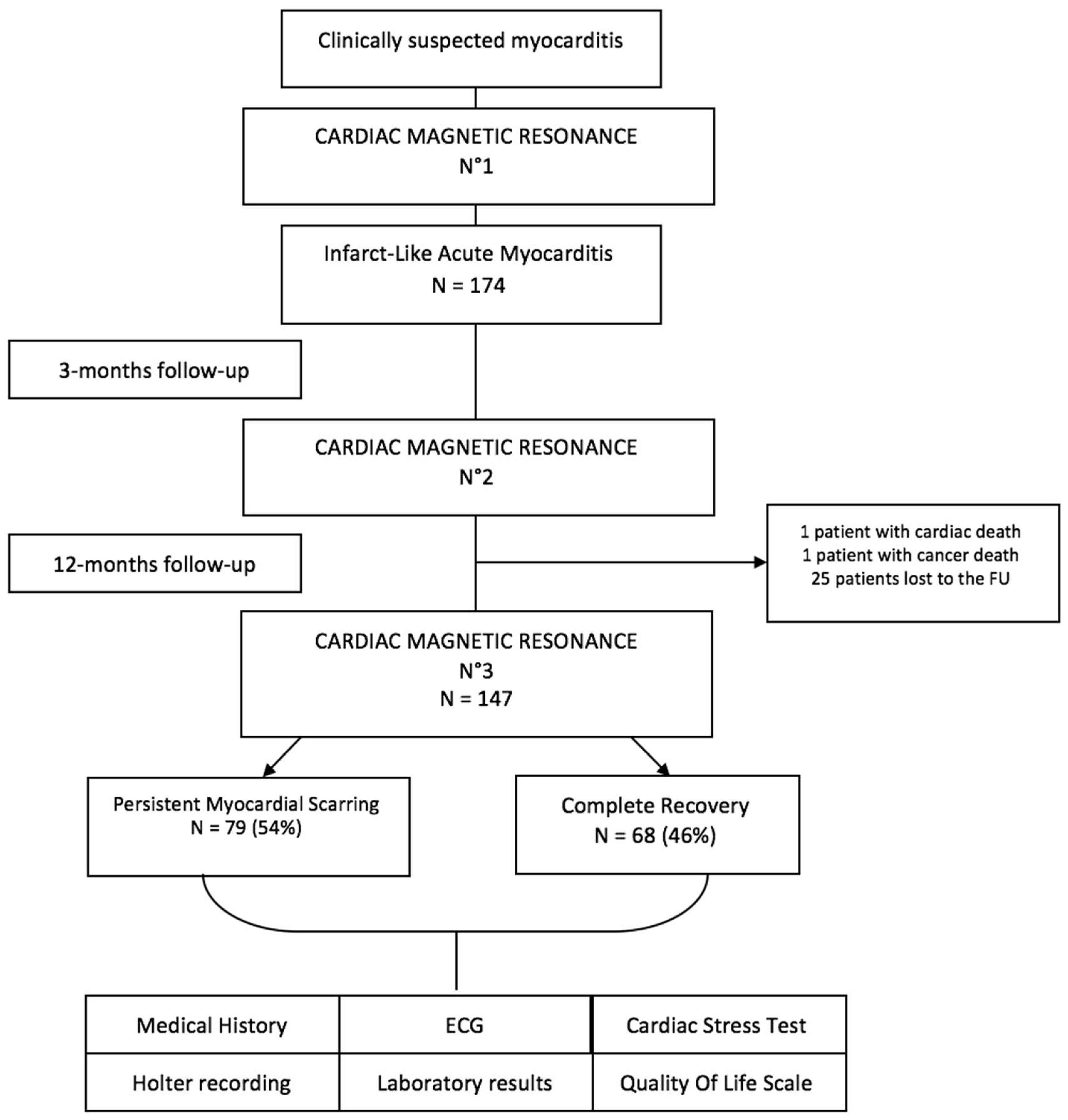
2.1. Study Flow Chart
2.2. CMR Analysis
2.3. Statistical Analysis
3. Results
3.1. Patients Baseline Characteristics
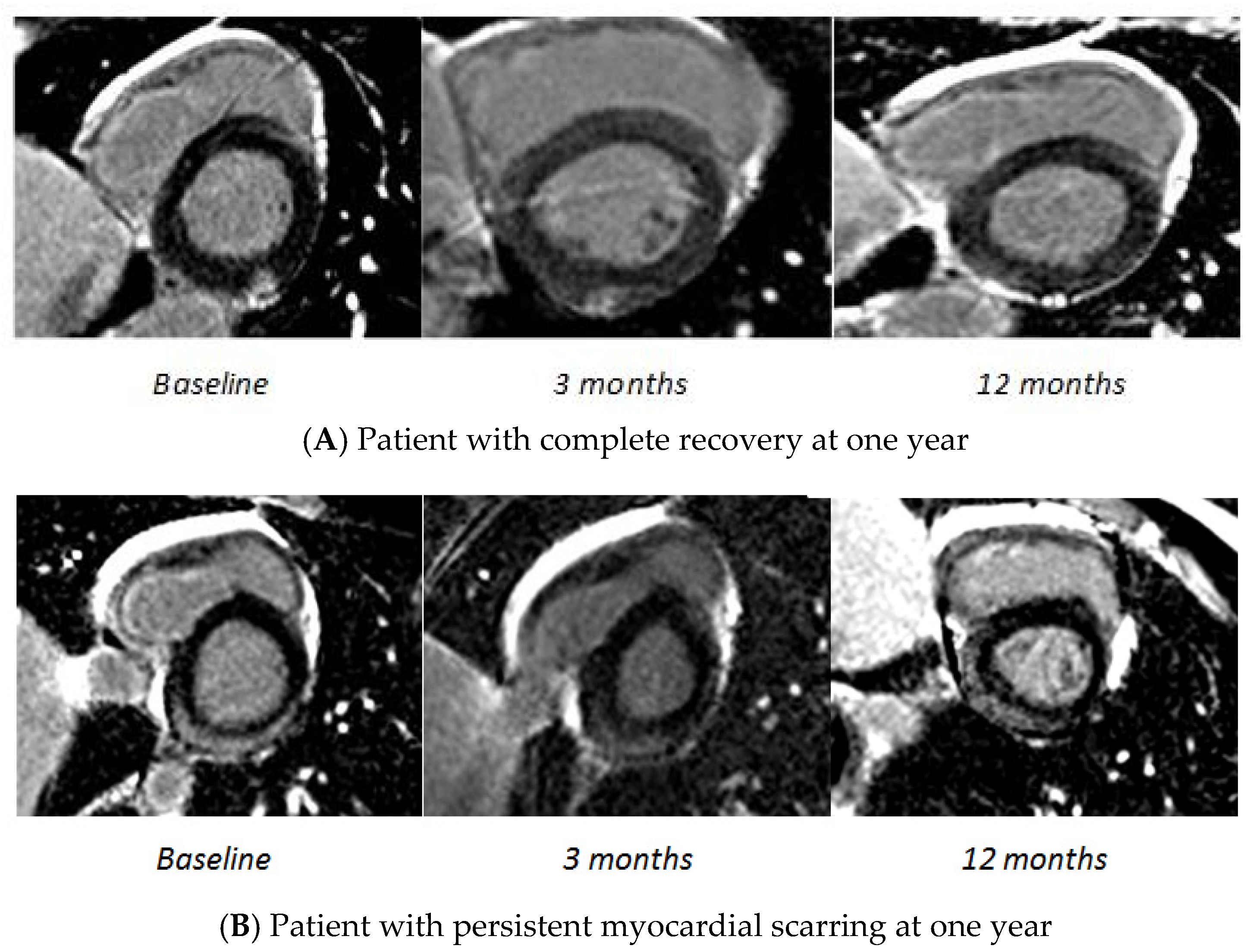
3.2. Follow-Up Results
4. Discussion
4.1. Infarct-like Acute Myocarditis and CMR Findings
4.2. Clinical Implications
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CMR | Cardiac Magnetic Resonance |
| CBC | Complete Blood Count |
| CRP | C-Reactive Protein |
| DCM | Dilated Cardiomyopathy ECG: Electrocardiogram |
| ESC | European Society of Cardiology FU: Follow-Up |
| LGE | Late Gadolinium Enhancement LV: Left Ventricle |
| LVEF | Left Ventricle Ejection Fraction |
| LVEDV | Left Ventricle End-Diastolic Volume |
| LVESV | Left Ventricle End-Systolic Volume |
| MACE | Major Adverse Cardiac Events |
| NYHA | New York Heart Association |
| NT-pro BNP | N-Terminal Pro-B-Type Natriuretic Peptide PVB19: Parvovirus B19 |
| PAC | Premature Atrial Contractions |
| PVC | Premature Ventricular Contractions |
| NSVT | Non-Sustained Ventricular Tachycardia |
| SCD | Sudden Cardiac Death |
Appendix A
CMR Protocol
References
- Caforio, A.L.P.; Marcolongo, R.; Basso, C.; Iliceto, S. Clinical presentation and diagnosis of myocarditis. Heart 2015, 101, 1332–1344. [Google Scholar] [CrossRef]
- Cooper, L.T. Myocarditis. N. Engl. J. Med. 2009, 360, 1526–1538. [Google Scholar] [CrossRef] [Green Version]
- Mahrholdt, H.; Wagner, A.; Deluigi, C.C.; Kispert, E.; Hager, S.; Meinhardt, G.; Vogelsberg, H.; Fritz, P.; Dippon, J.; Bock, C.-T.; et al. Presentation, Patterns of Myocardial Damage, and Clinical Course of Viral Myocarditis. Circulation 2006, 114, 1581–1590. [Google Scholar] [CrossRef] [Green Version]
- Eckart, R.E.; Scoville, S.L.; Campbell, C.L.; Shry, E.A.; Stajduhar, K.C.; Potter, R.N.; Pearse, L.A.; Virmani, R. Sudden Death in Young Adults: A 25-Year Review of Autopsies in Military Recruits. Ann. Intern. Med. 2004, 141, 829–834. [Google Scholar] [CrossRef]
- Kasper, E.K.; Agema, W.R.; Hutchins, G.M.; Deckers, J.W.; Hare, J.M.; Baughman, K.L. The causes of dilated cardiomyopathy: A clinicopathologic review of 673 consecutive patients. J. Am. Coll. Cardiol. 1994, 23, 586–590. [Google Scholar] [CrossRef] [Green Version]
- Friedrich, M.G.; Sechtem, U.; Schulz-Menger, J.; Holmvang, G.; Alakija, P.; Cooper, L.; White, J.A.; Abdel-Aty, H.; Gutberlet, M.; Prasad, S.; et al. Cardiovascular Magnetic Resonance in Myocarditis: A JACC White Paper. J. Am. Coll. Cardiol. 2009, 53, 1475–1487. [Google Scholar] [CrossRef] [Green Version]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Blanes, J.G.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef] [PubMed]
- Gräni, C.; Eichhorn, C.; Bière, L.; Murthy, V.L.; Agarwal, V.; Kaneko, K.; Cuddy, S.; Aghayev, A.; Steigner, M.; Blankstein, R.; et al. Prognostic Value of Cardiac Magnetic Resonance Tissue Characterization in Risk Stratifying Patients With Suspected Myocarditis. J. Am. Coll. Cardiol. 2017, 70, 1964–1976. [Google Scholar] [CrossRef] [PubMed]
- Aquaro, G.D.; Perfetti, M.; Camastra, G.; Monti, L.; Dellegrottaglie, S.; Moro, C.; Pepe, A.; Todiere, G.; Lanzillo, C.; Scatteia, A.; et al. Cardiac MR With Late Gadolinium Enhancement in Acute Myocarditis With Preserved Systolic Function: ITAMY Study. J. Am. Coll. Cardiol. 2017, 70, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
- Barone-Rochette, G.; Augier, C.; Rodière, M.; Quesada, J.-L.; Foote, A.; Bouvaist, H.; Marlière, S.; Fagret, D.; Baguet, J.P.; Vanzetto, G. Potentially simple score of late gadolinium enhancement cardiac MR in acute myocarditis outcome. J. Magn. Reson. Imaging 2014, 40, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Chopra, H.; Arangalage, D.; Bouleti, C.; Zarka, S.; Fayard, F.; Chillon, S.; Laissy, J.-P.; Henry-Feugeas, M.-C.; Steg, P.-G.; Vahanian, A.; et al. Prognostic value of the infarct- and non-infarct like patterns and cardiovascular magnetic resonance parameters on long-term outcome of patients after acute myocarditis. Int. J. Cardiol. 2016, 212, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Caforio, A.L.; Calabrese, F.; Angelini, A.; Tona, F.; Vinci, A.; Bottaro, S.; Ramondo, A.; Carturan, E.; Iliceto, S.; Thiene, G.; et al. A prospective study of biopsy-proven myocarditis: Prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur. Heart J. 2007, 28, 1326–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danti, M.; Sbarbati, S.; Alsadi, N.; Di Filippo, A.; Gangitano, G.; Giglio, L.; Salvini, V.; Amoruso, M.; Camastra, G.S.; Ansalone, G.; et al. Cardiac magnetic resonance imaging: Diagnostic value and utility in the follow-up of patients with acute myocarditis mimicking myocardial infarction. Radiol. Med. 2009, 114, 229–238. [Google Scholar] [CrossRef]
- Faletti, R.; Gatti, M.; Baralis, I.; Bergamasco, L.; Bonamini, R.; Ferroni, F.; Imazio, M.; Stola, S.; Gaita, F.; Fonio, P. Clinical and magnetic resonance evolution of “infarct-like” myocarditis. Radiol. Med. 2017, 122, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Grün, S.; Schumm, J.; Greulich, S.; Wagner, A.; Schneider, S.; Bruder, O.; Kispert, E.-M.; Hill, S.; Ong, P.; Klingel, K.; et al. Long-Term Follow-Up of Biopsy-Proven Viral Myocarditis: Predictors of Mortality and Incomplete Recovery. J. Am. Coll. Cardiol. 2012, 59, 1604–1615. [Google Scholar] [CrossRef]
- Løgstrup, B.; Nielsen, J.; Kim, W.; Poulsen, S. Myocardial oedema in acute myocarditis detected by echocardiographic 2D myocardial deformation analysis. Eur. Heart J.-Cardiovasc. Imaging 2016, 17, 1018–1026. [Google Scholar] [CrossRef] [Green Version]
- Radunski, U.K.; Lund, G.K.; Säring, D.; Bohnen, S.; Stehning, C.; Schnackenburg, B.; Avanesov, M.; Tahir, E.; Adam, G.; Blankenberg, S.; et al. T1 and T2 mapping cardiovascular magnetic resonance imaging techniques reveal unapparent myocardial injury in patients with myocarditis. Clin. Res. Cardiol. 2017, 106, 10–17. [Google Scholar] [CrossRef]
- Berg, J.; Kottwitz, J.; Baltensperger, N.; Kissel, C.K.; Lovrinovic, M.; Mehra, T.; Scherff, F.; Schmied, C.; Templin, C.; Lüscher, T.F.; et al. Cardiac Magnetic Resonance Imaging in Myocarditis Reveals Persistent Disease Activity Despite Normalization of Cardiac Enzymes and Inflammatory Parameters at 3-Month Follow-Up. Circ. Heart Fail. 2017, 10, e004262. [Google Scholar] [CrossRef]
- Imazio, M.; Trinchero, R. Myopericarditis: Etiology, management, and prognosis. Int. J. Cardiol. 2008, 127, 17–26. [Google Scholar] [CrossRef]
- Baughman, K.L. Diagnosis of myocarditis: Death of Dallas criteria. Circulation 2006, 113, 593–595. [Google Scholar] [CrossRef]
- Bohnen, S.; Radunski, U.K.; Lund, G.K.; Kandolf, R.; Stehning, C.; Schnackenburg, B.; Adam, G.; Blankenberg, S.; Muellerleile, K. Performance of T1 and T2 Mapping Cardiovascular Magnetic Resonance to Detect Active Myocarditis in Patients With Recent-Onset Heart Failure. Circ. Cardiovasc. Imaging 2015, 8, e003073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| N = 174 | |
|---|---|
| Demographic data | |
| Age, years | 39 ± 17 |
| Males | 123 (71%) |
| Smoking | 67 (40%) |
| Hypertension | 26 (15%) |
| Diabetes | 6 (4%) |
| Hyperlipidemia | 17 (10%) |
| Family history of CAD | 22 (13%) |
| Overweight—Obesity | 59 (35%) |
| Prior autoimmune disease | 14 (8%) |
| Clinical parameters | |
| Chest pain | 174 (100%) |
| Dyspnea | 25 (15%) |
| Palpitations | 11 (7%) |
| Recent viral history | 99 (59%) |
| ECG—TTE—Coronary artery angiography | |
| ECG abnormalities | 118 (72%) |
| Repolarization abnormalities | 109 (66%) |
| LVEF at echocardiography, % | 60 (55–65) |
| Coronary artery angiography performed | 83 (50%) |
| Laboratory tests | |
| Inflammatory syndrome (CRP > 5 mg/L) | 134 (83%) |
| Troponin Ic, peak (ng/mL) | 7.4 (2.2–12.0) |
| Elevated troponin Ic (cTnI > 0.1 ng/mL) | 161 (93%) |
| NT-pro BNP, peak (pg/mL) | 433 (172–968) |
| Medical prescription | |
| Beta blocker | 148 (89%) |
| ACE inhibitor | 151 (92%) |
| Aspirin (anti-inflammatory dose) | 63 (38%) |
| Baseline | 3 Months | 12 Months | p | |
|---|---|---|---|---|
| LVEF, % | 57 (52–62) | 59 (56–64) | 60 (55–65) | |
| LV dysfunction (<50%) | 27 (16%) | 10 (6%) | 10 (7%) | |
| Wall motion abnormalities | 32 (19%) | 10 (6%) | 10 (7%) | |
| LVEDVi, mL/m² | 83 (67–96) | 81 (67–92) | 77 (67–92) | |
| LVESVi, mL/m² | 36 (28–43) | 33 (26–39) | 32 (25–39) | |
| LV mass index, g/m² | 73 (64–86) | 72 (61–82) | 70 (58–80) | |
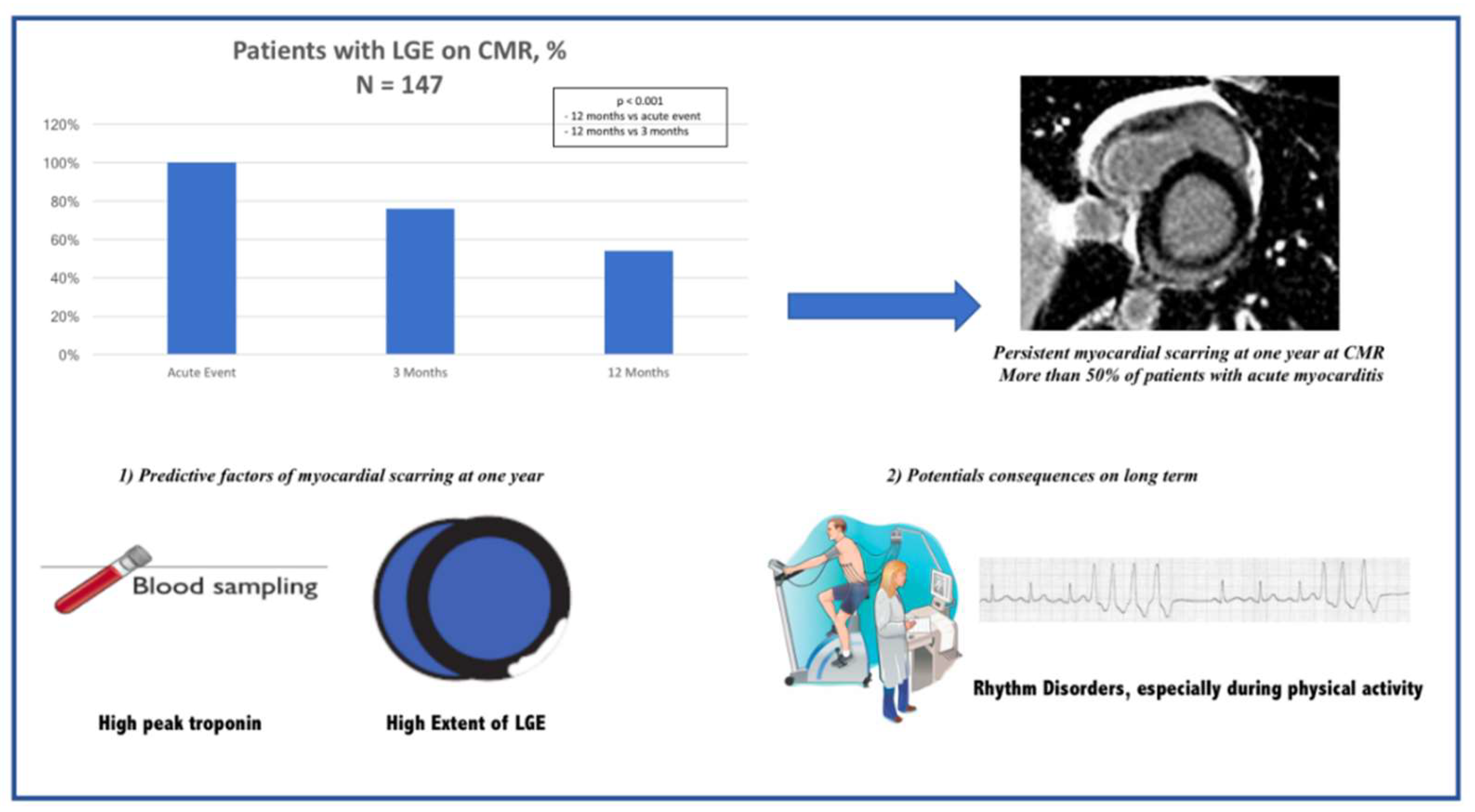
| LGE present | 174 (100%) | 127 (76%) * | 79 (54%) * $ | <0.001 |
| LGE extent (%) | 7.35 (4.41–12.5) | 2.94 (1.47–5.88) * | 1.47 (0.00–4.41) * $ | <0.001 |
| No. of segments with LGE | 3 ± 2 | 2 ± 2 | 1 ± 2 | |
| Edema on CMR | 71 (58%) | 7 (5%) * | 3 (4%) * $ | <0.001 |
| Pericardial effusion | 30 (19%) | 6 (5%) | 5 (3%) | |
| LV dilation | 32 (19%) | 23 (14%) | 22 (15%) | |
| Predominant Location | ||||
| Infero-lateral | 133 (77%) | 139 (83%) | 132 (89%) | |
| Antero-septo-apical | 14 (8%) | 12 (7%) | 9 (6%) | |
| Diffuse | 27 (15%) | 16 (10%) | 8 (5%) |
| Complete Recovery (N = 68) | Persistent Myocarditis (N = 79) | p | |
|---|---|---|---|
| Demographic data | |||
| Age, years | 40 ± 18 | 39 ± 16 | 0.848 |
| Males | 45 (66%) | 59 (75%) | 0.258 |
| Smoking | 27 (42%) | 27 (35%) | 0.355 |
| Hypertension | 12 (19%) | 12 (15%) | 0.594 |
| Diabetes | 3 (5%) | 1 (1%) | 0.327 |
| Hyperlipidemia | 4 (6%) | 10 (13%) | 0.191 |
| Family history of CAD | 7(11%) | 13 (17%) | 0.329 |
| Overweight—Obesity | 19 (30%) | 29 (37%) | 0.348 |
| Prior autoimmune disease | 5 (8%) | 6 (8%) | 1 |
| Clinical parameters | |||
| Chest pain | 66 (100%) | 77 (99%) | 1 |
| Dyspnea | 11 (17%) | 11 (14%) | 0.652 |
| Palpitations | 4 (6%) | 7 (9%) | 0.529 |
| Recent viral history | 33 (51%) | 47 (60%) | 0.255 |
| ECG and TTE | |||
| ECG abnormalities | 43 (71%) | 59 (76%) | 0.495 |
| Sinus tachycardia | 5 (8%) | 0 (0%) | 0.015 |
| Sustained VT | 1 (2%) | 0 (0%) | 0.439 |
| Repolarization abnormalities | 39 (63%) | 54 (69%) | 0.431 |
| LVEF at echocardiography | 60 (60–65) | 60 (56–65) | 0.197 |
| Laboratory tests | |||
| Inflammatory syndrome (CRP > 5 mg/L) | 46 (77%) | 61 (80%) | 0.611 |
| Troponin Ic, peak (ng/mL) | 4.4 (1.4–8.0) | 9.2 (3.9–17.5) | <0.001 |
| Elevated troponin Ic | 52 (90%) | 74 (96%) | 0.172 |
| GFR (mL/min/1.73 m2) | 106.4 ± 29.6 | 107.5 ± 22.9 | 0.808 |
| NT-pro BNP, peak (pg/mL) | 456 (218–1000) | 432 (181–954) | 0.931 |
| Medical prescription | |||
| Beta blocker | 58 (91%) | 67 (87%) | 0.501 |
| ACE inhibitor | 59 (94%) | 69 (90%) | 0.396 |
| Aspirin (anti-inflammatory dose) | 22 (34%) | 35 (45%) | 0.204 |
| CMR | Baseline | ||
|---|---|---|---|
| Complete Recovery (N = 68) | Persistent Myocarditis (N = 79) | p | |
| LVEF, % | 57 ± 9 | 56 ± 8 | 0.238 |
| LV dysfunction | 6 (9%) | 15 (19%) | 0.092 |
| Wall motion abnormalities | 7 (10%) | 16 (20%) | 0.122 |
| LVEDVi, mL/m² | 80 ± 21 | 86 ± 19 | 0.049 |
| LVESVi, mL/m² | 31 (23–41) | 40 (31–45) | 0.001 |
| LV dilation | 11 (17%) | 15 (20%) | 0.727 |
| LV mass index, g/m² | 72 ± 15 | 76 ± 18 | 0.140 |
| Predominant location LGE | |||
| Infero-lateral | 50 (76%) | 62 (79%) | 0.697 |
| Antero-septo-apical | 9 (14%) | 3 (4%) | 0.032 |
| Diffuse | 7 (11%) | 14 (18%) | 0.225 |
| LGE present | 68 (100%) | 79 (100%) | 1 |
| LGE extent (%) | 5.88 (2.94–8.82) | 8.82 (5.88–17.65) | <0.001 |
| No. of segments with LGE | 2 (1–4) | 3 (2–5) | 0.004 |
| Edema on CMR | 23 (51%) | 37/57 (65%) | 0.160 |
| Pericardial effusion | 15 (25%) | 14/70 (20%) | 0.462 |
| Global native T1, ms | 1046 (1017–1098) | 1105 (1052–1150) | 0.019 |
| Extra cellular volume | 0.29 ± 0.04 | 0.33 ± 0.05 | 0.038 |
| Univariable | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age, years | 0.998 (0.979–1.018) | 0.846 | 1.016 (0.992–1.040) | 0.199 |
| Males | 1.508 (0.738–3.079) | 0.260 | 0.753 (0.308–1.840) | 0.534 |
| Troponin Ic, peak > 10 ng/mL | 4.305 (1.856–9.983) | 0.001 | 3.032 (1.155–7.964) | 0.024 |
| LVEDVi, mL/m | 1.017 (1–1.035) | 0.052 | 1.015 (0.995–1.035) | 0.155 |
| LGE extent (%) | 1.123 (1.056–1.194) | <0.001 | 1.105 (1.029–1.187) | 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pommier, T.; Leclercq, T.; Guenancia, C.; Tisserand, S.; Lairet, C.; Carré, M.; Lalande, A.; Bichat, F.; Maza, M.; Zeller, M.; et al. More than 50% of Persistent Myocardial Scarring at One Year in “Infarct-like” Acute Myocarditis Evaluated by CMR. J. Clin. Med. 2021, 10, 4677. https://doi.org/10.3390/jcm10204677
Pommier T, Leclercq T, Guenancia C, Tisserand S, Lairet C, Carré M, Lalande A, Bichat F, Maza M, Zeller M, et al. More than 50% of Persistent Myocardial Scarring at One Year in “Infarct-like” Acute Myocarditis Evaluated by CMR. Journal of Clinical Medicine. 2021; 10(20):4677. https://doi.org/10.3390/jcm10204677
Chicago/Turabian StylePommier, Thibaut, Thibault Leclercq, Charles Guenancia, Simon Tisserand, Céline Lairet, Max Carré, Alain Lalande, Florence Bichat, Maud Maza, Marianne Zeller, and et al. 2021. "More than 50% of Persistent Myocardial Scarring at One Year in “Infarct-like” Acute Myocarditis Evaluated by CMR" Journal of Clinical Medicine 10, no. 20: 4677. https://doi.org/10.3390/jcm10204677
APA StylePommier, T., Leclercq, T., Guenancia, C., Tisserand, S., Lairet, C., Carré, M., Lalande, A., Bichat, F., Maza, M., Zeller, M., Cochet, A., & Cottin, Y. (2021). More than 50% of Persistent Myocardial Scarring at One Year in “Infarct-like” Acute Myocarditis Evaluated by CMR. Journal of Clinical Medicine, 10(20), 4677. https://doi.org/10.3390/jcm10204677







