Why the Term MINOCA Does Not Provide Conceptual Clarity for Actionable Decision-Making in Patients with Myocardial Infarction with No Obstructive Coronary Artery Disease
Abstract
:1. Introduction
2. Discussion
2.1. Myocardial Infarction with No Obstructive Coronary Artery Disease
2.2. The Emerging Concept of MINOCA as a “Working Diagnosis”
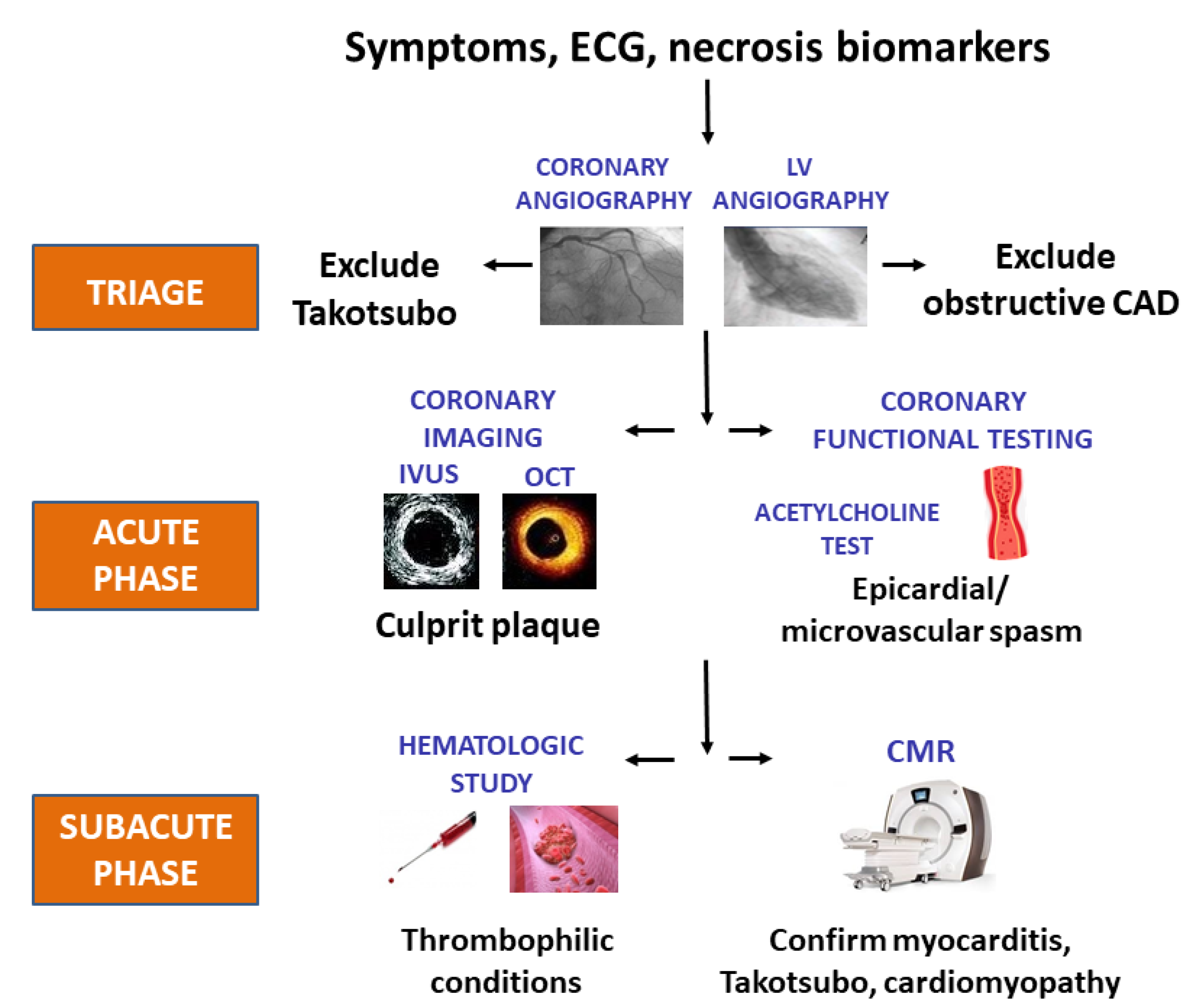
2.3. Back to the Future: Clinical Triage in Provisional MINOCA
2.4. The Crucial Role of a Complete Diagnostic Work-Up in MINOCA
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Libby, P. Mechanisms of Acute Coronary Syndromes and Their Implications for Therapy. N. Engl. J. Med. 2013, 368, 2004–2013. [Google Scholar] [CrossRef] [Green Version]
- Rothberg, M.B. Coronary artery disease as clogged pipes. A misconceptual model. Circ. Cardiovasc. Qual. Outcomes 2013, 36, 129–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzilli, M.; Merz, C.N.; Boden, W.E.; Bonow, R.O.; Capozza, P.G.; Chilian, W.M.; DeMaria, A.N.; Guarini, G.; Huqi, A.; Morrone, D.; et al. Obstructive coronary atherosclerosis and ischemic heart disease: An elusive link. J. Am. Coll. Cardiol. 2012, 60, 951–956. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Marzilli, M.; Crea, F.; Morrone, D.; Bonow, R.O.; Brown, D.L.; Camici, P.G.; Chilian, W.M.; DeMaria, A.; Guarini, G.; Huqi, A.; et al. Myocardial ischemia: From disease to syndrome. Int. J. Cardiol. 2020, 314, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Ahn, J.M.; Kang, S.J. Paradigm shift to functional angioplasty: New insights for fractional flow reserve- and intravascular ultrasound-guided percutaneous coronary intervention. Circulation 2011, 124, 951–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roffi, M.; Patrono, C.; Collet, J.-P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2016, 37, 267–315. [Google Scholar] [CrossRef]
- Agewall, S.; Beltrame, J.F.; Reynolds, H.R.; Niessner, A.; Rosano, G.; Caforio, A.L.; De Caterina, R.; Zimarino, M.; Roffi, M.; Kjeldsen, K.; et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur. Heart J. 2017, 38, 143–153. [Google Scholar] [CrossRef]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Tamis-Holland, J.E.; Jneid, H.; Reynolds, H.R.; Agewall, S.; Brilakis, E.S.; Brown, T.M.; Lerman, A.; Cushman, M.; Kumbhani, D.J.; Arslanian-Engoren, C.; et al. Contemporary Diagnosis and Management of Patients With Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease: A Scientific Statement From the American Heart Association. Circulation 2019, 139, e891–e908. [Google Scholar] [CrossRef]
- Maseri, A.; L’Abbate, A.; Baroldi, G.; Chierchia, S.; Marzilli, M.; Ballestra, A.M.; Severi, S.; Parodi, O.; Biagini, A.; Distante, A.; et al. Coronary vasospasm as a possible cause of myocardial infarction. A conclusion derived from the study of “preinfarction” angina. N. Engl. J. Med. 1978, 299, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Raymond, R.; Lynch, J.; Underwood, D.; Leatherman, J.; Razavi, M. Myocardial infarction and normal coronary arteriography: A 10 year clinical and risk analysis of 74 patients. J. Am. Coll. Cardiol. 1988, 11, 471–477. [Google Scholar] [CrossRef] [Green Version]
- Kereiakes, D.J.; Topol, E.J.; George, B.S.; Stack, R.S.; Abbottsmith, C.W.; Ellis, S.; Candela, R.J.; Harrelson, L.; Martin, L.H.; Califf, R.M. Myocardial infarction with minimal coronary atherosclerosis in the era of thrombolytic reperfusion. The Thrombolysis and Angioplasty in Myocardial Infarction (TAMI) Study Group. J. Am. Coll. Cardiol. 1991, 17, 304–312. [Google Scholar] [CrossRef]
- Alpert, J.S. Myocardial infarction with angiographically normal coronary arteries. Arch. Intern. Med. 1994, 154, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Ammann, P.; Marschall, S.; Kraus, M.; Schmid, L.; Angehrn, W.; Krapf, R.; Rickli, H. Characteristics and prognosis of myocardial infarction in patients with normal coronary arteries. Chest 2000, 117, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, J.F. Assessing Patients with Myocardial Infarction and Non-obstructed Coronary Arteries (MINOCA). J. Intern. Med. 2013, 273, 182–185. [Google Scholar] [CrossRef]
- Pasupathy, S.; Tavella, R.; Beltrame, J.F. Myocardial infarction with nonobstructive coronary arteries (MINOCA): The past, present, and future management. Circulation 2017, 135, 1490–1493. [Google Scholar] [CrossRef]
- Smilowitz, N.R.; Sampson, B.A.; Abrecht, C.R.; Siegfried, J.S.; Hochman, J.S.; Reynolds, H.R. Women have less severe and extensive coronary atherosclerosis in fatal cases of ischemic heart disease: An autopsy study. Am. Heart J. 2011, 161, 681–688. [Google Scholar] [CrossRef]
- Safdar, B.; Spatz, E.S.; Dreyer, R.P.; Beltrame, J.F.; Lichtman, J.H.; Spertus, J.A.; Reynolds, H.R.; Geda, M.; Bueno, H.; Dziura, J.D.; et al. Presentation, Clinical Profile, and Prognosis of Young Patients With Myocardial Infarction With Nonobstructive Coronary Arteries (MINOCA): Results From the VIRGO Study. J. Am. Heart Assoc. 2018, 7, e009174. [Google Scholar] [CrossRef] [Green Version]
- Kang, W.Y.; Jeong, M.H.; Ahn, Y.K.; Kim, J.H.; Chae, S.C.; Kim, Y.J.; Hur, S.H.; Seong, I.W.; Hong, T.J.; Choi, D.H.; et al. Are patients with angiographically near-normal coronary arteries who present as acute myocardial infarction actually safe? Int. J. Cardiol. 2011, 146, 207–212. [Google Scholar] [CrossRef]
- Grodzinsky, A.; Arnold, S.V.; Gosch, K.; Spertus, J.A.; Foody, J.M.; Beltrame, J.; Maddox, T.M.; Parashar, S.; Kosiborod, M. Angina Frequency After Acute Myocardial Infarction In Patients Without Obstructive Coronary Artery Disease. Eur. Heart J. Qual. Care Clin. Outcomes 2015, 1, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Gould, K.L.; Lipscomb, K.; Hamilton, G.W. Physiologic basis for assessing critical coronary stenosis. Instantaneous flow response and regional distribution during coronary hyperemia as measures of coronary flow reserve. Am. J. Cardiol. 1974, 33, 87–94. [Google Scholar] [CrossRef]
- Uren, N.G.; Melin, J.A.; De Bruyne, B.; Wijns, W.; Baudhuin, T.; Camici, P.G. Relation between myocardial blood flow and the severity of coronary-artery stenosis. N. Engl. J. Med. 1994, 330, 1782–1788. [Google Scholar] [CrossRef]
- Naya, M.; Murthy, V.L.; Blankstein, R.; Sitek, A.; Hainer, J.; Foster, C.; Gaber, M.; Fantony, J.M.; Dorbala, S.; Di Carli, M.F. Quantitative relationship between the extent and morphology of coronary atherosclerotic plaque and downstream myocardial perfusion. J. Am. Coll. Cardiol. 2011, 58, 1807–1816. [Google Scholar] [CrossRef] [Green Version]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [PubMed] [Green Version]
- Barbato, E.; Mehilli, J.; Sibbing, D.; Siontis, G.C.M.; Collet, J.P.; Thiele, H.; ESC Scientific Document Group. Questions and answers on antithrombotic therapy and revascularization strategies in non-ST-elevation acute coronary syndrome (NSTE-ACS): A companion document of the 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1368–1378. [Google Scholar] [PubMed]
- Niccoli, G.; Scalone, G.; Crea, F. Acute myocardial infarction with no obstructive coronary atherosclerosis: Mechanisms and management. Eur. Heart J. 2015, 36, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; ESC Scientific Document Group. Fourth universal definition of myocardial infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef] [Green Version]
- Kuhl, U.; Pauschinger, M.; Bock, T.; Klingel, K.; Schwimmbeck, C.P.; Seeberg, B.; Krautwurm, L.; Poller, W.; Schultheiss, H.P.; Kandolf, R. Parvovirus B19 infection mimicking acute myocardial infarction. Circulation 2003, 108, 945–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crea, F.; Montone, R.A.; Niccoli, G. Myocardial infarction with non-obstructive coronary arteries: Dealing with pears and apples. Eur. Heart J. 2020, 41, 879–881. [Google Scholar] [CrossRef]
- Pelliccia, F.; Sinagra, G.; Elliott, P.; Parodi, G.; Basso, C.; Camici, P.G. Takotsubo is not a cardiomyopathy. Int. J. Cardiol. 2018, 254, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Niccoli, G.; Russo, M.; Giaccari, M.; Del Buono, M.G.; Meucci, M.C.; Gurguglione, F.; Vergallo, R.; D’Amario, D.; Buffon, A.; et al. Clinical, angiographic and echocardiographic correlates of epicardial and microvascular spasm in patients with myocardial ischaemia and non-obstructive coronary arteries. Clin. Res. Cardiol. 2020, 109, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, F.; Parodi, G.; Greco, C.; Antoniucci, D.; Brenner, R.; Bossone, E.; Cacciotti, L.; Capucci, A.; Citro, R.; Delmas, C.; et al. Comorbidities frequency in Takotsubo syndrome: An international collaborative systematic review including 1109 patients. Am. J. Med. 2015, 128, 654.e11–654.e19. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.; Núñez Gil, I.J.; Santoro, F.; Madias, J.E.; Pelliccia, F.; Brunetti, N.D.; Salmoirago-Blotcher, E.; Sharkey, S.W.; Eitel, I.; Akashi, Y.J.; et al. Takotsubo syndrome: State-of-the-art review by an expert panel—Part 1. Cardiovasc. Revasc. Med. 2019, 20, 70–79. [Google Scholar] [CrossRef]
- Dias, A.; Núñez Gil, I.J.; Santoro, F.; Madias, J.E.; Pelliccia, F.; Brunetti, N.D.; Salmoirago-Blotcher, E.; Sharkey, S.W.; Eitel, I.; Akashi, Y.J.; et al. Takotsubo syndrome: State-of-the-art review by an expert panel—Part 2. Cardiovasc. Revasc. Med. 2019, 20, 153–166. [Google Scholar] [CrossRef]
- Ammirati, E.; Frigerio, M.; Adler, E.D.; Basso, C.; Birnie, D.H.; Brambatti, M.; Friedrich, M.G.; Klingel, K.; Lehtonen, J.; Moslehi, J.J.; et al. Management of Acute Myocarditis and Chronic Inflammatory Cardiomyopathy: An Expert Consensus Document. Circ. Heart Fail. 2020, 13, e007405. [Google Scholar] [CrossRef]
- Spoletini, I.; Vitale, C.; Pelliccia, F.; Fossati, C.; Rosano, G.M. Androgens and cardiovascular disease in postmenopausal women: A systematic review. Climacteric 2014, 17, 625–634. [Google Scholar] [CrossRef]
- Waterbury, T.M.; Tarantini, G.; Vogel, B.; Mehran, R.; Gersh, B.J.; Gulati, R. Non-atherosclerotic causes of acute coronary syndromes. Nat. Rev. Cardiol. 2020, 17, 229–241. [Google Scholar] [CrossRef]
- Montone, R.A.; Niccoli, G.; Fracassi, F.; Russo, M.; Gurgoglione, F.; Cammà, G.; Lanza, G.A.; Crea, F. Patients with acute myocardial infarction and non-obstructive coronary arteries: Safety and prognostic relevance of invasive coronary provocative tests. Eur. Heart J. 2018, 39, 91–98. [Google Scholar] [CrossRef]
- Probst, S.; Seitz, A.; Martínez Pereyra, V.; Hubert, A.; Becker, A.; Storm, K.; Bekeredjian, R.; Sechtem, U.; Ong, P. Safety assessment and results of coronary spasm provocation testing in patients with myocardial infarction with unobstructed coronary arteries compared to patients with stable angina and unobstructed coronary arteries. Eur. Heart J. Acute Cardiovasc. Care 2020, 10, 380–387. [Google Scholar] [CrossRef]
- Khan, S.; Dickerman, J.D. Hereditary thrombophilia. Thromb. J. 2006, 4, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelliccia, F.; Pepine, C.J.; Berry, C.; Camici, P.G. The role of a comprehensive two-step diagnostic evaluation to unravel the pathophysiology of MINOCA: A review. Int. J. Cardiol. 2021, 336, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Occhipinti, G.; Bucciarelli-Ducci, C.; Capodanno, D. Diagnostic pathways in myocardial infarction with non-obstructive coronary artery disease (MINOCA). Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Pasupathy, S.; Beltrame, J.F. Refining the Role of CMR Imaging in MINOCA. JACC Cardiovasc. Imaging 2021, 14, 1784–1786. [Google Scholar] [CrossRef]
- Reynolds, H.R.; Maehara, A.; Kwong, R.Y.; Sedlak, T.; Saw, J.; Smilowitz, N.R.; Mahmud, E.; Wei, J.; Marzo, K.; Matsumura, M.; et al. Coronary Optical Coherence Tomography and Cardiac Magnetic Resonance Imaging to Determine Underlying Causes of Myocardial Infarction With Nonobstructive Coronary Arteries in Women. Circulation 2021, 143, 624–640. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelliccia, F.; Marzilli, M.; Boden, W.E.; Camici, P.G. Why the Term MINOCA Does Not Provide Conceptual Clarity for Actionable Decision-Making in Patients with Myocardial Infarction with No Obstructive Coronary Artery Disease. J. Clin. Med. 2021, 10, 4630. https://doi.org/10.3390/jcm10204630
Pelliccia F, Marzilli M, Boden WE, Camici PG. Why the Term MINOCA Does Not Provide Conceptual Clarity for Actionable Decision-Making in Patients with Myocardial Infarction with No Obstructive Coronary Artery Disease. Journal of Clinical Medicine. 2021; 10(20):4630. https://doi.org/10.3390/jcm10204630
Chicago/Turabian StylePelliccia, Francesco, Mario Marzilli, William E. Boden, and Paolo G. Camici. 2021. "Why the Term MINOCA Does Not Provide Conceptual Clarity for Actionable Decision-Making in Patients with Myocardial Infarction with No Obstructive Coronary Artery Disease" Journal of Clinical Medicine 10, no. 20: 4630. https://doi.org/10.3390/jcm10204630
APA StylePelliccia, F., Marzilli, M., Boden, W. E., & Camici, P. G. (2021). Why the Term MINOCA Does Not Provide Conceptual Clarity for Actionable Decision-Making in Patients with Myocardial Infarction with No Obstructive Coronary Artery Disease. Journal of Clinical Medicine, 10(20), 4630. https://doi.org/10.3390/jcm10204630






