The Retrobulbar Spot Sign and Prominent Middle Limiting Membrane as Prognostic Markers in Non-Arteritic Retinal Artery Occlusion
Abstract
1. Introduction
2. Materials and Methods
Prospective Trial
Inclusion and Exclusion Criteria
Retrospective Trial
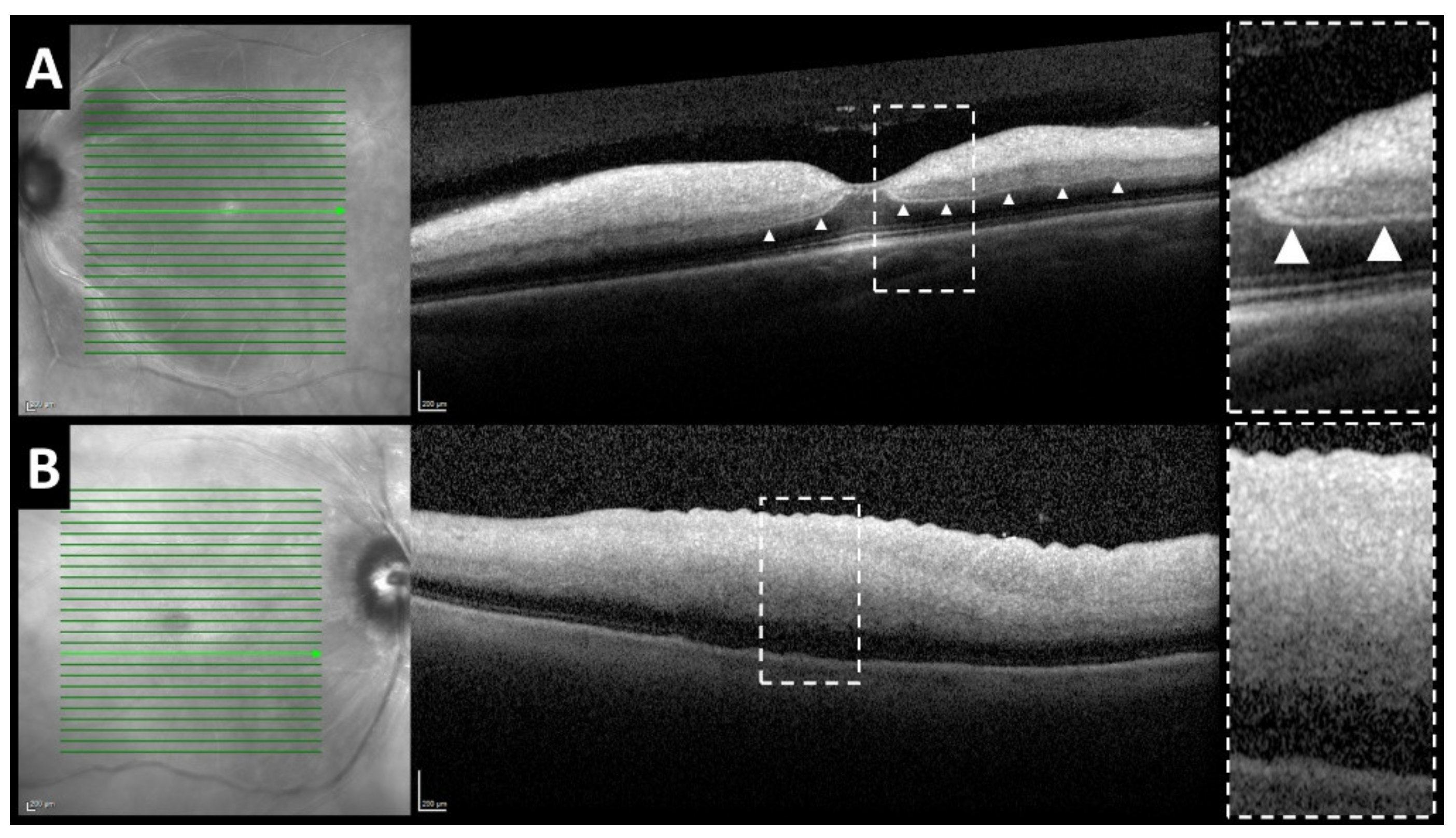
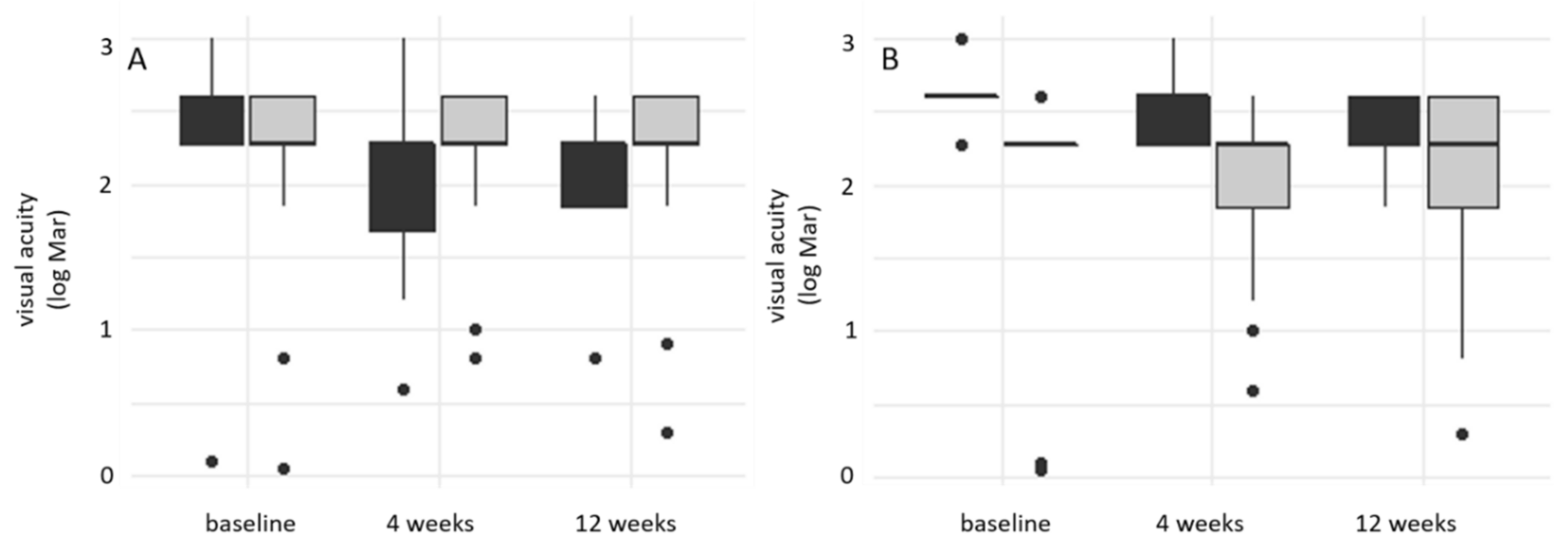
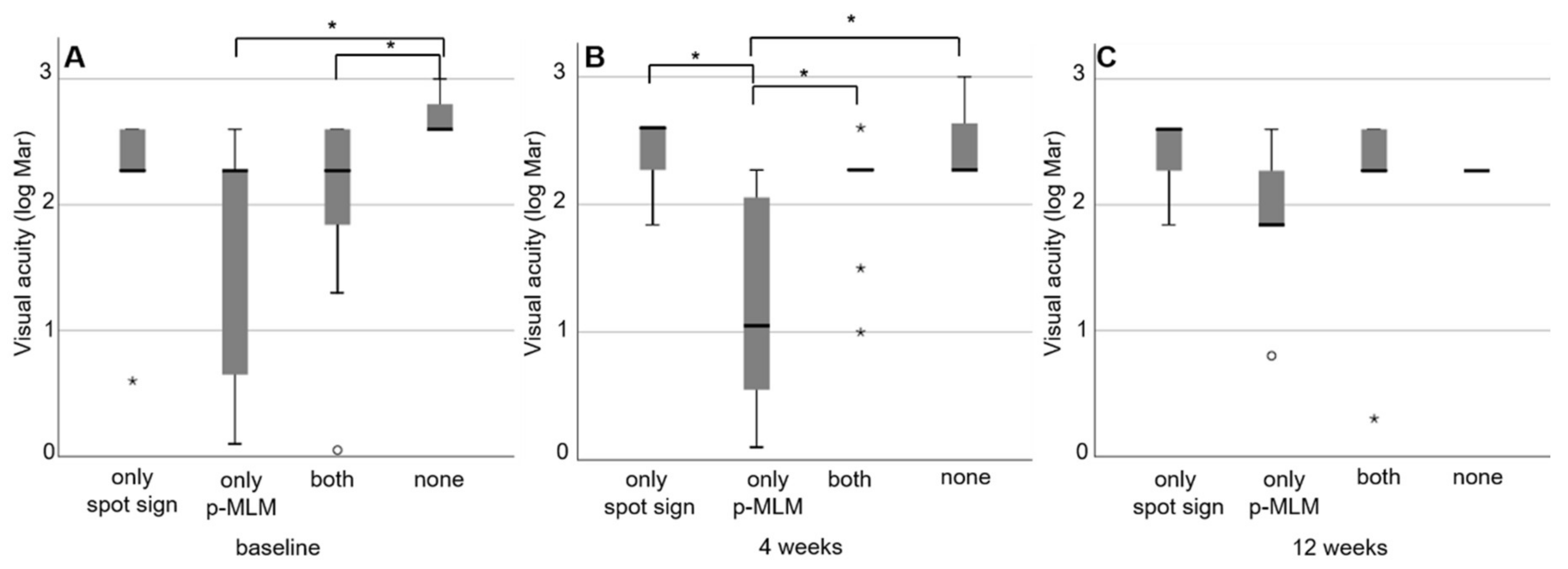
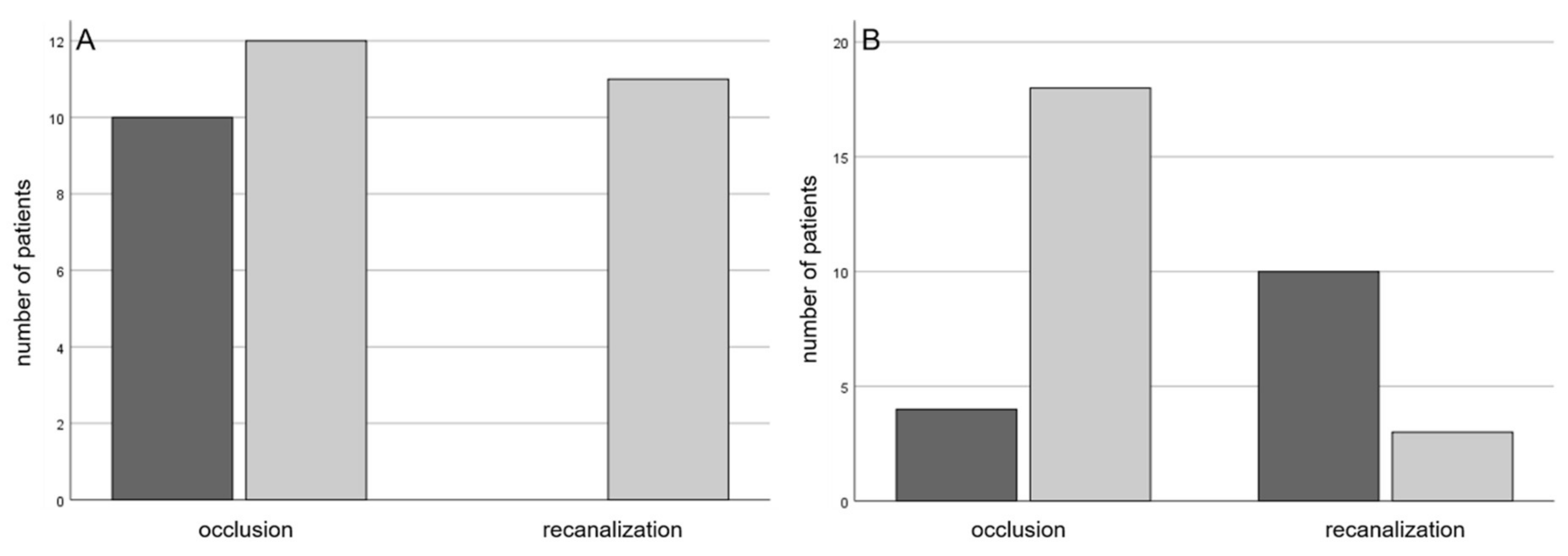
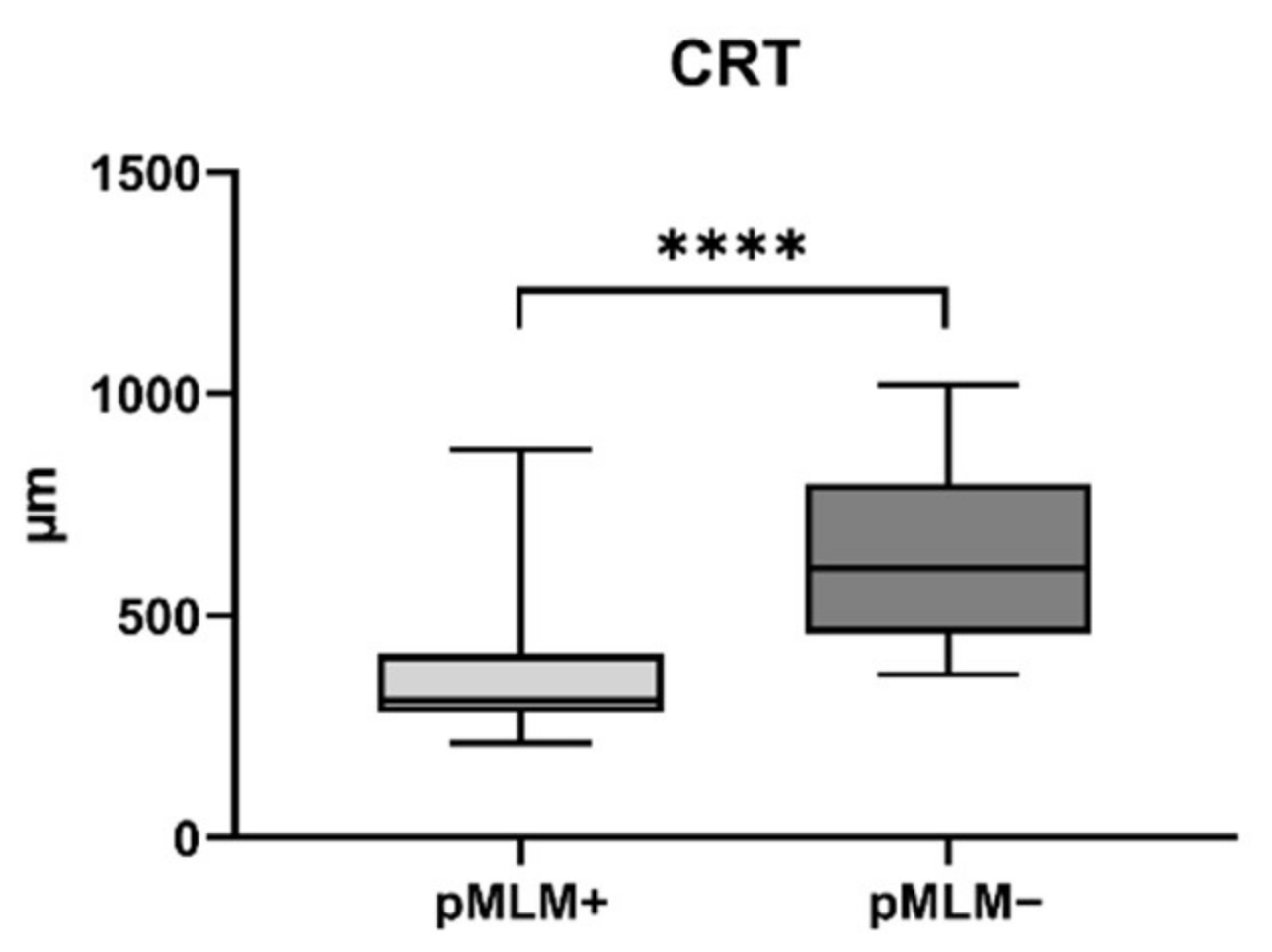
3. Results
3.1. Prospective Trial
3.2. Retrospective Trial
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dattilo, M.; Newman, N.J.; Biousse, V. Acute retinal arterial ischemia. Ann. Eye Sci. 2018, 3, 28. [Google Scholar] [CrossRef]
- Hayreh, S.S.; Zimmerman, M.B. Central Retinal Artery Occlusion: Visual Outcome. Am. J. Ophthalmol. 2005, 140, 376–e1. [Google Scholar] [CrossRef]
- Rudkin, A.K.; Lee, A.W.; Chen, C.S. Vascular risk factors for central retinal artery occlusion. Eye 2010, 24, 678–681. [Google Scholar] [CrossRef]
- Callizo, J.; Feltgen, N.; Ammermann, A.; Ganser, J.; Bemme, S.; Bertelmann, T.; Pfeiffer, S.; Duvinage, A.; Gröschel, K.; Hoerauf, H.; et al. Atrial fibrillation in retinal vascular occlusion disease and non-Arteritic anterior ischemic optic neuropathy. PLoS ONE 2017, 12, e0181766. [Google Scholar] [CrossRef]
- Schorr, E.M.; Rossi, K.C.; Stein, L.K.; Park, B.L.; Tuhrim, S.; Dhamoon, M.S. Characteristics and Outcomes of Retinal Artery Occlusion. Stroke 2020, 51, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Callizo, J.; Feltgen, N.; Pantenburg, S.; Wolf, A.; Neubauer, A.S.; Jurklies, B.; Wachter, R.; Schmoor, C.; Schumacher, M.; Junker, B.; et al. Cardiovascular Risk Factors in Central Retinal Artery Occlusion: Results of a Prospective and Standardized Medical Examination. Ophthalmology 2015, 122, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Chodnicki, K.D.; Pulido, J.S.; Hodge, D.O.; Klaas, J.P.; Chen, J.J. Stroke Risk Before and After Central Retinal Artery Occlusion in a US Cohort. Mayo Clin. Proc. 2019, 94, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Fallico, M.; Lotery, A.J.; Longo, A.; Avitabile, T.; Bonfiglio, V.; Russo, A.; Murabito, P.; Palmucci, S.; Pulvirenti, A.; Reibaldi, M. Risk of acute stroke in patients with retinal artery occlusion: A systematic review and meta-analysis. Eye 2020, 34, 683–689. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Zhang, J.; Kim, R.K.; Matthews, J.L.; Rudich, D.S.; Greer, D.M.; Lesser, R.L.; Amin, H. Risk of Acute Ischemic Stroke in Patients With Monocular Vision Loss of Vascular Etiology. J. Neuro Ophthalmol. Off. J. North Am. Neuro Ophthalmol. Soc. 2018, 38, 328–333. [Google Scholar] [CrossRef]
- Vodopivec, I.; Cestari, D.M.; Rizzo, J.F., III. Management of Transient Monocular Vision Loss and Retinal Artery Occlusions. Semin. Ophthalmol. 2017, 32, 125–133. [Google Scholar] [CrossRef]
- Sharma, R.A.; Dattilo, M.; Newman, N.J.; Biousse, V. Treatment of Nonarteritic Acute Central Retinal Artery Occlusion. Asia Pac. J. Ophthalmol. 2018, 7, 235–241. [Google Scholar] [CrossRef]
- Olsen, T.W.; Pulido, J.S.; Folk, J.C.; Hyman, L.; Flaxel, C.J.; Adelman, R.A. Retinal and Ophthalmic Artery Occlusions Preferred Practice Pattern®. Ophthalmology 2017, 124, 120–143. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Schmidt, D.; Jurklies, B.; Gall, C.; Wanke, I.; Schmoor, C.; Maier-Lenz, H.; Solymosi, L.; Brueckmann, H.; Neubauer, A.S.; et al. Central Retinal Artery Occlusion: Local Intra-Artzerial Fibrinolysis versus Conservative Treatment, a Multicenter Randomized Trial. Ophthalmology 2010, 117, 1367–1375.e1. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.; Lee, A.W.; Campbell, B.; Lee, T.; Paine, M.; Fraser, C.; Grigg, J.; Markus, R. Efficacy of Intravenous Tissue-Type Plasminogen Activator in Central Retinal Artery Occlusion: Report from a Randomized, Controlled Trial. Stroke 2011, 42, 2229–2234. [Google Scholar] [CrossRef] [PubMed]
- Biousse, V.; Calvetti, O.; Bruce, B.B.; Newman, N.J. Thrombolysis for Central Retinal Artery Occlusion. J. Neuroophthalmol. 2007, 27, 215. [Google Scholar] [CrossRef] [PubMed]
- Ertl, M.; Altmann, M.; Torka, E.; Helbig, H.; Bogdahn, U.; Gamulescu, A.; Schlachetzki, F. The Retrobulbar “Spot Sign” as a Discriminator Between Vasculitic and Thrombo-Embolic Affections of the Retinal Blood Supply. Ultraschall Med. Eur. J. Ultrasound 2012, 33, E263–E267. [Google Scholar] [CrossRef]
- Nedelmann, M.; Graef, M.; Weinand, F.; Wassill, K.-H.; Kaps, M.; Lorenz, B.; Tanislav, C. Retrobulbar Spot Sign Predicts thrombolytic Treatment Effects and Etiology in Central Retinal Artery Occlusion. Stroke 2015, 46, 2322–2324. [Google Scholar] [CrossRef]
- Smith, A.T.; Wilbert, C.D.; Ferre, R.M. Using the Retrobulbar Spot Sign to Assist in Diagnosis and Management of Central Retinal Artery Occlusions. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2020, 39, 197–202. [Google Scholar] [CrossRef]
- Altmann, M.; Ertl, M.; Helbig, H.; Schömig, B.; Bogdahn, U.; Gamulescu, M.-A.; Schlachetzki, F. Low Endogenous Recanalization in Embolic Central Retinal Artery Occlusion—The Retrobulbar “Spot Sign”. J. Neuroimaging 2015, 25, 251–256. [Google Scholar] [CrossRef]
- Chu, Y.K.; Hong, Y.T.; Byeon, S.H.; Kwon, O.W. IN VIVO DETECTION OF ACUTE ISCHEMIC DAMAGES IN RETINAL ARTERIAL OCCLUSION WITH OPTICAL COHERENCE TOMOGRAPHY: A “Prominent Middle Limiting Membrane Sign”. RETINA 2013, 33, 2110. [Google Scholar] [CrossRef]
- Schulze-Bonsel, K.; Feltgen, N.; Burau, H.; Hansen, L.; Bach, M. Visual Acuities “Hand Motion” and “Counting Fingers” Can Be Quantified with the Freiburg Visual Acuity Test. Invest. Ophthalmol. Vis. Sci. 2006, 47, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; Feltgen, N.; Junker, B.; Schulze-Bonsel, K.; Bach, M. Resolving the clinical acuity categories “hand motion” and “counting fingers” using the Freiburg Visual Acuity Test (FrACT). Graefes Arch. Clin. Exp. Ophthalmol. 2009, 247, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.P.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- Beneficial Effect of Carotid Endarterectomy in Symptomatic Patients with High-Grade Carotid Stenosis. N. Engl. J. Med. 1991, 325, 445–453. [CrossRef]
- Ko, J.; Kwon, O.W.; Byeon, S.H. Optical Coherence Tomography Predicts Visual Outcome in acute central retinal vein occlusion. RETINA 2014, 34, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, X.; Li, H.; Xu, J.; Wang, F. Optical coherence tomography angiography characteristics of acute retinal arterial occlusion. BMC Ophthalmol. 2019, 19, 147. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-N.; Hwang, J.-F.; Chen, Y.-T. Macular thickness measurements in central retinal artery occlusion by optical coherence tomography. RETINA 2011, 31, 730–737. [Google Scholar] [CrossRef]
- Hart, R.G.; Diener, H.-C.; Coutts, S.B.; Easton, J.D.; Granger, C.B.; O’Donnell, M.J.; Sacco, R.L.; Connolly, S.J. Embolic strokes of undetermined source: The case for a new clinical construct. Lancet Neurol. 2014, 13, 429–438. [Google Scholar] [CrossRef]
- Mac Grory Brian; Yaghi Shadi; Flood Shane; Silver Brian; Schrag Matthew Competing Embolic Mechanisms in Acute Central Retinal Artery Occlusion. Stroke 2019, 50, e253–e256. [CrossRef]


| Baseline Characteristics | |
|---|---|
| age (years; IQR) | 75 (11) |
| female | 14 (34.1%) |
| male | 27 (65.9%) |
| left eye | 18 (43.9%) |
| right eye | 23 (56.1%) |
| spot sign | 24 (58.5%) |
| p-MLM sign | 26 (63.4%) |
| spot sign and p-MLM sign | 11(26.8%) |
| secondary retinal ischemia confirmed by UWFA | 8/18 (44%) |
| recanalization | 13 (31.7%) |
| central macular thickness in µm (std) | 507.8 (255.6) |
| central macular volume in mm2 (std) | 0.4267 (0.19) |
| time since onset of symptoms (hours; IQR) | 8 (17) |
| hypertension | 27 (64.3%) |
| hyperlipidemia | 20 (47.6%) |
| diabetes mellitus | 7 (16.7%) |
| anti-coagulant medication | 6 (14.3%) |
| Etiology | |
| macroangiopathy | 10 (24.4%) |
| cardio-embolic | 6 (14.6%) |
| microangiopathy | 5 (12.2%) |
| other | 2 (4.9%) |
| cryptogenic | 18 (43.9%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schnieder, M.; Fischer-Wedi, C.V.; Bemme, S.; Kortleben, M.-L.; Feltgen, N.; Liman, J. The Retrobulbar Spot Sign and Prominent Middle Limiting Membrane as Prognostic Markers in Non-Arteritic Retinal Artery Occlusion. J. Clin. Med. 2021, 10, 338. https://doi.org/10.3390/jcm10020338
Schnieder M, Fischer-Wedi CV, Bemme S, Kortleben M-L, Feltgen N, Liman J. The Retrobulbar Spot Sign and Prominent Middle Limiting Membrane as Prognostic Markers in Non-Arteritic Retinal Artery Occlusion. Journal of Clinical Medicine. 2021; 10(2):338. https://doi.org/10.3390/jcm10020338
Chicago/Turabian StyleSchnieder, Marlena, Charlotte V. Fischer-Wedi, Sebastian Bemme, Mai-Linh Kortleben, Nicolas Feltgen, and Jan Liman. 2021. "The Retrobulbar Spot Sign and Prominent Middle Limiting Membrane as Prognostic Markers in Non-Arteritic Retinal Artery Occlusion" Journal of Clinical Medicine 10, no. 2: 338. https://doi.org/10.3390/jcm10020338
APA StyleSchnieder, M., Fischer-Wedi, C. V., Bemme, S., Kortleben, M.-L., Feltgen, N., & Liman, J. (2021). The Retrobulbar Spot Sign and Prominent Middle Limiting Membrane as Prognostic Markers in Non-Arteritic Retinal Artery Occlusion. Journal of Clinical Medicine, 10(2), 338. https://doi.org/10.3390/jcm10020338





