Relation of Dietary Fatty Acids and Vitamin D to the Prevalence of Meibomian Gland Dysfunction in Japanese Adults: The Hirado–Takushima Study
Abstract
1. Introduction
2. Experimental Section
2.1. Study Design and Subjects
2.2. Dietary Assessment
2.3. Assessment of Subjective Symptoms and Other Variables
2.4. Ocular Examinations
2.5. Statistical Analysis
3. Results
3.1. Subsection
3.2. Daily Intake of Fatty Acids and Vitamin D in Subjects with or Without MGD
3.3. Multivariate Adjusted ORs for Fatty Acid and Vitamin D Intake with Regard to MGD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lam, S.M.; Tong, L.; Duan, X.; Petznick, A.; Wenk, M.R.; Shui, G. Extensive characterization of human tear fluid collected using different techniques unravels the presence of novel lipid amphiphiles. J. Lipid Res. 2014, 55, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Butovich, I.A.; Arciniega, J.C.; Lu, H.; Molai, M. Evaluation and quantitation of intact wax esters of human meibum by gas-liquid chromatography-ion trap mass spectrometry. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3766–3781. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, L.; Fuller, G.G. Consequences of interfacial viscoelasticity on thin film stability. Langmuir 2012, 28, 14238–14244. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, G.A.; Yokoi, N.; Ivanova, S.; Tonchev, V.; Nencheva, Y.; Krastev, R. Surface relaxations as a tool to distinguish the dynamic interfacial properties of films formed by normal and diseased meibomian lipids. Soft Matter 2014, 10, 5579–5588. [Google Scholar] [CrossRef]
- Tomlinson, A.; Doane, M.G.; McFadyen, A. Inputs and outputs of the lacrimal system: Review of production and evaporative loss. Ocul. Surf. 2009, 7, 186–198. [Google Scholar] [CrossRef]
- Nelson, J.D.; Shimazaki, J.; Benitez-Del-Castillo, J.M.; Craig, J.P.; McCulley, J.P.; Den, S.; Foulks, G.N. The international workshop on meibomian gland dysfunction: Report of the definition and classification subcommittee. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1930–1937. [Google Scholar] [CrossRef] [PubMed]
- Nichols, K.K.; Foulks, G.N.; Bron, A.J.; Glasgow, B.J.; Dogru, M.; Tsubota, K.; Lemp, M.A.; Sullivan, D.A. The international workshop on meibomian gland dysfunction: Executive summary. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- Lemp, M.A. Report of the national eye institute/industry workshop on clinical trials in dry eyes. CLAO J. 1995, 21, 221–232. [Google Scholar]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Fiona, S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II epidemiology report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- Arita, R.; Mizoguchi, T.; Kawashima, M.; Fukuoka, S.; Koh, S.; Shirakawa, R.; Suzuki, T.; Morishige, N. Meibomian gland dysfunction and dry eye are similar but different based on a population-based study: The hirado-takushima study in Japan. Am. J. Ophthalmol. 2019, 207, 410–418. [Google Scholar] [CrossRef]
- Geerling, G.; Tauber, J.; Baudouin, C.; Goto, E.; Matsumoto, Y.; O’Brien, T.; Rolando, M.; Tsubota, K.; Nichols, K.K. The international workshop on meibomian gland dysfunction: Report of the subcommittee on management and treatment of mei-bomian gland dysfunction. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2050–2064. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-Del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. TFOS DEWS II management and therapy report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [CrossRef] [PubMed]
- Macsai, M.S. The Role of omega-3 dietary supplementation in blepharitis and meibomian gland dysfunction (an AOS thesis). Trans. Am. Ophthalmol. Soc. 2008, 106, 336–356. [Google Scholar] [PubMed]
- Oleñik, A.; Jiménez-Alfaro, I.; Alejandre-Alba, N.; Mahillo-Fernández, I. A randomized, double-masked study to evaluate the effect of omega-3 fatty acids supplementation in meibomian gland dysfunction. Clin. Interv. Aging 2013, 8, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, C.; Singh, S.; Chakma, P.; Jain, A.K. Effect of oral omega-3 Fatty Acid supplementation on contrast sensitivity in patients with moderate meibomian gland dysfunction: A prospective placebo-controlled study. Cornea 2015, 34, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Miljanovicć, B.; Trivedi, K.A.; Dana, M.R.; Gilbard, J.P.; Buring, J.E.; Schaumberg, D.A. Relation between dietary n−3 and n−6 fatty acids and clinically diagnosed dry eye syndrome in women. Am. J. Clin. Nutr. 2005, 82, 887–893. [Google Scholar] [CrossRef]
- Ziemanski, J.F.; Wolters, L.R.; Jones-Jordan, L.; Nichols, J.J.; Nichols, K.K. Relation between dietary essential fatty acid intake and dry eye disease and meibomian gland dysfunction in postmenopausal women. Am. J. Ophthalmol. 2018, 189, 29–40. [Google Scholar] [CrossRef]
- Arita, R.; Kawashima, M.; Ito, M.; Tsubota, K. Clinical safety and efficacy of vitamin D3 analog ointment for treatment of obstructive meibomian gland dysfunction. BMC Ophthalmol. 2017, 17, 1–6. [Google Scholar] [CrossRef]
- Karaca, E.E.; Kemer, Ö.E.; Özek, D.; Berker, D.; Imga, N.N. Clinical outcomes of ocular surface in patients treated with vitamin D oral replacement. Arq. Bras. Oftalmol. 2020, 83, 312–317. [Google Scholar] [CrossRef]
- Kobayashi, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 d dietary records in Japanese adults. Public Health Nutr. 2011, 14, 1200–1211. [Google Scholar] [CrossRef]
- Kobayashi, S.; Honda, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Both comprehensive and brief self-administered diet history questionnaires satisfactorily rank nutrient intakes in japanese adults. J. Epidemiol. 2012, 22, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Science and Technology Agency. Standard Tables of Food Composition in Japan 2015; 7th Revised Rersion; Ministry of Education, Culture, Sports, Science and Technology: Tokyo, Japan, 2015.
- Sakane, Y.; Yamaguchi, M.; Yokoi, N.; Uchino, M.; Dogru, M.; Oishi, T.; Ohashi, Y.; Ohashi, Y. Development and validation of the dry eye–related quality-of-life score questionnaire. JAMA Ophthalmol. 2013, 131, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Amano, S.; Arita, R.; Kinoshita, S.; Yokoi, N.; Sotozono, C.; Komuro, A.; Suzuki, T.; Shimazaki, J. Definition and diagnostic criteria for meibomian gland dysfunction. Atarashii Ganka 2010, 27, 627–631. [Google Scholar]
- Shimazaki, J.; Sakata, M.; Tsubota, K. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch. Ophthalmol. 1995, 113, 1266–1270. [Google Scholar] [CrossRef]
- Tomlinson, A.; Bron, A.J.; Korb, D.R.; Amano, S.; Paugh, J.R.; Pearce, E.I.; Yee, R.; Yokoi, N.; Arita, R.; Dogru, M. The international workshop on meibomian gland dysfunction: Report of the diagnosis subcommittee. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2006–2049. [Google Scholar] [CrossRef]
- Galor, A.; Gardener, H.; Pouyeh, B.; Feuer, W.; Florez, H. Effect of a mediterranean dietary pattern and vitamin d levels on dry eye syndrome. Cornea 2014, 33, 437–441. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Mediterranean Diets: What is so special about the diet of Greece? The scientific evidence. J. Nutr. 2001, 131, 3065S–3073S. [Google Scholar] [CrossRef]
- James, M.J.; Gibson, R.A.; Cleland, L.G. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am. J. Clin. Nutr. 2000, 71, 343s–348s. [Google Scholar] [CrossRef]
- Molina-Leyva, I.; Molina-Leyva, A.; Riquelme-Gallego, B.; Cano-Ibáñez, N.; García-Molina, L.; Bueno-Cavanillas, A. Effectiveness of mediterranean diet implementation in dry eye parameters: A study of PREDIMED-PLUS trial. Nutrients 2020, 12, 1289. [Google Scholar] [CrossRef]
- Panagiotakos, D.; Pitsavos, C.; Arvaniti, F.; Stefanadis, C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev. Med. 2007, 44, 335–340. [Google Scholar] [CrossRef]
- Hu, N.; Yu, J.-T.; Tan, L.; Wang, Y.-L.; Sun, L.; Tan, L. Nutrition and the risk of Alzheimer’s disease. Biomed. Res. Int. 2013, 2013, 524820. [Google Scholar] [CrossRef] [PubMed]
- Korb, D.R.; Blackie, C.A.; Finnemore, V.M.; Douglass, T. Effect of using a combination of lid wipes, eye drops, and omega-3 supplements on meibomian gland functionality in patients with lipid deficient/evaporative dry eye. Cornea 2015, 34, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Epitropoulos, A.T.; Donnenfeld, E.D.; Shah, Z.A.; Holland, E.J.; Gross, M.; Faulkner, W.J.; Matossian, C.; Lane, S.S.; Toyos, M.; Bucci, F.A.; et al. Effect of oral re-esterified omega-3 nutritional supplementation on dry eyes. Cornea 2016, 35, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Asbell, P.A.; Maguire, M.G.; Pistilli, M.; Ying, G.S.; Szczotka-Flynn, L.B.; Hardten, D.R.; Lin, M.C.; Shtein, R.M. n-3 fatty acid supplementation for the treatment of dry eye disease. N. Engl. J. Med. 2018, 378, 1681–1690. [Google Scholar] [PubMed]
- Orsavova, J.; Misurcova, L.; Ambrozova, J.V.; Vicha, R.; Mlcek, J. Fatty acids composition of vegetable oils and its con-tribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef]
- Dougherty, J.M.; McCulley, J.P. Analysis of the free fatty acid component of meibomian secretions in chronic blepharitis. Investig. Ophthalmol. Vis. Sci. 1986, 27, 52–56. [Google Scholar]
- Joffre, C.; Souchier, M.; Gregoire, S.; Viau, S.; Bretillon, L.; Acar, N.; Bron, A.M.; Creuzot-Garcher, C. Differences in meibomian fatty acid composition in patients with meibomian gland dysfunction and aqueous-deficient dry eye. Br. J. Ophthalmol. 2008, 92, 116–119. [Google Scholar] [CrossRef]
- Gillingham, L.G.; Harris-Janz, S.; Jones, P.J. Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids 2011, 46, 209–228. [Google Scholar] [CrossRef]
- Knop, E.; Knop, N.; Millar, T.; Obata, H.; Sullivan, D.A. The International workshop on meibomian gland dysfunction: Report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1938–1978. [Google Scholar] [CrossRef] [PubMed]
- Shine, W.E.; McCulley, J.P. Association of meibum oleic acid with meibomian seborrhea. Cornea 2000, 19, 72–74. [Google Scholar] [CrossRef]
- Bowen, K.J.; Kris-Etherton, P.M.; West, S.G.; Fleming, J.A.; Connelly, P.W.; Lamarche, B.; Couture, P.; Jenkins, D.J.A.; Taylor, C.G.; Zahradka, P.; et al. Diets enriched with conventional or high-oleic acid canola oils lower atherogenic lipids and lipoproteins compared to a diet with a western fatty acid profile in adults with central adiposity. J. Nutr. 2019, 149, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Dao, A.H.; Spindle, J.D.; Harp, B.A.; Jacob, A.; Chuang, A.Z.; Yee, R.W. Association of dyslipidemia in moderate to severe meibomian gland dysfunction. Am. J. Ophthalmol. 2010, 150, 371–375.e1. [Google Scholar] [CrossRef]
- Módulo, C.M.; Machado Filho, E.B.; Malki, L.T.; Dias, A.C.; de Souza, J.C.; Oliveira, H.C.; Jorge, Í.C.; Santos Gomes, I.B.; Meyrelles, S.S.; Rocha, E.M. The role of dyslipidemia on ocular surface, lacrimal and meibomian gland structure and function. Curr. Eye Res. 2012, 37, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, A.A. Associations between the grade of meibomian gland dysfunction and dyslipidemia. Ophthalmic Plast. Reconstr. Surg. 2013, 29, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Braich, P.S.; Howard, M.K.; Singh, J.S. Dyslipidemia and its association with meibomian gland dysfunction. Int. Ophthalmol. 2016, 36, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Naik, M.; Guliani, B.P.; Bhalla, A. Association of the severity of meibomian gland dysfunction with dyslipidemia in Indian population. Indian J. Ophthalmol. 2018, 66, 1411–1416. [Google Scholar] [CrossRef]
- Liu, Y.; Kam, W.R.; Sullivan, D.A. Influence of omega 3 and 6 fatty acids on human meibomian gland epithelial cells. Cornea 2016, 35, 1122–1126. [Google Scholar] [CrossRef]
- Sullivan, B.D.; Cermak, J.M.; Sullivan, R.M.; Papas, A.S.; Evans, J.E.; Dana, M.R.; Sullivan, D.A. Correlations between nutrient intake and the polar lipid profiles of meibomian gland secretions in women with Sjogren’s syndrome. Adv. Exp. Med. Biol. 2002, 506 Pt A, 441–447. [Google Scholar]
- Lichtenstein, A.H.; Appel, L.J.; Brands, M.; Carnethon, M.; Daniels, S.; Franch, H.A.; Franklin, B.; Kris-Etherton, P.; Harris, W.S.; Howard, B.; et al. Diet and lifestyle recommendations revision 2006: A scientific statement from the American Heart Association Nutrition Committee. Circulation 2006, 114, 82–96. [Google Scholar] [CrossRef]
- McGuire, S.U.S. Department of Agriculture and U.S. Department of Health and Human Services, dietary guidelines for Americans, 2010. 7th Edition, Washington, DC: U.S. Government Printing Office, January 2011. Adv. Nutr. 2011, 2, 293–294. [Google Scholar] [CrossRef]
- De Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schunemann, H.J.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Bo, Y.; Liu, Y. Dietary total fat, fatty acids intake, and risk of cardiovascular disease: A dose-response meta-analysis of cohort studies. Lipids Health Dis. 2019, 18, 91. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Hou, L.; Wang, W. Dietary total fat and fatty acids intake, serum fatty acids and risk of breast cancer: A meta-analysis of prospective cohort studies. Int. J. Cancer 2016, 138, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-H.; Lin, J.-S.; Yang, G.; Aris, I.M.; Aris, I.M.; Chen, W.-Q.; Li, L.-J. Circulating saturated fatty acids and incident type 2 diabetes: A systematic review and meta-analysis. Nutrients 2019, 11, 998. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, P.; Garip, Y.; Karci, A.A.; Guler, T. Dry eye in vitamin D deficiency: More than an incidental association. Int. J. Rheum. Dis. 2016, 19, 49–54. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Bae, S.H.; Shin, Y.J.; Park, S.G.; Hwang, S.-H.; Hyon, J.Y.; Wee, W.R. Low Serum 25-Hydroxyvitamin D Levels Are Associated with Dry Eye Syndrome. PLoS ONE 2016, 11, e0147847. [Google Scholar] [CrossRef]
- Shetty, R.; Deshpande, K.; Deshmukh, R.; Jayadev, C.; Shroff, R. Bowman break and subbasal nerve plexus changes in a patient with dry eye presenting with chronic ocular pain and vitamin D deficiency. Cornea 2016, 35, 688–691. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Jester, J.V.; Parfitt, G.J.; Brown, D.J. Meibomian gland dysfunction: Hyperkeratinization or atrophy? BMC Ophthalmol. 2015, 15, 156. [Google Scholar] [CrossRef]
- Morimoto, S.; Kumahara, Y. A patient with psoriasis cured by 1 alpha-hydroxyvitamin D3. Med. J. Osaka Univ. 1985, 35, 51–54. [Google Scholar]
- Kragballe, K.; Beck, H.I.; Søgaard, H. Improvement of psoriasis by a topical vitamin D3 analogue (MC 903) in a double-blind study. Br. J. Dermatol. 1988, 119, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D: A calciotropic hormone regulating calcium-induced keratinocyte differentiation. J. Am. Acad. Dermatol. 1997, 37 3 Pt 2, 42–52. [Google Scholar]
- Cianferotti, L.; Cox, M.; Skorija, K.; DeMay, M.B. Vitamin D receptor is essential for normal keratinocyte stem cell function. Proc. Natl. Acad. Sci. USA 2007, 104, 9428–9433. [Google Scholar] [CrossRef] [PubMed]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and immune function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef] [PubMed]
- Savastano, S.B.L.; Barrea, L.; Savanelli, M.C.; Nappi, F.; Di Somma, C.; Orio, F.; Colao, A. Low vitamin D status and obesity: Role of nutritionist. Rev. Endocr. Metab. Disord. 2017, 18, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Gummert, J.F.; Börgermann, J. The role of vitamin D in dyslipidemia and cardiovascular disease. Curr. Pharm. Des. 2011, 17, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Pannu, P.K.; Calton, E.K.; Soares, M.J. Calcium and vitamin D in obesity and related chronic disease. Adv. Food Nutr. Res. 2016, 77, 57–100. [Google Scholar] [CrossRef]
| Characteristic | Total (n = 300) | Non-MGD (n = 194) | MGD (n = 106) | p |
|---|---|---|---|---|
| Age (years) | 61.7 ± 15.6 | 57.9 ± 15.7 | 68.2 ± 12.8 | <0.001 ** |
| Male sex, n (%) | 109 (36.3) | 59 (30.4) | 50 (47.2) | 0.006 † |
| Body height (cm) | 158.2 ± 8.2 | 158.5 ± 8.1 | 157.7 ± 8.3 | 0.51 |
| Body weight (kg) | 60.2 ± 11.6 | 69.5 ± 12.2 | 59.5 ± 10.5 | 0.72 |
| Body mass index (kg/m2) | 23.9 ± 3.5 | 24.0 ± 3.5 | 23.8 ± 3.4 | 0.99 |
| Occupation, n (%) | ||||
| Fisherman | 38 (12.7) | 27 (13.9) | 11 (10.4) | 0.52 |
| Farmer | 28 (9.3) | 8 (4.1) | 5 (4.7) | |
| Local government official | 13 (4.3) | 15 (7.7) | 13 (12.3) | |
| Other | 221 (73.7) | 144 (74.2) | 77 (72.6) | |
| History of ocular surgery, n (%) | 51 (17.0) | 27 (13.9) | 24 (22.6) | 0.076 |
| Eyedrop use, n (%) | 131 (43.7) | 81 (41.8) | 50 (47.2) | 0.40 |
| Contact lens wear, n (%) | 15 (5.0) | 13 (6.7) | 2 (1.9) | 0.095 |
| History of chronic systemic disease, n (%) | 171 (57.0) | 93 (47.9) | 78 (73.6) | <0.001 †† |
| History of dyslipidemia, n (%) | 13 (4.3) | 7 (3.6) | 6 (5.7) | 0.39 |
| Taking lipid-lowering agents, n (%) | 3 (1.0) | 2 (1.0) | 1 (0.9) | 1.0 |
| Current smoking, n (%) | 26 (8.7) | 19 (9.8) | 7 (6.6) | 0.40 |
| Alcohol drinking, n (%) | 254 (84.7) | 165 (85.1) | 89 (84.0) | 0.87 |
| Dietary supplement use, n (%) | 77 (25.7) | 49 (25.3) | 28 (26.4) | 0.89 |
| Dietary Component | Total (n = 300) | Non-MGD (n = 194) | MGD (n = 106) | p |
|---|---|---|---|---|
| Energy intake (kcal/day) | 1782.9 ± 564.3 | 1744.6 ± 550.4 | 1853.0 ± 585.0 | 0.057 |
| Total fat (g/day) | 50.7 ± 11.7 | 52.1 ± 11.1 | 48.0 ± 12.4 | 0.007 * |
| Animal fat (g/day) | 23.3 ± 8.3 | 24.5 ± 8.3 | 21.0 ± 8.0 | 0.002 * |
| Plant fat (g/day) | 27.4 ± 7.2 | 27.6 ± 6.9 | 27.0 ± 7.9 | 0.60 |
| Saturated fatty acids (g/day) | 13.7 ± 4.0 | 14.2 ± 4.0 | 12.8 ± 3.9 | 0.015 * |
| Monounsaturated fatty acids (g/day) | 18.1 ± 4.5 | 18.7 ± 4.2 | 17.1 ± 4.7 | 0.005 * |
| Oleic acid (g/day) | 16.4 ± 4.1 | 16.9 ± 3.9 | 15.5 ± 4.3 | 0.007 * |
| Polyunsaturated fatty acids (g/day) | 12.3 ± 2.9 | 12.6 ± 2.8 | 11.8 ± 3.1 | 0.039 * |
| n-3 Polyunsaturated fatty acids (g/day) | 2.6 ± 1.0 | 2.7 ± 1.0 | 2.5 ± 0.9 | 0.017 * |
| α-Linolenic acid (g/day) | 1.5 ± 0.4 | 1.6 ± 0.4 | 1.5 ± 0.5 | 0.10 |
| Eicosapentaenoic acid (g/day) | 0.33 ± 0.27 | 0.35 ± 0.30 | 0.30 ± 0.22 | 0.044 * |
| Docosahexaenoic acid (g/day) | 0.55 ± 0.40 | 0.58 ± 0.44 | 0.50 ± 0.33 | 0.051 |
| n-6 Polyunsaturated fatty acids (g/day) | 9.7 ± 2.4 | 9.8 ± 2.3 | 9.3 ± 2.5 | 0.10 |
| Linoleic acid (g/day) | 9.4 ± 2.3 | 9.5 ± 2.2 | 9.1 ± 2.5 | 0.11 |
| Arachidonic acid (g/day) | 0.16 ± 0.05 | 0.17 ± 0.05 | 0.16 ± 0.06 | 0.17 |
| n-6/n-3 Polyunsaturated fatty acid ratio | 4.1 ± 1.3 | 4.0 ± 1.2 | 4.0 ± 1.6 | 0.40 |
| Cholesterol (mg/day) | 377.3 ± 132.6 | 381.8 ± 126.6 | 369.0 ± 143.1 | 0.49 |
| Vitamin D (µg/day) | 15.4 ± 11.9 | 16.4 ± 13.0 | 13.6 ± 9.6 | 0.039 * |
| Food Group | Total Fat (%) | Vitamin D (%) |
|---|---|---|
| Cooking oil | 20.2 ± 9.1 | 0.0 ± 0.0 |
| Animal food | ||
| Meat | 15.4 ± 10.9 | 0.4 ± 4.7 |
| Fish and shellfish | 11.8 ± 10.1 | 92.4 ± 55.4 |
| Dairy products | 10.8 ± 8.7 | 0.7 ± 11.2 |
| Eggs | 7.4 ± 5.0 | 3.8 ± 34.8 |
| Plant food | ||
| Seasonings and spices | 10.3 ± 7.6 | 0.3 ± 1.9 |
| Confectionaries | 8.9 ± 9.6 | 0.6 ± 13.7 |
| Cereals | 7.5 ± 4.3 | 0.2 ± 1.9 |
| Pulses | 6.7 ± 4.1 | 0.0 ± 0.0 |
| Vegetables | 0.8 ± 0.5 | 1.5 ± 5.1 |
| Fruits | 0.2 ± 0.3 | 0.0 ± 0.0 |
| Potatoes | 0.1 ± 0.1 | 0.0 ± 0.0 |
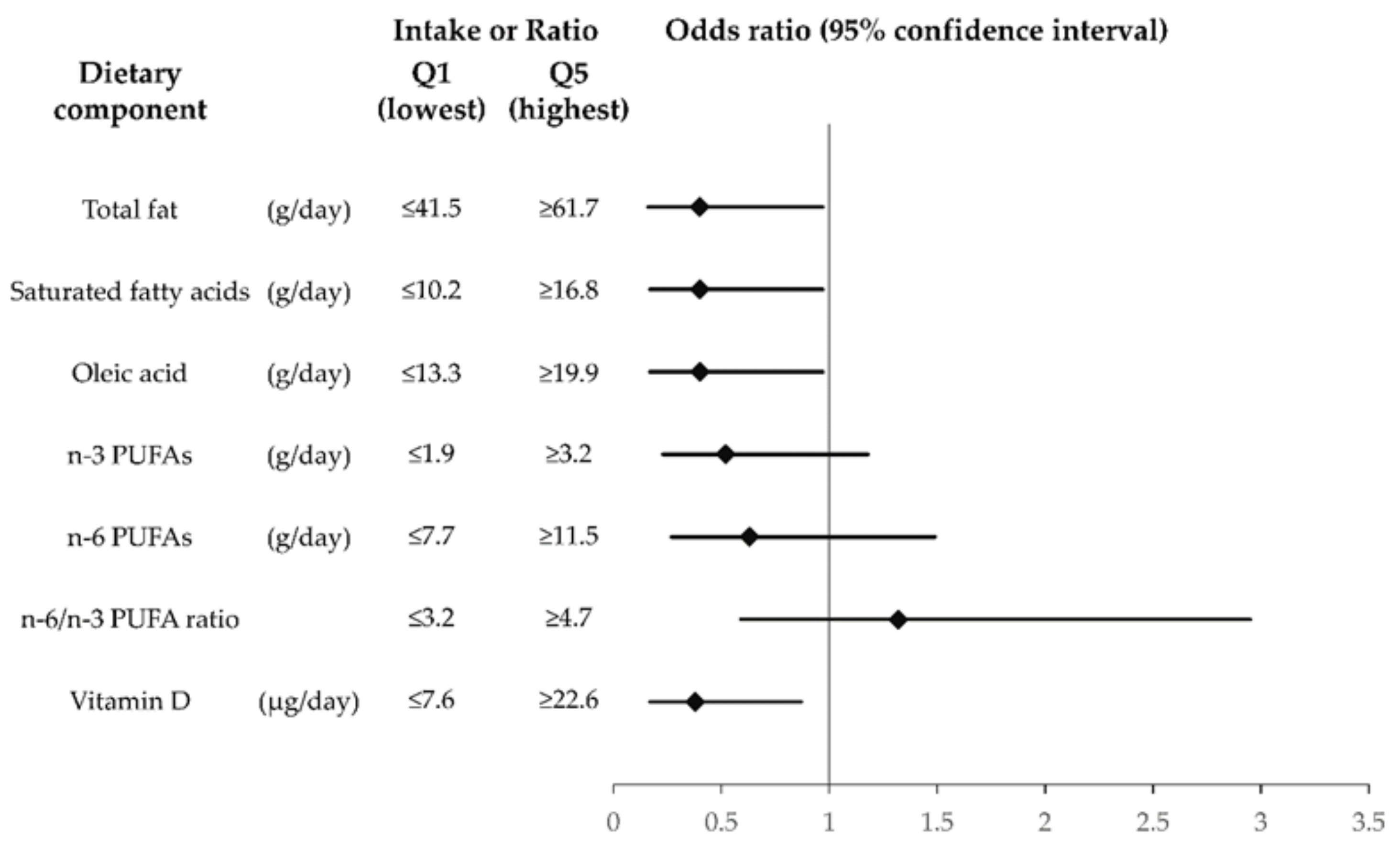
| Q1 (Lowest) | Q2 | Q3 | Q4 | Q5 (Highest) | p for Trend | |||
|---|---|---|---|---|---|---|---|---|
| Dietary Component | n = 60) | (n = 60) | (n = 60) | (n = 60) | n = 60) | |||
| Total fat | ||||||||
| Intake | (g/day) | ≤41.5 | 41.5–47.7 | 47.9–53.9 | 54.1–61.7 | ≥61.7 | ||
| MGD | (%) | 48.3 | 31.7 | 41.7 | 33.3 | 21.7 | ||
| Model 1 | OR (95% CI) | 1.00 | 0.61 (0.28–1.34) | 1.07 (0.49–2.34) | 0.94 (0.42–2.13) | 0.46 (0.20–1.07) | 0.20 | |
| Model 2 | OR (95% CI) | 1.00 | 0.60 (0.27–1.35) | 1.02 (0.46–2.26) | 0.86 (0.37–1.97) | 0.40 (0.16–0.97) | 0.16 | |
| Animal fat | ||||||||
| Intake | (g/day) | ≤16.9 | 16.9–21.0 | 21.2–25.0 | 25.1–29.8 | ≥29.8 | ||
| MGD | (%) | 46.7 | 40.0 | 36.7 | 30.0 | 23.3 | ||
| Model 1 | OR (95% CI) | 1.00 | 0.92 (0.42–2.00) | 0.76 (0.35–1.66) | 0.87 (0.38–1.97) | 0.59 (0.25–1.36) | 0.75 | |
| Model 2 | OR (95% CI) | 1.00 | 0.93 (0.42–2.06) | 0.69 (0.31–1.53) | 0.75 (0.32–1.76) | 0.55 (0.23–1.32) | 0.66 | |
| Plant fat | ||||||||
| Intake | (g/day) | ≤21.5 | 21.9–25.8 | 25.9–28.7 | 28.9–33.0 | ≥33.1 | ||
| MGD | (%) | 41.7 | 35.0 | 36.7 | 23.3 | 40.0 | ||
| Model 1 | OR (95% CI) | 1.00 | 0.87 (0.39–1.93) | 1.10 (0.49–2.46) | 0.64 (0.27–1.49) | 1.09 (0.50–2.39) | 0.70 | |
| Model 2 | OR (95% CI) | 1.00 | 0.79 (0.35–1.79) | 0.99 (0.43–2.28) | 0.54 (0.22–1.30) | 0.95 (0.42–2.16) | 0.58 | |
| Saturated fatty acids | ||||||||
| Intake | (g/day) | ≤10.2 | 10.2–12.6 | 12.6–14.5 | 14.5–16.7 | ≥16.8 | ||
| MGD | (%) | 53.3 | 26.7 | 31.7 | 41.7 | 23.3 | ||
| Model 1 | OR (95% CI) | 1.00 | 0.34 (0.15–0.77) | 0.55 (0.25–1.21) | 1.04 (0.47–2.28) | 0.46 (0.20–1.09) | 0.026 * | |
| Model 2 | OR (95% CI) | 1.00 | 0.34 (0.15–0.78) | 0.44 (0.19–1.02) | 0.94 (0.42–2.13) | 0.40 (0.17–0.97) | 0.020 * | |
| Monounsaturated fatty acids | ||||||||
| Intake | (g/day) | ≤14.4 | 14.5–17.1 | 17.1–19.5 | 19.5–22.1 | ≥22.1 | ||
| MGD | (%) | 46.7 | 30.0 | 50.0 | 23.3 | 26.7 | ||
| Model 1 | OR (95% CI) | 1.00 | 0.57 (0.25–1.27) | 1.33 (0.62–2.88) | 0.62 (0.27–1.46) | 0.58 (0.25–1.30) | 0.13 | |
| Model 2 | OR (95% CI) | 1.00 | 0.55 (0.24–1.25) | 1.27 (0.58–2.80) | 0.58 (0.24–1.38) | 0.50 (0.21–1.17) | 0.094 | |
| Oleic acid | ||||||||
| Intake | (g/day) | ≤13.3 | 13.4–15.2 | 15.3–17.6 | 17.6–19.9 | ≥19.9 | ||
| MGD | (%) | 48.3 | 30.0 | 46.7 | 28.3 | 23.3 | ||
| Model 1 | OR (95% CI) | 1.00 | 0.50 (0.22–1.11) | 1.14 (0.53–2.49) | 0.74 (0.33–1.69) | 0.46 (0.20–1.07) | 0.10 | |
| Model 2 | OR (95% CI) | 1.00 | 0.54 (0.24–1.21) | 1.08 (0.49–2.39) | 0.68 (0.29–1.58) | 0.40 (0.17–0.97) | 0.11 | |
| Polyunsaturated fatty acids | ||||||||
| Intake | (g/day) | ≤9.9 | 10.0–11.5 | 11.5–13.1 | 13.2–14.7 | ≥14.7 | ||
| MGD | (%) | 45.0 | 35.0 | 40.0 | 31.7 | 25.0 | ||
| Model 1 | OR (95% CI) | 1.00 | 0.82 (0.37–1.82) | 1.20 (0.54–2.64) | 0.93 (0.41–2.12) | 0.51 (0.22–1.15) | 0.31 | |
| Model 2 | OR (95% CI) | 1.00 | 0.79 (0.35–1.78) | 1.16 (0.51–2.62) | 0.83 (0.36–1.93) | 0.46 (0.19–1.07) | 0.25 | |
| n-3 Polyunsaturated fatty acids | ||||||||
| Intake | (g/day) | ≤1.9 | 1.9–2.4 | 2.4–2.7 | 2.7–3.1 | ≥3.2 | ||
| MGD | (%) | 45.0 | 48.3 | 23.3 | 31.7 | 28.3 | ||
| Model 1 | OR (95% CI) | 1.00 | 1.33 (0.60–2.94) | 0.45 (0.19–1.05) | 0.66 (0.29–1.48) | 0.53 (0.24–1.18) | 0.049 * | |
| Model 2 | OR (95% CI) | 1.00 | 1.29 (0.57–2.89) | 0.47 (0.20–1.12) | 0.64 (0.28–1.47) | 0.52 (0.23–1.18) | 0.077 | |
| α-Linolenic acid | ||||||||
| Intake | (g/day) | ≤1.2 | 1.2–1.4 | 1.4–1.6 | 1.6–1.9 | ≥1.9 | ||
| MGD | (%) | 45.0 | 33.3 | 36.7 | 31.7 | 30.0 | ||
| Model 1 | OR (95% CI) | 1.00 | 0.71 (0.32–1.57) | 1.01 (0.45–2.23) | 0.75 (0.34–1.68) | 0.67 (0.30–1.50) | 0.77 | |
| Model 2 | OR (95% CI) | 1.00 | 0.75 (0.34–1.67) | 0.96 (0.42–2.18) | 0.68 (0.30–1.55) | 0.62 (0.27–1.43) | 0.74 | |
| Eicosapentaenoic acid | ||||||||
| Intake | (g/day) | ≤0.15 | 0.15–0.26 | 0.26–0.34 | 0.34–0.47 | ≥0.47 | ||
| MGD | (%) | 45.0 | 40.0 | 35.0 | 21.7 | 35.0 | ||
| Model 1 | OR (95% CI) | 1.00 | 0.87 (0.39–1.91) | 0.90 (0.40–2.02) | 0.40 (0.17–0.94) | 0.63 (0.29–1.36) | 0.20 | |
| Model 2 | OR (95% CI) | 1.00 | 0.84 (0.37–1.87) | 0.93 (0.41–2.12) | 0.41 (0.17–0.97) | 0.68 (0.31–1.50) | 0.24 | |
| Docosahexaenoic acid | ||||||||
| Intake | (g/day) | ≤0.28 | 0.28–0.44 | 0.44–0.57 | 0.57–0.75 | ≥0.76 | ||
| MGD | (%) | 43.3 | 46.7 | 23.3 | 31.7 | 31.7 | ||
| Model 1 | OR (95% CI) | 1.00 | 1.18 (0.54–2.58) | 0.55 (0.24–1.30) | 0.70 (0.31–1.56) | 0.57 (0.26–1.26) | 0.24 | |
| Model 2 | OR (95% CI) | 1.00 | 1.20 (0.54–2.66) | 0.58 (0.24–1.38) | 0.74 (0.33–1.67) | 0.61 (0.28–1.37) | 0.34 | |
| n-6 Polyunsaturated fatty acids | ||||||||
| Intake | (g/day) | ≤7.7 | 7.7–9.2 | 9.2–10.2 | 10.2–11.5 | ≥11.5 | ||
| MGD | (%) | 43.3 | 33.3 | 40.0 | 31.7 | 28.3 | ||
| Model 1 | OR (95% CI) | 1.00 | 0.79 (0.36–1.76) | 1.22 (0.55–2.71) | 1.54 (0.70–3.41) | 0.97 (0.43–2.02) | 0.74 | |
| Model 2 | OR (95% CI) | 1.00 | 0.71 (0.31–1.62) | 1.16 (0.51–2.63) | 0.82 (0.35–1.92) | 0.63 (0.27–1.49) | 0.61 | |
| Linoleic acid | ||||||||
| Intake | (g/day) | ≤7.4 | 7.5–8.9 | 8.9–9.9 | 9.9–11.1 | ≥11.2 | ||
| MGD | (%) | 43.3 | 31.7 | 41.7 | 31.7 | 28.3 | ||
| Model 1 | OR (95% CI) | 1.00 | 0.72 (0.32–1.62) | 1.31 (0.59–2.90) | 1.00 (0.44–2.28) | 0.74 (0.32–1.68) | 0.57 | |
| Model 2 | OR (95% CI) | 1.00 | 0.66 (0.29–1.50) | 1.25 (0.56–2.83) | 0.83 (0.35–1.94) | 0.63 (0.27–1.49) | 0.44 | |
| Arachidonic acid | ||||||||
| Intake | (g/day) | ≤0.12 | 0.12–0.15 | 0.15–0.17 | 0.17–0.20 | ≥0.20 | ||
| MGD | (%) | 45.0 | 38.3 | 21.7 | 36.7 | 35.0 | ||
| Model 1 | OR (95% CI) | 1.00 | 0.87 (0.40–1.91) | 0.42 (0.18–0.99) | 0.95 (0.43–2.11) | 0.78 (0.35–1.69) | 0.27 | |
| Model 2 | OR (95% CI) | 1.00 | 0.94 (0.42–2.09) | 0.44 (0.18–1.03) | 0.46 (0.20–1.10) | 1.00 (0.44–2.25) | 0.26 | |
| n-6/n-3 Polyunsaturated fatty acid ratio | ||||||||
| Ratio | ≤3.2 | 3.2–3.6 | 3.6–4.0 | 4.0–4.7 | ≥4.7 | |||
| MGD | (%) | 36.7 | 31.7 | 28.3 | 38.3 | 41.7 | ||
| Model 1 | OR (95% CI) | 1.00 | 0.89 (0.40–1.99) | 0.97 (0.43–2.21) | 1.44 (0.65–3.21) | 1.53 (0.70–3.34) | 0.58 | |
| Model 2 | OR (95% CI) | 1.00 | 0.86 (0.38–1.95) | 0.93 (0.40–2.17) | 1.36 (0.60–3.07) | 1.32 (0.59–2.95) | 0.75 | |
| Cholesterol | ||||||||
| Intake | (mg/day) | ≤270.6 | 270.8–330.7 | 331.2–395.0 | 399.1–479.8 | ≥480.6 | ||
| MGD | (%) | 40.0 | 35.0 | 26.7 | 40.0 | 35.0 | ||
| Model 1 | OR (95% CI) | 1.00 | 1.04 (0.47–2.33) | 0.63 (0.27–1.45) | 1.41 (0.63–3.14) | 0.73 (0.33–1.63) | 0.33 | |
| Model 2 | OR (95% CI) | 1.00 | 1.07 (0.47–2.44) | 0.60 (0.25–1.41) | 1.36 (0.60–3.11) | 0.70 (0.31–1.58) | 0.30 | |
| Vitamin D | ||||||||
| Intake | (μg/day) | ≤7.6 | 7.6–11.7 | 11.7–14.9 | 15.0–22.3 | ≥22.6 | ||
| MGD | (%) | 46.7 | 35.0 | 38.3 | 28.3 | 28.3 | ||
| Model 1 | OR (95% CI) | 1.00 | 0.55 (0.24–1.23) | 0.98 (0.44–2.20) | 0.55 (0.24–1.24) | 0.38 (0.17–0.86) | 0.087 | |
| Model 2 | OR (95% CI) | 1.00 | 0.56 (0.25–1.27) | 1.00 (0.44–2.27) | 0.51 (0.22–1.19) | 0.38 (0.17–0.87) | 0.081 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukuoka, S.; Arita, R.; Mizoguchi, T.; Kawashima, M.; Koh, S.; Shirakawa, R.; Suzuki, T.; Sasaki, S.; Morishige, N. Relation of Dietary Fatty Acids and Vitamin D to the Prevalence of Meibomian Gland Dysfunction in Japanese Adults: The Hirado–Takushima Study. J. Clin. Med. 2021, 10, 350. https://doi.org/10.3390/jcm10020350
Fukuoka S, Arita R, Mizoguchi T, Kawashima M, Koh S, Shirakawa R, Suzuki T, Sasaki S, Morishige N. Relation of Dietary Fatty Acids and Vitamin D to the Prevalence of Meibomian Gland Dysfunction in Japanese Adults: The Hirado–Takushima Study. Journal of Clinical Medicine. 2021; 10(2):350. https://doi.org/10.3390/jcm10020350
Chicago/Turabian StyleFukuoka, Shima, Reiko Arita, Takanori Mizoguchi, Motoko Kawashima, Shizuka Koh, Rika Shirakawa, Takashi Suzuki, Satoshi Sasaki, and Naoyuki Morishige. 2021. "Relation of Dietary Fatty Acids and Vitamin D to the Prevalence of Meibomian Gland Dysfunction in Japanese Adults: The Hirado–Takushima Study" Journal of Clinical Medicine 10, no. 2: 350. https://doi.org/10.3390/jcm10020350
APA StyleFukuoka, S., Arita, R., Mizoguchi, T., Kawashima, M., Koh, S., Shirakawa, R., Suzuki, T., Sasaki, S., & Morishige, N. (2021). Relation of Dietary Fatty Acids and Vitamin D to the Prevalence of Meibomian Gland Dysfunction in Japanese Adults: The Hirado–Takushima Study. Journal of Clinical Medicine, 10(2), 350. https://doi.org/10.3390/jcm10020350





