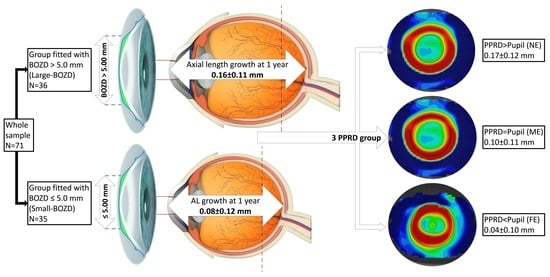
The Role of Back Optic Zone Diameter in Myopia Control with Orthokeratology Lenses
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Subjects
2.3. OK Lens Characteristics
2.4. Outcomes
2.5. Statistical Analysis
3. Results
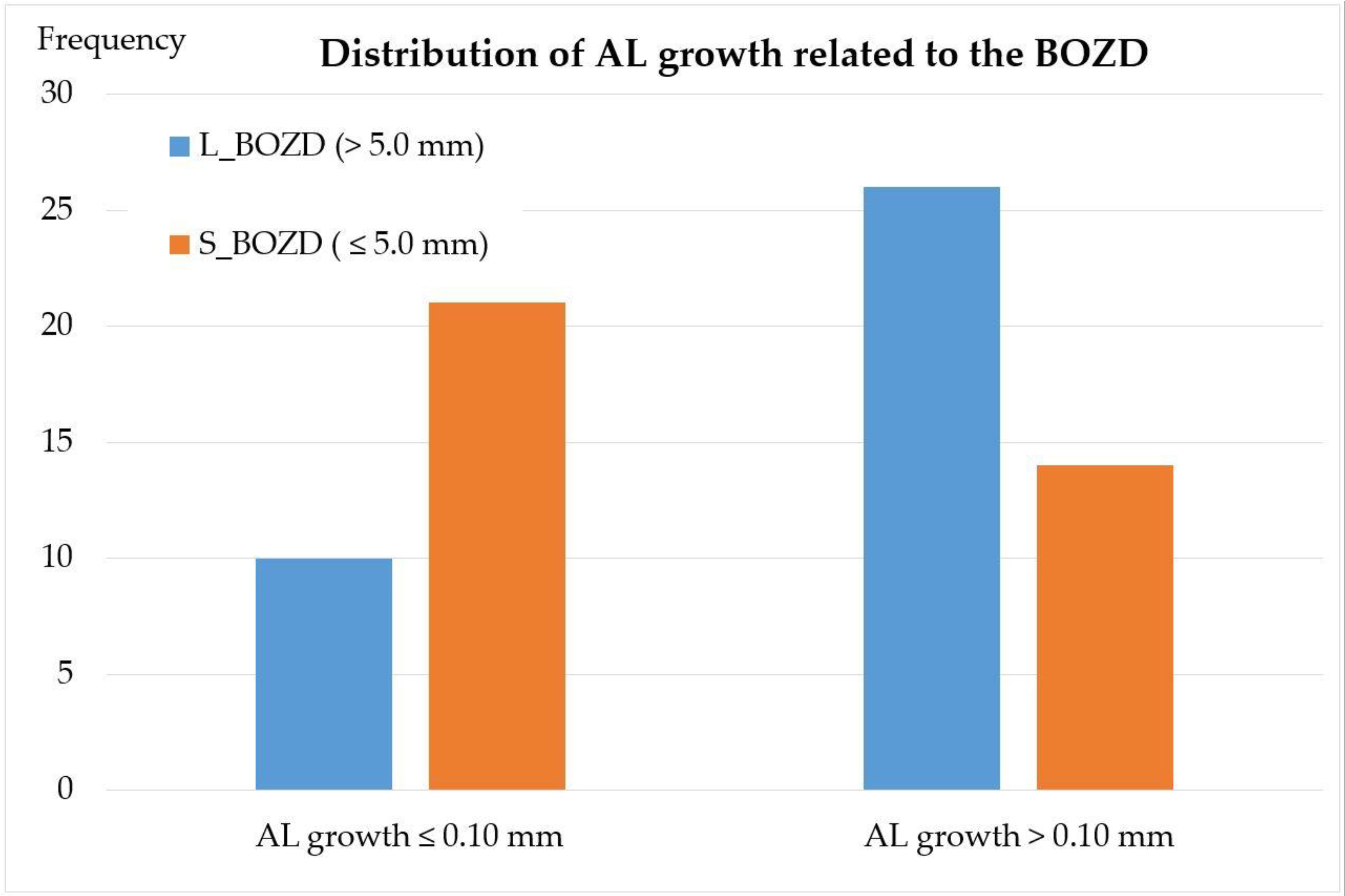
3.1. BOZD
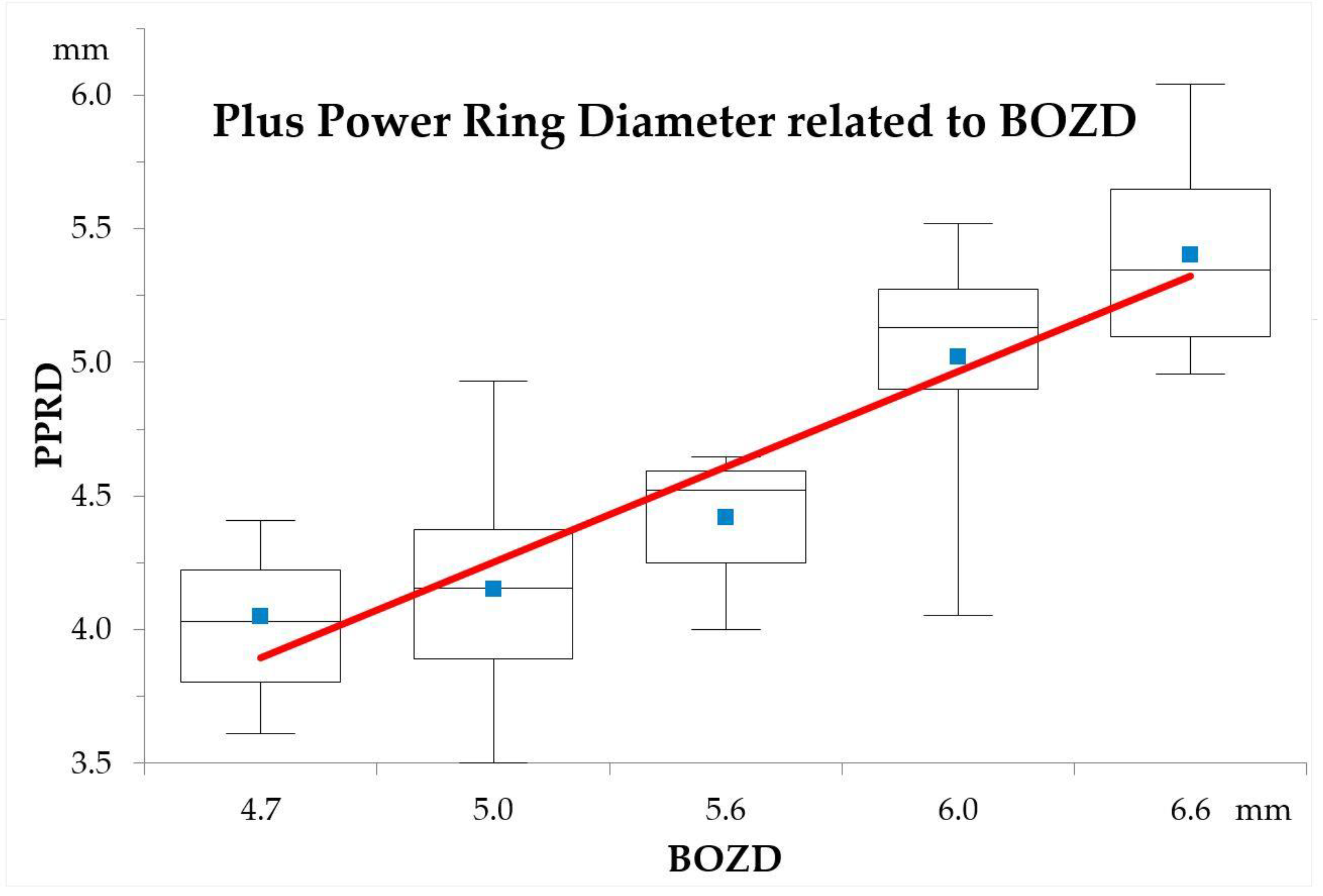
3.2. PPRD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolgin, E. The myopia boom. Nature 2015, 519, 9–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourne, R.R.; Stevens, G.A.; White, R.A.; Smith, J.L.; Flaxman, S.R.; Price, H.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K. Causes of vision loss worldwide, 1990-2010: A systematic analysis. Lancet Glob. Health 2013, 1, 339–349. [Google Scholar] [CrossRef] [Green Version]
- Holden, B.; Sankaridurg, P.; Smith, E.; Aller, T.; Jong, M.; He, M. Myopia, an underrated global challenge to vision: Where the current data takes us on myopia control. Eye 2014, 28, 142–146. [Google Scholar] [CrossRef] [Green Version]
- Flitcroft, D.I. The complex interactions of retinal, optical and environmental factors in myopia aetiology. Prog. Retin. Eye Res. 2012, 31, 622–660. [Google Scholar] [CrossRef]
- Cheng, D.; Schmid, K.; Woo, G.; Drobe, B. Randomized trial of effect of bifocal and prismatic bifocal spectacles on myopic progression: Two-year results. Arch. Ophthalmol. 2010, 128, 12–19. [Google Scholar] [CrossRef]
- Lam, C.S.Y.; Tang, W.C.; Tse, D.Y.; Lee, R.P.K.; Chun, R.K.M.; Hasegawa, K.; Qi, H.; Hatanaka, T.; To, C.H. Defocus incorporated multiple segments (DIMS) spectacle lenses slow myopia progression: A 2-year randomised clinical trial. Br. J. Ophthalmol. 2020, 104, 363–368. [Google Scholar] [CrossRef] [Green Version]
- Aller, T.A.; Liu, M.; Wildsoet, C.F. Myopia control with bifocal contact lenses. Optom. Vis. Sci. 2016, 93, 344–352. [Google Scholar] [CrossRef] [Green Version]
- Pauné, J.; Morales, H.; Armengol, J.; Quevedo, L.; Faria-Ribeiro, M.; González-Méijome, J.M. Myopia control with a novel peripheral gradient soft lens and orthokeratology: A 2-year clinical trial. Biomed Res. Int. 2015, 2015, 507572. [Google Scholar] [CrossRef] [Green Version]
- Cho, P.; Cheung, S.-W.W. Retardation of myopia in orthokeratology (ROMIO) study: A 2-year randomized clinical trial. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7077–7085. [Google Scholar] [CrossRef]
- Santodomingo-Rubido, J.; Villa-Collar, C.; Gilmartin, B.; Gutierrez-Ortega, R. Myopia control with orthokeratology contact lenses in Spain: Refractive and biometric changes. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5060–5065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiraoka, T.; Kakita, T.; Okamoto, F.; Takahashi, H.; Oshika, T. Long-term effect of overnight orthokeratology on axial length elongation in childhood myopia: A 5-year follow-up study. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3913–3919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Wen, D.; Wang, Q.; McAlinden, C.; Flitcroft, I.; Chen, H.; Saw, S.M.; Chen, H.; Bao, F.; Zhao, Y.; et al. Efficacy comparison of 16 interventions for myopia control in children: A network meta-analysis. Ophthalmology 2016, 123, 697–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinstein, D.D.Z.; Gobbe, M.; Archer, T.J.; Couch, D.; Bloom, B. Epithelial, stromal, and corneal pachymetry changes during orthokeratology. Optom. Vis. Sci. 2009, 86, 1006–1014. [Google Scholar] [CrossRef]
- Queiros, A.; Gonzalez-Meijome, J.M.; Villa-Collar, C.; Gutierrez, A.R.; Jorge, J. Local steepening in peripheral corneal curvature after corneal refractive therapy and LASIK. Optom. Vis. Sci. 2010, 87, 432–439. [Google Scholar] [CrossRef]
- Smith, E.L. Prentice Award Lecture 2010: A case for peripheral optical treatment strategies for myopia. Optom. Vis. Sci. 2011, 88, 1029–1044. [Google Scholar] [CrossRef]
- Lau, J.K.; Vincent, S.J.; Collins, M.J.; Cheung, S.W.; Cho, P. Ocular higher-order aberrations and axial eye growth in young Hong Kong children. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Hiraoka, T.; Kakita, T.; Okamoto, F.; Oshika, T. Influence of ocular wavefront aberrations on axial length elongation in myopic children treated with overnight orthokeratology. Ophthalmology 2015, 122, 1–8. [Google Scholar] [CrossRef]
- Kim, J.; Lim, D.H.; Han, S.H.; Chung, T.-Y.Y. Predictive factors associated with axial length growth and myopia progression in orthokeratology. PLoS ONE 2019, 14, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.; Yang, C. Influence of overnight orthokeratology lens treatment zone decentration on myopia progression. J. Ophthalmol. 2019, 2019, 2596953. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, Z.; Xue, F.; Zhou, J.; Niu, L.; Zhou, X. Corneal power change is predictive of myopia progression in orthokeratology. Optom. Vis. Sci. 2014, 91, 404–411. [Google Scholar] [CrossRef] [PubMed]
- González-Méijome, J.M.; Faria-Ribeiro, M.A.; Lopes-Ferreira, D.P.; Fernandes, P.; Carracedo, G.; Queiros, A. Changes in peripheral refractive profile after orthokeratology for different degrees of myopia. Curr. Eye Res. 2016, 41, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Queirós, A.; Lopes-Ferreira, D.; Yeoh, B.; Issacs, S.; Amorim-De-Sousa, A.; Villa-Collar, C.; González-Méijome, J. Refractive, biometric and corneal topographic parameter changes during 12 months of orthokeratology. Clin. Exp. Optom. 2020, 103, 454–462. [Google Scholar]
- Queirós, A.; González-Méijome, J.M.; Jorge, J.; Villa-Collar, C.; Gutiérrez, A.R. Peripheral refraction in myopic patients after orthokeratology. Optom. Vis. Sci. 2010, 87, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Queirós, A.; Amorim-de-Sousa, A.; Lopes-Ferreira, D.; Villa-Collar, C.; Gutiérrez, Á.R.; González-Méijome, J.M. Relative peripheral refraction across 4 meridians after orthokeratology and LASIK surgery. Eye Vis. 2018, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, D.; Bi, H.; Du, B.; Lin, W.; Gu, T.; Zhang, B.; Wei, R. A new method to analyze the relative corneal refractive power and its association to myopic progression control with orthokeratology. Transl. Vis. Sci. Technol. 2018, 7, 17. [Google Scholar] [CrossRef] [Green Version]
- Kang, P.; Gifford, P.; Swarbrick, H. Can manipulation of orthokeratology lens parameters modify peripheral refraction? Optom. Vis. Sci. 2013, 90, 1237–1248. [Google Scholar] [CrossRef]
- Kang, P.; Swarbrick, H. The influence of different ok lens designs on peripheral refraction. Optom. Vis. Sci. 2016, 93, 1–8. [Google Scholar] [CrossRef]
- Marcotte-Collard, R.; Simard, P.; Michaud, L. Analysis of two orthokeratology lens designs and comparison of their optical effects on the cornea. Eye Contact Lens 2018, 44, 1. [Google Scholar] [CrossRef]
- Yang, X.; Bi, H.; Li, L.; Li, S.; Chen, S.; Zhang, B.; Wang, Y. The effect of relative corneal refractive power shift distribution on axial length growth in myopic children undergoing orthokeratology treatment. Curr. Eye Res. 2020, 18, 1–9. [Google Scholar] [CrossRef]
- Carracedo, G.; Espinosa-Vidal, T.M.; Martínez-Alberquilla, I.; Batres, L. The topographical effect of optical zone diameter in orthokeratology contact lenses in high myopes. J. Ophthalmol. 2019, 2019, 1082472. [Google Scholar] [CrossRef] [PubMed]
- Gifford, P.; Tran, M.; Priestley, C.; Maseedupally, V.; Kang, P. Reducing treatment zone diameter in orthokeratology and its effect on peripheral ocular refraction. Contact Lens Anterior Eye 2019, 43, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Queirós, A.; González-Méijome, J.; Jorge, J. Influence of fogging lenses and cycloplegia on open-field automatic refraction. Ophthalmic Physiol. Opt. 2008, 28, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Şimşek, A.; Bilak, Ş.; Güler, M.; Çapkin, M.; Bilgin, B.; Reyhan, A.H. Comparison of central corneal thickness measurements obtained by RTVue OCT, Lenstar, Sirius topography, and ultrasound pachymetry in healthy subjects. Semin. Ophthalmol. 2016, 31, 467–472. [Google Scholar] [CrossRef]
- Chui, W.S.; Cho, P. A comparative study of the performance of different corneal topographers on children with respect to orthokeratology practice. Optom. Vis. Sci. 2005, 82, 420–427. [Google Scholar] [CrossRef]
- Periman, L.M.; Ambrosio, R., Jr.; Harrison, D.A.; Wilson, S.E. Correlation of pupil sizes measured with a mesopic infrared pupillometer and a photopic topographer. J Refract. Surg. 2003, 19, 555–559. [Google Scholar]
- Walline, J.J.; Walker, M.K.; Mutti, D.O.; Jones-Jordan, L.A.; Sinnott, L.T.; Giannoni, A.G.; Bickle, K.M.; Schulle, K.L.; Nixon, A.; Pierce, G.E.; et al. Effect of High Add Power, Medium Add Power, or Single-Vision Contact Lenses on Myopia Progression in Children: The BLINK Randomized Clinical Trial. JAMA 2020, 324, 571–580. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, F.; Zhang, T.; Liu, M.; Wang, D.; Chen, Y.; Liu, Q. Orthokeratology to control myopia progression: A meta-analysis. PLoS ONE 2015, 10, e0124535. [Google Scholar]
- Brennan, N.A.; Toubouti, M.Y.; Cheng, X.; Bullimore, M.A. Efficacy in myopia control. Prog. Retin. Eye Res. 2020, 100923. [Google Scholar] [CrossRef]
- Chen, Z.; Niu, L.; Xue, F. Impact of pupil diameter on axial growth. Optom. Vis. Sci. 2012, 89, 1636–1640. [Google Scholar] [CrossRef] [Green Version]
- Faria-Ribeiro, M.; Navarro, R.; González-Méijome, J.M. Effect of pupil size on wavefront refraction during orthokeratology. Optom. Vis. Sci. 2016, 93, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Hung, L.F. The role of optical defocus in regulating refractive development in infant monkeys. Vis. Res. 1999, 39, 1415–1435. [Google Scholar] [CrossRef] [Green Version]
- Benavente-Perez, A.; Nour, A.; Troilo, D. The effect of simultaneous negative and positive defocus on eye growth and development of refractive state in marmosets. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6479–6487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiraoka, T.; Kotsuka, J.; Kakita, T.; Okamoto, F.; Oshika, T. Relationship between higher-order wavefront aberrations and natural progression of myopia in schoolchildren. Sci. Rep. 2017, 7, 7876. [Google Scholar] [CrossRef] [Green Version]
- Koch, D.D.; Samuelson, S.W.; Haft, E.A.; Merin, L.M. Pupillary size and responsiveness. Implications for selection of a bifocal intraocular lens. Ophthalmology 1991, 98, 1030–1035. [Google Scholar] [CrossRef]
- Cardona, G.; López, S. Pupil diameter, working distance, and illumination during habitual tasks. Implications for simultaneous vision contact lenses for presbyopia. J. Optom. 2016, 9, 78–84. [Google Scholar] [CrossRef]
- Hsieh, Y.T.; Hu, F.R. The correlation of pupil size measured by Colvard pupillometer and Orbscan II. J Refract. Surg. 2007, 8, 789–795. [Google Scholar] [CrossRef]
- Tan, Q.; Ng, A.L.; Cheng, G.P.; Woo, V.C.; Cho, P. Repeatability of pupil size measurements with NIDEK OPD-Scan III in myopic children. Ophthalmic Physiol. Opt. 2020. [Google Scholar] [CrossRef]
- Jones, L.A.; Mitchell, G.L.; Mutti, D.O.; Hayes, J.R.; Moeschberger, M.L.; Zadnik, K. Comparison of ocular component growth curves among refractive error groups in children. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2317–2327. [Google Scholar] [CrossRef] [Green Version]
- Alfonso, J.F.; Ferrer-Blasco, T.; González-Méijome, J.M.; García-Manjarres, M.; Peixoto-de-Matos, S.C.; Montés-Micó, R. Pupil size, white-to-white corneal diameter, and anterior chamber depth in patients with myopia. J. Refract. Surg. 2010, 26, 891–898. [Google Scholar] [CrossRef]
- Chen, Z.; Xue, F.; Zhou, J.; Qu, X.; Zhou, X. Prediction of orthokeratology lens decentration with corneal elevation. Optom. Vis. Sci. 2017, 94, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Chen, Y.; Lipson, M.; Kang, P.; Lian, H.; Zhao, Y.; McAlinden, C.; Huang, J. The effect of treatment zone decentration on myopic progression during orthokeratology. Curr. Eye Res. 2020, 45, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Mutti, D.O.; Hayes, J.R.; Mitchell, G.L.; Jones, L.A.; Moeschberger, M.L.; Cotter, S.A.; Kleinstein, R.N.; Manny, R.E.; Twelker, J.D.; Zadnik, K. Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2510–2519. [Google Scholar] [CrossRef] [PubMed]
- Gwiazda, J.; Hyman, L.; Hussein, M.; Everett, D.; Norton, T.T.; Kurtz, D.; Leske, M.C.; Manny, R.; Marsh-Tootle, W.; Scheiman, M. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1492–1500. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, D.; Manny, R.; Hussein, M. Variability of the ocular component measurements in children using A-scan ultrasonography. Optom. Vis. Sci. 2004, 81, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Cho, P.; Cheung, S.; Edwards, M. The longitudinal orthokeratology research in children (LORIC) in Hong Kong: A pilot study on refractive changes and myopic control. Curr. Eye Res. 2005, 30, 71–80. [Google Scholar] [CrossRef]
- Chen, Z.; Xue, F.; Zhou, J.; Qu, X.; Zhou, X. Effects of orthokeratology on choroidal thickness and axial length. Optom. Vis. Sci. 2016, 93, 1064–1071. [Google Scholar] [CrossRef]
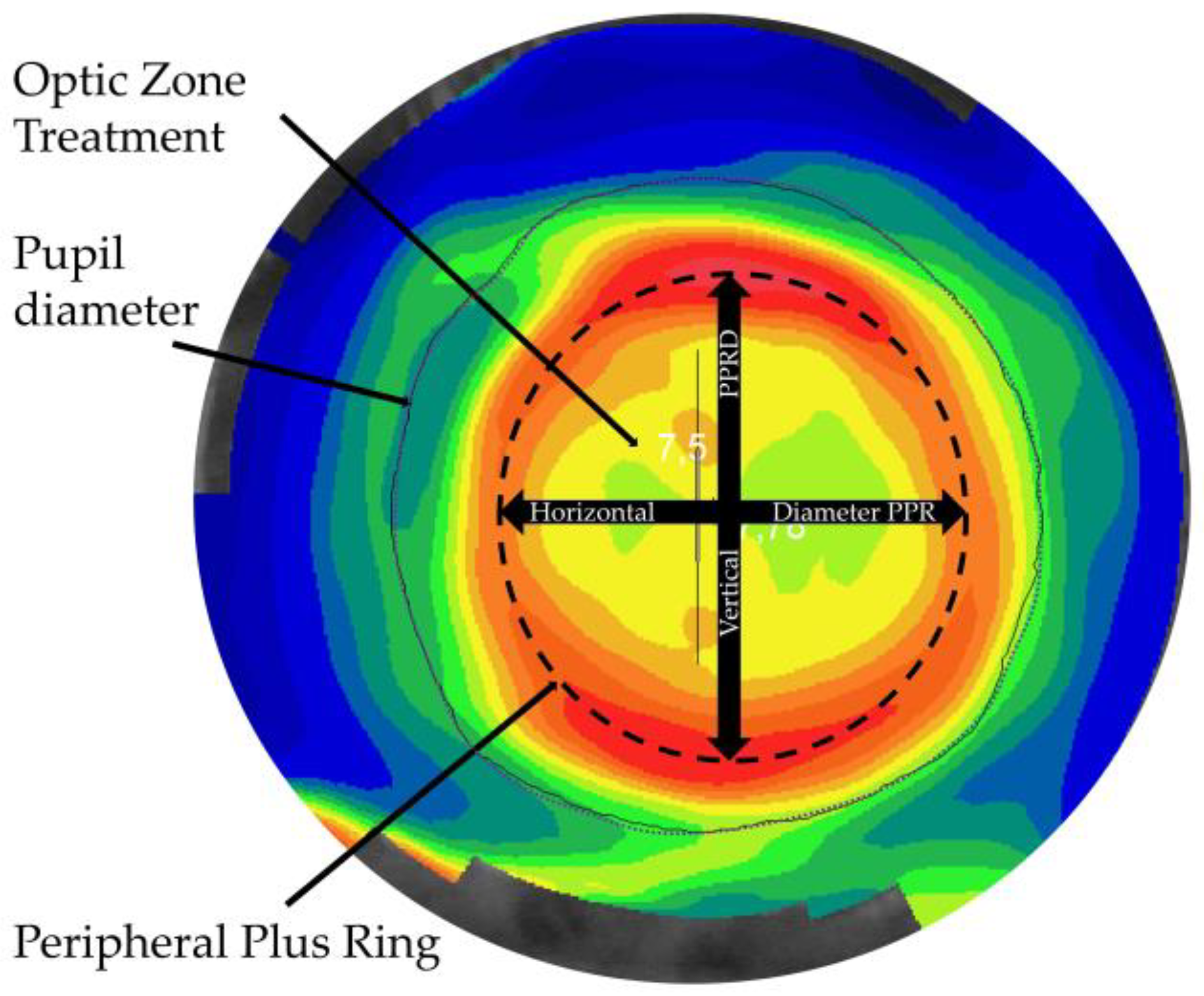
| Baseline | BZOD Group | Mean ± SD | Minimum | Maximum | p-Value |
|---|---|---|---|---|---|
| Age (years) | >5 | 13.27 ± 1.50 | 10.08 | 15.31 | 0.666 * |
| ≤5 | 13.41 ± 1.25 | 11.00 | 15.82 | ||
| Flat keratometry (mm) | >5 | 7.87 ± 0.27 | 7.47 | 8.45 | 0.580 * |
| ≤5 | 7.92 ± 0.37 | 7.02 | 8.48 | ||
| Steep keratometry (mm) | >5 | 7.72 ± 0.27 | 7.21 | 8.21 | 0.410 * |
| ≤5 | 7.78 ± 0.34 | 6.96 | 8.44 | ||
| Eccentricity flat | >5 | 0.43 ± 0.07 | 0.22 | 0.55 | 0.845 † |
| ≤5 | 0.43 ± 0.08 | 0.28 | 0.55 | ||
| Pupillary diameter (mm) | >5 | 4.01 ± 0.50 | 3.35 | 5.39 | 0.004 * |
| ≤5 | 4.45 ± 0.71 | 3.22 | 6.28 | ||
| BOZD (mm) | >5 | 6.11 ± 0.34 | 5.60 | 6.60 | 0.000 † |
| ≤5 | 4.91 ± 0.14 | 4.70 | 5.00 | ||
| PPRD Horizontal | >5 | 5.13 ± 0.46 | 4.03 | 6.08 | 0.000 * |
| ≤5 | 4.21 ± 0.30 | 3.73 | 4.88 | ||
| PPRD Vertical | >5 | 4.96 ± 0.49 | 3.97 | 6.00 | 0.000 * |
| ≤5 | 4.02 ± 0.39 | 3.17 | 4.98 | ||
| Mean PPRD (mm) | >5 | 5.05 ± 0.47 | 4.00 | 6.04 | 0.000 * |
| ≤5 | 4.12 ± 0.32 | 3.50 | 4.93 | ||
| Axial Length (mm) | >5 | 24.68 ± 0.94 | 23.14 | 26.37 | 0.789 * |
| ≤5 | 24.61 ± 0.83 | 23.16 | 26.14 |
| BOZD (mm) | Baseline | 12 Months | 12-Month-Baseline | p Value | |
|---|---|---|---|---|---|
| Spherical equivalent (Diopters) | >5 | −3.41 ± 1.51 | −3.68 ± 1.51 | −0.27 ± 0.23 | <0.001 * |
| ≤5 | −2.80 ± 1.37 | −2.64 ± 1.45 | 0.16 ± 0.34 | 0.013 † | |
| p | 0.079 ‡ | 0.003 § | <0.001 ‡ | ||
| Vitreous chamber depth (mm) | >5 | 17.31 ± 0.95 | 17.40 ± 0.99 | 0.09 ± 0.12 | <0.001 * |
| ≤5 | 17.16 ± 0.77 | 17.21 ± 0.77 | 0.05 ± 0.12 | 0.012 † | |
| p | 0.468 ‡ | 0.359 ‡ | 0.311 § | ||
| Axial Length (mm) | >5 | 24.69 ± 0.94 | 24.84 ± 0.96 | 0.16 ± 0.11 | <0.001 * |
| ≤5 | 24.61 ± 0.83 | 24.69 ± 0.85 | 0.08 ± 0.12 | <0.001 * | |
| p | 0.723 ‡ | 0.488 ‡ | 0.007 ‡ |
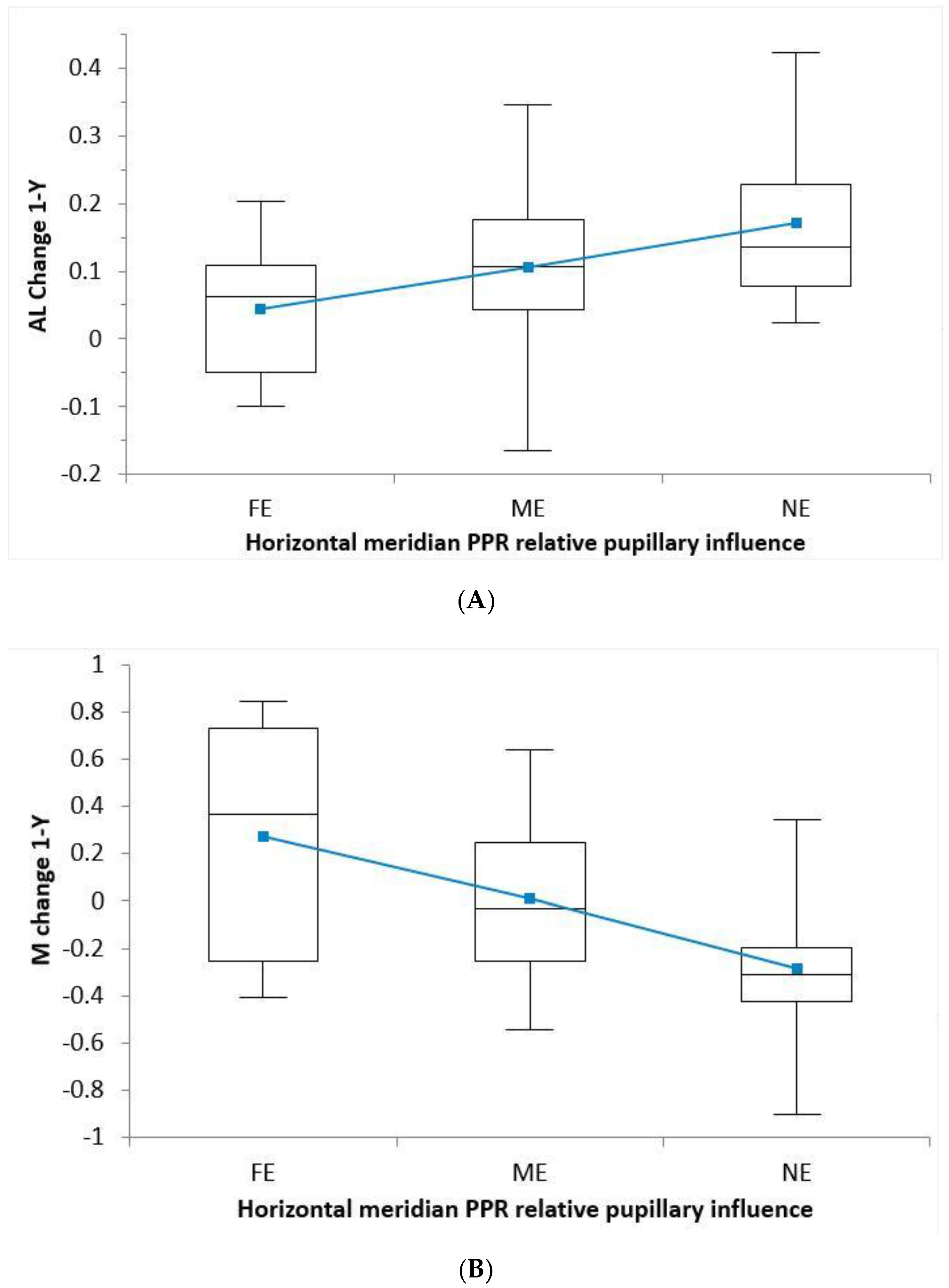
| PPRD | FE | ME | NE | p | |
|---|---|---|---|---|---|
| AL | Horizontal | 0.04 ± 0.10 | 0.10 ± 0.11 | 0.17 ± 0.12 | 0.035 * |
| Vertical | 0.06 ± 0.11 | 0.11 ± 0.11 | 0.16 ± 0.12 | 0.158 * | |
| Mean | 0.04 ± 0.10 | 0.11 ± 0.11 | 0.17 ± 0.12 | 0.056 * | |
| M | Horizontal | 0.27 ± 0.50 | 0.01 ± 0.30 | −0.29 ± 0.26 | <0.001 † |
| Vertical | 0.28 ± 0.47 | −0.02 ± 0.29 | −0.24 ± 0.31 | <0.001 † | |
| Mean | 0.27 ± 0.50 | −0.01 ± 0.29 | −0.25 ± 0.31 | <0.001 † |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pauné, J.; Fonts, S.; Rodríguez, L.; Queirós, A. The Role of Back Optic Zone Diameter in Myopia Control with Orthokeratology Lenses. J. Clin. Med. 2021, 10, 336. https://doi.org/10.3390/jcm10020336
Pauné J, Fonts S, Rodríguez L, Queirós A. The Role of Back Optic Zone Diameter in Myopia Control with Orthokeratology Lenses. Journal of Clinical Medicine. 2021; 10(2):336. https://doi.org/10.3390/jcm10020336
Chicago/Turabian StylePauné, Jaume, Silvia Fonts, Lina Rodríguez, and Antonio Queirós. 2021. "The Role of Back Optic Zone Diameter in Myopia Control with Orthokeratology Lenses" Journal of Clinical Medicine 10, no. 2: 336. https://doi.org/10.3390/jcm10020336
APA StylePauné, J., Fonts, S., Rodríguez, L., & Queirós, A. (2021). The Role of Back Optic Zone Diameter in Myopia Control with Orthokeratology Lenses. Journal of Clinical Medicine, 10(2), 336. https://doi.org/10.3390/jcm10020336