Packing Technique with or without Remodeling for Endovascular Coil Embolization of Renal Artery Aneurysms: Safety, Efficacy and Mid-Term Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Endovascular Procedure
2.3. Angiographic Outcomes, Complications, and Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Patients
3.2. Aneurysms
3.3. Coil Embolization and Outcomes
3.4. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tham, G.; Ekelund, L.; Herrlin, K.; Lindstedt, E.L.; Olin, T.; Bergentz, S.E. Renal artery aneurysms. Natural history and prognosis. Ann. Surg. 1983, 197, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Henke, P.K.; Cardneau, J.D.; Welling, T.H., 3rd; Upchurch, G.R., Jr.; Wakefield, T.W.; Jacobs, L.A.; Proctor, S.B.; Greenfield, L.J.; Stanley, J.C. Renal artery aneurysms: A 35-year clinical experience with 252 aneurysms in 168 patients. Ann. Surg. 2001, 234, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.C.; Rhodes, E.L.; Gewertz, B.L.; Chang, C.Y.; Walter, J.F.; Fry, W.J. Renal artery aneurysms. Arch. Surg. 1975, 110, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Hageman, J.H.; Smith, R.F.; Szilagyi, E.; Elliott, J.P. Aneurysms of the renal artery: Problems of prognosis and surgical management. Surgery 1978, 84, 563–572. [Google Scholar] [PubMed]
- Henriksson, C.; Björkerud, S.; Nilson, A.E.; Pettersson, S. Natural history of renal artery aneurysm elucidated by repeated angiography and pathoanatomical studies. Eur. Urol. 1985, 11, 244–248. [Google Scholar] [CrossRef]
- Vaughan, T.J.; Barry, W.F.; Jeffords, D.L.; Johnsrude, I.S. Renal artery aneurysms and hypertension. Radiology 1971, 99, 287–293. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Z.; Qin, F.; Wei, X.; Sun, Y.; Liu, J.; Feng, J.; Zhou, J.; Feng, R.; Jing, Z. Outcomes of endovascular treatment and open repair for renal artery aneurysms: A single-center retrospective comparative analysis. J. Vasc. Interv. Radiol. 2018, 29, 62–70. [Google Scholar] [CrossRef]
- Irace, L.; Ben Hamida, J.; Martinelli, O.; Stumpo, R.; Irace, F.G.; Venosi, S.; Gattuso, R.; Berloco, P.B.; Gossetti, B. Open and endovascular treatment by covered and multilayer stents in the therapy of renal artery aneurysms: Mid and long-term outcomes in a single center experience. G. Chir. 2017, 38, 219–224. [Google Scholar] [CrossRef]
- Tsilimparis, N.; Reeves, J.G.; Dayama, A.; Perez, S.D.; Debus, E.S.; Ricotta, J.J., 2nd. Endovascular vs open repair of renal artery aneurysms: Outcomes of repair and long-term renal function. J. Am. Coll. Surg. 2013, 217, 263–269. [Google Scholar] [CrossRef]
- Hislop, S.J.; Patel, S.A.; Abt, P.L.; Singh, M.J.; Illig, K.A. Therapy of renal artery aneurysms in New York State: Outcomes of patients undergoing open and endovascular repair. Ann. Vasc. Surg. 2009, 23, 194–200. [Google Scholar] [CrossRef]
- Guglielmi, G.; Viñuela, F.; Briganti, F.; Duckwiler, G. Carotid-cavernous fistula caused by a ruptured intracavernous aneurysm: Endovascular treatment by electrothrombosis with detachable coils. Neurosurgery 1992, 31, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Moret, J.; Cognard, C.; Weill, A.; Castaings, L.; Rey, A. The “remodelling technique” in the treatment of wide neck intracranial aneurysms. angiographic results and clinical follow-up in 56 cases. Interv. Neuroradiol. 1997, 3, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Manninen, H.I.; Berg, M.; Vanninen, R.L. Stent-assisted coil embolization of wide-necked renal artery bifurcation aneurysms. J. Vasc. Interv. Radiol. 2008, 19, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, O.; Nakasone, Y.; Tamura, Y.; Yamashita, Y. Endovascular management of visceral artery pseudoaneurysms: Transcatheter coil embolization using the isolation technique. Cardiovasc. Intervent. Radiol. 2010, 33, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, T.; Osuga, K.; Yamamoto, H.; Ono, Y.; Masada, M.; Mikami, K.; Kanamori, D.; Nakamura, M.; Tanaka, K.; Nakazawa, T.; et al. Long-term outcomes of coil packing for visceral aneurysms: Correlation between packing density and incidence of coil compaction or recanalization. J. Vasc. Interv. Radiol. 2013, 24, 1798–1807. [Google Scholar] [CrossRef]
- Li, Z.; Hu, L.; Chen, C.; Wang, Z.; Zhou, Z.; Chen, Y. Hemodynamic performance of multilayer stents in the treatment of aneurysms with a branch attached. Sci. Rep. 2019, 9, 10193. [Google Scholar] [CrossRef]
- Alherz, A.I.; Tanweer, O.; Flamini, V.J. A numerical framework for the mechanical analysis of dual-layer stents in intracranial aneurysm treatment. J. Biomech. 2016, 49, 2420–2427. [Google Scholar] [CrossRef]
- Balderi, A.; Antonietti, A.; Pedrazzini, F.; Sortino, D.; Vinay, C.; Grosso, M. Treatment of visceral aneurysm using multilayer stent: Two-year follow-up results in five consecutive patients. Cardiovasc. Intervent. Radiol. 2013, 36, 1256–1261. [Google Scholar] [CrossRef]
- Meyer, C.; Verrel, F.; Weyer, G.; Wilhelm, K. Endovascular management of complex renal artery aneurysms using the multilayer stent. Cardiovasc. Intervent. Radiol. 2011, 34, 637–641. [Google Scholar] [CrossRef]
- Mascitelli, J.R.; Moyle, H.; Oermann, E.K.; Polykarpou, M.F.; Patel, A.A.; Doshi, A.H.; Gologorsky, Y.; Bederson, J.B.; Patel, A.B. An update to the Raymond-Roy Occlusion Classification of intracranial aneurysms treatd with coil embolization. J. Neurointerv. Surg. 2015, 7, 496–502. [Google Scholar] [CrossRef]
- Patel, N.; Sacks, D.; Patel, R.I.; Moresco, K.P.; Ouriel, K.; Gray, R.; Ambrosius, W.T.; Lewis, C.A.; Society of Interventional Radiology Technology Assessment Committee. SIR reporting standards for the treatment of acute limb ischemia with use of transluminal removal of arterial thrombus. J. Vasc. Interv. Radiol. 2003, 14, S453–S465. [Google Scholar] [CrossRef] [PubMed]
- Coleman, D.M.; Stanley, J.C. Renal artery aneurysms. J. Vasc. Surg. 2015, 62, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Wayne, E.J.; Edwards, M.S.; Stafford, J.M.; Hansen, K.J.; Corriere, M.A. Anatomic characteristics and natural history of renal artery aneurysms during longitudinal imaging surveillance. J. Vasc. Surg. 2014, 60, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Klausner, J.Q.; Harlander-Locke, M.P.; Plotnik, A.N.; Lehrman, E.; DeRubertis, B.G.; Lawrence, P.F. Current treatment of renal artery aneurysms may be too aggressive. J. Vasc. Surg. 2014, 59, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.S., 3rd; Meacham, P.W.; Ditesheim, J.A.; Mulherin, J.L., Jr.; Edwards, W.H. Renal artery aneurysm: Selective treatment for hypertension and prevention of rupture. J. Vasc. Surg. 1989, 92, 6–34. [Google Scholar]
- Roberts, W.C. The hypertensive diseases. Evidence that systemic hypertension is a greater risk factor to the development of other cardiovascular diseases than previously suspected. Am. J. Med. 1975, 59, 523–532. [Google Scholar] [CrossRef]
- Dustan, H.P. Atherosclerosis complicating chronic hypertension. Circulation 1974, 50, 871–879. [Google Scholar] [CrossRef][Green Version]
- Hollander, W. Role of hypertension in atherosclerosis and cardiovascular disease. Am. J. Cardiol. 1976, 38, 786–800. [Google Scholar] [CrossRef]
- Spittell, J.A. Hypertension and arterial aneurysm. J. Am. Coll. Cardiol. 1983, 1, 533. [Google Scholar] [CrossRef]
- Klausner, J.Q.; Lawrence, P.F.; Harlander Locke, M.P.; Coleman, D.M.; Stanley, J.C.; Fujimura, N. The contemporary management of renal artery aneurysms. J. Vasc. Surg. 2014, 61, 978–984. [Google Scholar] [CrossRef]
- Stanley, J.C.; Rhodes, E.L.; Gewertz, B.L.; Chang, C.Y.; Walter, J.F.; Fry, W.J. Renal artery aneurysms. Significance of macroaneurysms exclusive of dissections and fibrodysplastic mural dilations. Arch. Surg. 1975, 110, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Sédat, J.; Chau, Y.; Baque, J. Endovascular treatment of renal aneurysms: A series of 18 cases. Eur. J. Radiol. 2012, 81, 3973–3978. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.P., 3rd; Bafford, R.; Belkin, M.; Menard, M.T. Favorable outcomes with in situ techniques for surgical repair of complex renal artery aneurysms. J. Vasc. Surg. 2011, 53, 684–691. [Google Scholar] [CrossRef]
- Ngninkeu, N.B.; Eucher, P.; Vandenbossche, P.; Lacrosse, M.; Van Cangh, P.J.; Lorge, F. Ruptured aneurysm of the renal artery: A rare cause of macroscopic hematuria. Prog. Urol. 2002, 12, 454–458. [Google Scholar]
- Dean, R.H.; Meacham, P.W.; Weaver, F.A. Ex vivo renal artery reconstructions: Indications and techniques. J. Vasc. Surg. 1986, 4, 546–552. [Google Scholar] [CrossRef][Green Version]
- Li, G.; Sun, Y.; Song, H.; Wang, Y. Embolization of ruptured renal artery aneurysms. Clin. Exp. Nephrol. 2015, 19, 901–908. [Google Scholar] [CrossRef]
- Sojka, M.; Szmygin, M.; Pyra, K.; Kuczyńska, M.; Jargiełło, T. Acute renal artery stenting recovered renal function after spontaneous rupture of renal artery aneurysm—case report. Pol. J. Radiol. 2020, 85, e29–e31. [Google Scholar] [CrossRef]
- Atlı, E.; Uyanık, S.A.; Öğüşlü, U.; Cenkeri, Ç.H.; Yılmaz, B.; Gümüş, B. The endovascular management of postpartum ruptured renal artery aneurysm by parent artery occlusion. J. Obstet. Gynaecol. 2020, 40, 1164–1165. [Google Scholar] [CrossRef]
- Yadav, S.; Sharma, S.; Singh, P.; Nayak, B. Pregnancy with a ruptured renal artery aneurysm: Management concerns and endovascular management. BMJ Case Rep. 2015, 2015, bcr2015211884. [Google Scholar] [CrossRef]
- Duan, Z.; Li, Y.; Guan, S.; Ma, C.; Han, Y.; Ren, X.; Wei, L.; Li, W.; Lou, J.; Yang, Z. Morphological parameters and anatomical locations associated with rupture status of small intracranial aneurysms. Sci. Rep. 2018, 8, 6440. [Google Scholar] [CrossRef]
- Rahman, M.; Smietana, J.; Hauck, E.; Hoh, B.; Hopkins, N.; Siddiqui, A.; Levy, E.I.; Meng, H.; Mocco, J. Size ratio correlates with intracranial aneurysm rupture status. Stroke 2010, 41, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Tremmel, M.; Mocco, J.; Kim, M.; Yamamoto, J.; Siddiqui, A.H.; Hopkins, L.N.; Meng, H. Morphology parameters for intracranial aneurysm rupture risk assessment. Neurosurgery 2008, 63, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Künzle, S.; Glenck, M.; Puippe, G.; Schadde, E.; Mayer, D.; Pfammatter, T. Stent-graft repairs of visceral and renal artery aneurysms are effective and result in long-term patency. J. Vasc. Interv. Radiol. 2013, 24, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Loffroy, R.; Rao, P.; Ota, S.; De Lin, M.; Kwak, B.K.; Krause, D.; Geschwind, J.F. Packing technique for endovascular coil embolisation of peripheral arterial pseudo-aneurysms with preservation of the parent artery: Safety, efficacy and outcomes. Eur. J. Vasc. Endovasc. Surg. 2010, 40, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Loffroy, R.; Guiu, B.; Cercueil, J.P.; Lepage, C.; Cheynel, N.; Steinmetz, E.; Ricolfi, F.; Krausé, D. Transcatheter arterial embolization of splenic artery aneurysms and pseudoaneurysms: Short- and long-term results. Ann. Vasc. Surg. 2008, 22, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Bui, B.T.; Oliva, V.L.; Leclerc, G.; Courteau, M.; Harel, C.; Plante, R.; Giroux, D.; Carignan, L. Renal artery aneurysm: Treatment with percutaneous placement of a stent-graft. Radiology 1995, 195, 181–182. [Google Scholar] [CrossRef]
- Saltzberg, S.S.; Maldonado, T.S.; Lamparello, P.J.; Cayne, N.S.; Nalbandian, M.M.; Rosen, R.J.; Jacobowitz, G.R.; Adelman, M.A.; Gagne, P.J.; Riles, T.S.; et al. Is endovascular therapy the preferred treatment for all visceral artery aneurysms? Ann. Vasc. Surg. 2005, 19, 507–515. [Google Scholar] [CrossRef]
- Tang, H.; Tang, X.; Fu, W.; Luo, J.; Shi, Z.; Wang, L.; Liu, F.; Guo, D. Coil embolization of renal artery bifurcation and branch aneurysms with flow preservation. J. Vasc. Surg. 2018, 68, 451–458. [Google Scholar] [CrossRef]
- Abath, C.; Andrade, G.; Cavalcanti, D.; Brito, N.; Marques, R. Complex renal artery aneurysms: Liquids or coils? Tech. Vasc. Interv. Radiol. 2007, 10, 299–307. [Google Scholar] [CrossRef]
- Chung, R.; Touska, P.; Morgan, R.; Belli, A.M. Endovascular management of true renal arterial aneurysms: Results from a single centre. Cardiovasc. Intervent. Radiol. 2016, 39, 36–43. [Google Scholar] [CrossRef]
- Venturini, M.; Della Corte, A.; Lanza, C.; Fontana, F.; Chiesa, R.; De Cobelli, F. Embolization of 2 coexisting intraparenchymal renal artery aneurysms with an ethylene vinyl alcohol copolymer agent (Squid) and coils. Cardiovasc. Intervent. Radiol. 2020, 43, 942–944. [Google Scholar] [CrossRef] [PubMed]
- Buck, D.B.; Curran, T.; McCallum, J.C.; Darling, J.; Mamtani, R.; Van Herwaarden, J.A.; Moll, F.L.; Schermerhorn, M.L. Management and outcomes of isolated renal artery aneurysms in the endovascular era. J. Vasc. Surg. 2016, 63, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.; Kavanagh, E.P.; Hynes, N.; Diethrich, E.B. Evaluation of functionality and biological response of the multilayer flow modulator in porcine animal models. Int. Angiol. 2016, 35, 31–39. [Google Scholar] [PubMed]
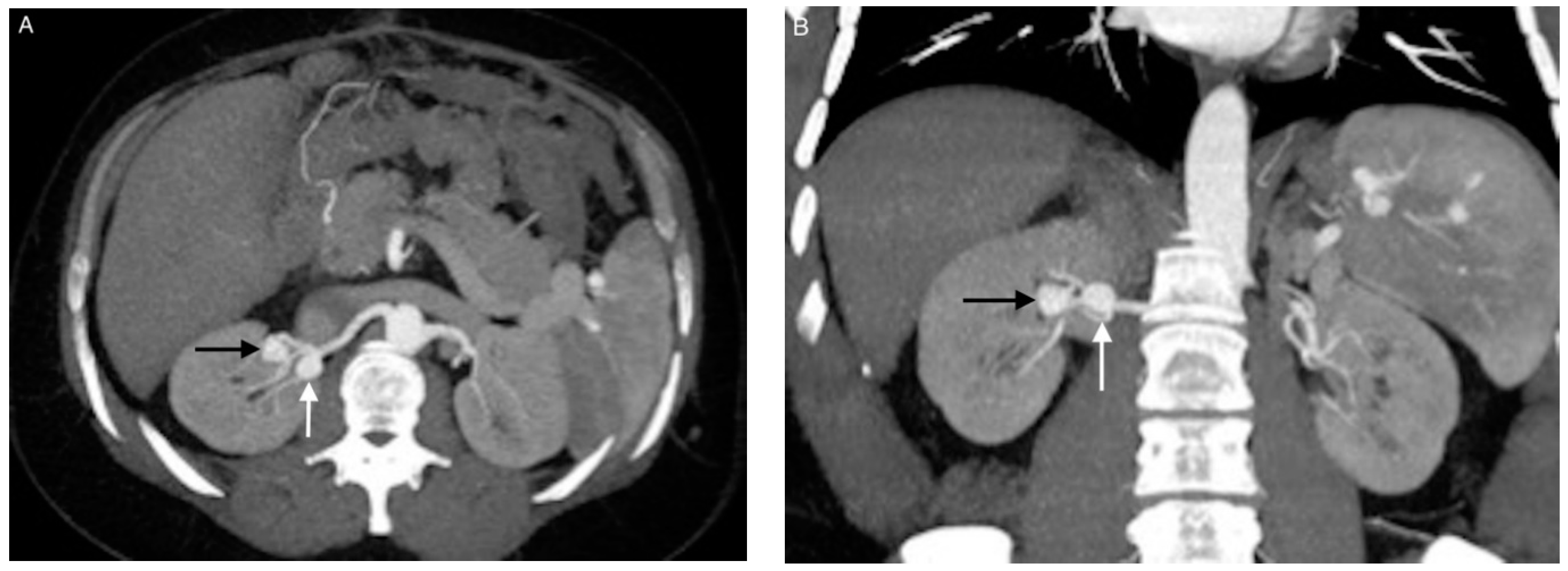
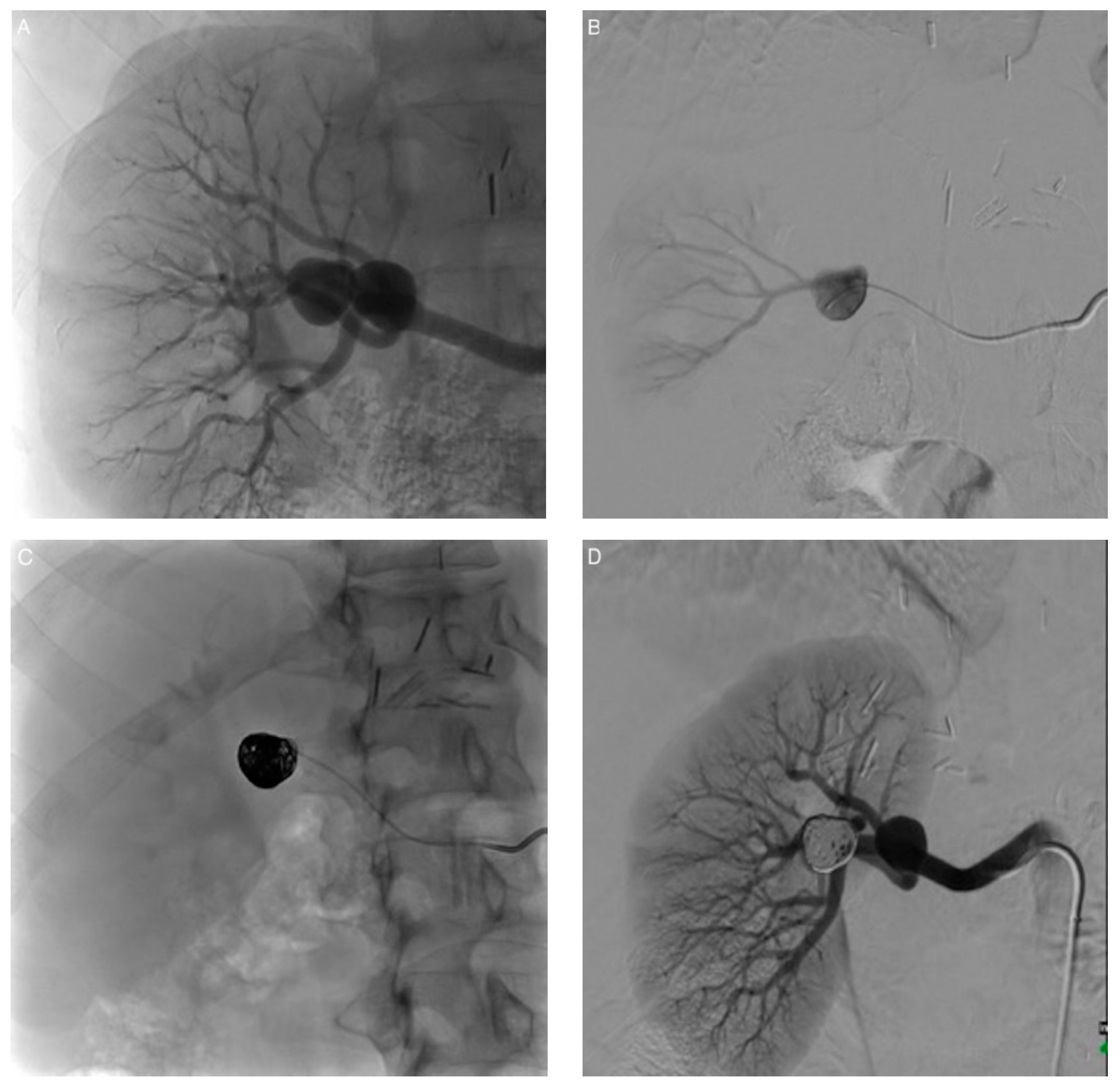
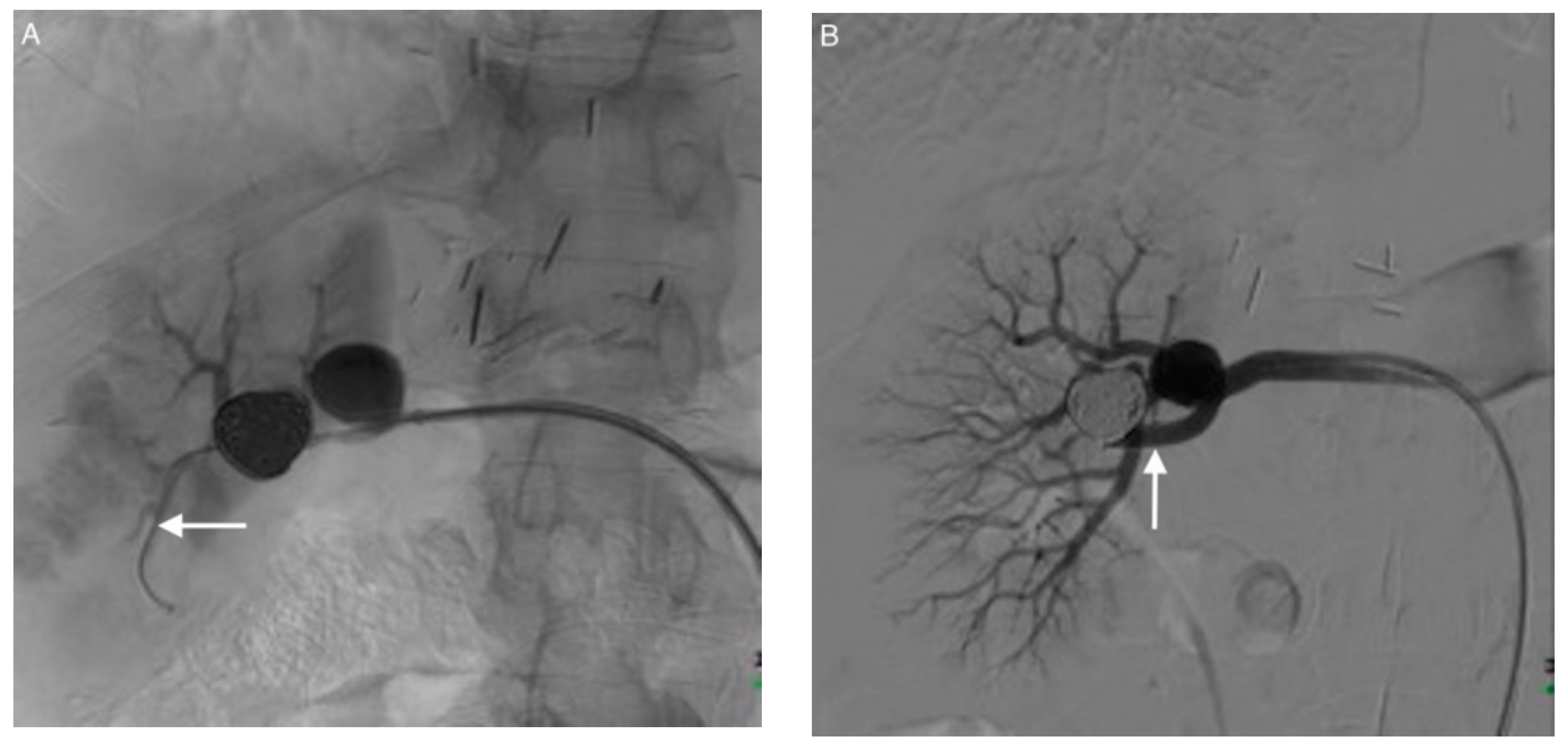

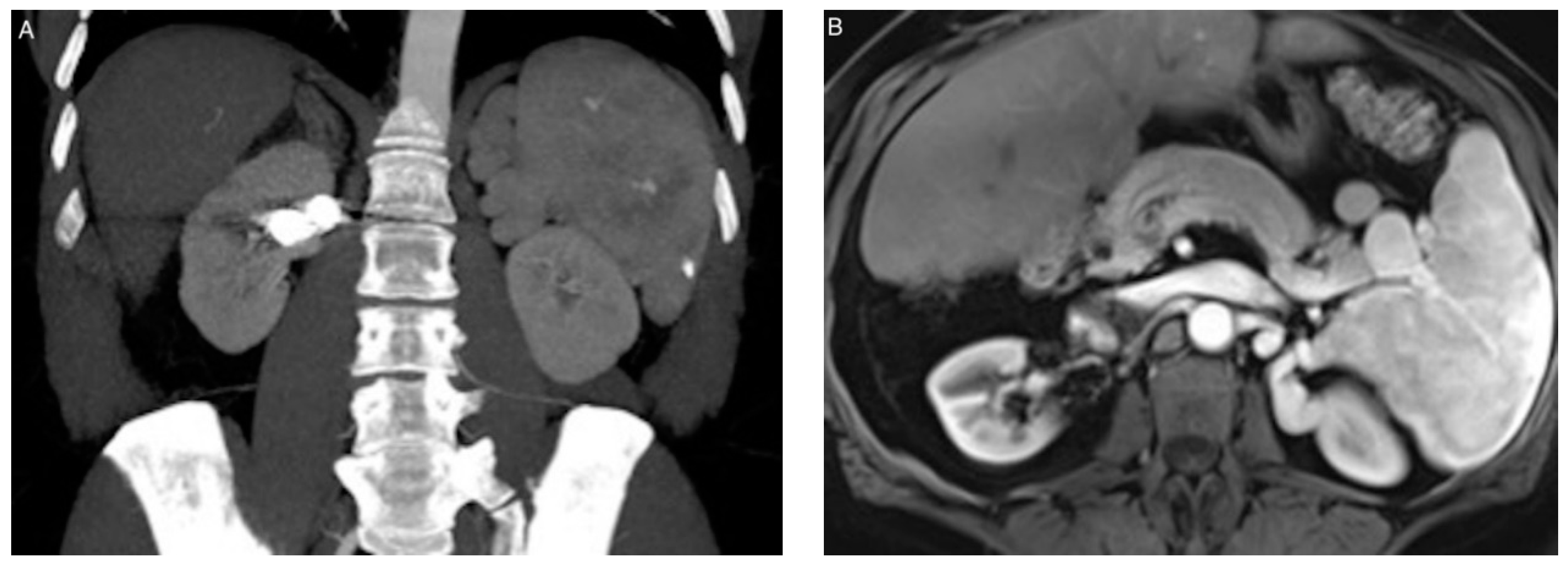
| Patient/ Aneurysm | Age (years) | Sex | Side (Right–Left) | Size (mm) | Neck Size (mm) | Parent Artery Size (mm) | Number of Efferences | Location |
|---|---|---|---|---|---|---|---|---|
| 1/1 | 83 | Female | R | 23 | 15 | 6 | 1 | Ostial |
| 2/2 | 55 | Female | L | 11 | 6 | 5 | 1 | Hilar |
| 3/3 | 58 | Female | R | 15 | 6 | 4 | 2 | Hilar |
| 4/4 | 60 | Female | R | 13 | 4 | 3 | 2 | Hilar |
| 5/5 | 85 | Female | L | 18 | 5 | 3 | 2 | Intrarenal |
| 6/6 | 39 | Female | R | 15 | 9 | 5 | 1 | Hilar |
| 6/7 | 39 | Female | R | 13 | 5 | 5 | 1 | Hilar |
| 7/8 | 54 | Female | R | 20 | 7 | 6 | 2 | Hilar |
| 8/9 | 44 | Female | L | 21 | 6 | 6 | 2 | Hilar |
| 9/10 | 79 | Female | R | 13 | 3 | 3 | 1 | Hilar |
| 10/11 | 62 | Female | L | 14 | 9 | 3 | 2 | Hilar |
| 11/12 | 55 | Female | R | 14 | 6 | 4 | 2 | Hilar |
| 12/13 | 65 | Female | L | 16 | 7 | 5 | 2 | Hilar |
| 13/14 | 57 | Female | L | 17 | 6 | 4 | 1 | Truncal |
| 14/15 | 66 | Male | L | 10 | 5 | 5 | 1 | Hilar |
| 15/16 | 70 | Female | L | 12 | 5 | 5 | 2 | Hilar |
| 16/17 | 71 | Male | R | 18 | 9 | 6 | 2 | Hilar |
| 17/18 | 78 | Male | L | 20 | 8 | 8 | 2 | Hilar |
| Patient/Aneurysm | History | Clinical Presentation | Indications for Treatment |
|---|---|---|---|
| 1/1 | Hypertension | Incidental discovery | Size ≥ 15 mm |
| 2/2 | Hypertension | Vascular steal syndrome | Symptomatic aneurysm |
| 3/3 | None | Incidental discovery | Size ≥ 15 mm |
| 4/4 | None | Hematuria | Symptomatic aneurysm |
| 5/5 | Hypertension | Assessment of hypertension | Renovascular hypertension, Size ≥ 15 mm |
| 6/6 | Liver transplantation | Incidental discovery | Size ≥ 15 mm |
| 6/7 | Liver transplantation | Incidental discovery | Aneurysm-to-parent artery size ratio > 2, Treatment of another RAA |
| 7/8 | Hypertension | Flank pain | Symptomatic aneurysm |
| 8/9 | Fibromuscular dysplasia | Incidental discovery | Size ≥ 15 mm |
| 9/10 | None | Incidental discovery | Aneurysm-to-parent artery size ratio > 2 |
| 10/11 | Hypertension | Assessment of hypertension | Renovascular hypertension |
| 11/12 | None | Incidental discovery | Size ≥ 15 mm |
| 12/13 | None | Incidental discovery | Size ≥ 15 mm |
| 13/14 | None | Incidental discovery | Size ≥ 15 mm |
| 14/15 | None | Incidental discovery | Aneurysm-to-parent artery size ratio > 2 |
| 15/16 | None | Incidental discovery | Aneurysm-to-parent artery size ratio > 2 |
| 16/17 | None | Incidental discovery | Progressiveness |
| 17/18 | Hypertension | Hematuria | Symptomatic aneurysm |
| Patient/ Aneurysm | Endovascular Technique | Postoperative Angiographic Results (RROC) | Perioperative Minor Complications | Perioperative Major Complications |
|---|---|---|---|---|
| 1/1 | Stenting + coiling | Class 1 | None | None |
| 2/2 | Coiling | Class 1 | Pain | None |
| 3/3 | Coiling | Class 1 | None | None |
| 4/4 | Balloon + coiling | Class 1 | Pain | None |
| 5/5 | Coiling | Class 1 | None | None |
| 6/6 | Stenting + coiling | Class 1 | None | None |
| 6/7 | Coiling | Class 1 | None | None |
| 7/8 | Coiling | Class 1 | None | None |
| 8/9 | Coiling | Class 1 | None | None |
| 9/10 | Coiling | Class 1 | None | None |
| 10/11 | Stenting + coiling | Class 1 | Mild decompensated heart failure | None |
| 11/12 | Coiling | Class 1 | None | None |
| 12/13 | Coiling | Class 1 | None | None |
| 13/14 | Coiling | Class 1 | None | None |
| 14/15 | Stenting + coiling | Class 1 | None | None |
| 15/16 | Stenting + coiling | Class 1 | None | None |
| 16/17 | Stenting + coiling | Class 2 | Transient renal failure | None |
| 17/18 | Coiling | Class 1 | None | None |
| Patient/ Aneurysm | Short-Term Imaging Results | Long-Term Imaging Results | Duration of Follow-Up (months) |
|---|---|---|---|
| 1/1 | Incomplete | Complete occlusion after reintervention | 99 |
| 2/2 | Complete | Complete | 79 |
| 3/3 | Complete | Complete | 78 |
| 4/4 | Complete | Complete | 59 |
| 5/5 | Complete | Complete | 53 |
| 6/6 | Complete | Complete | 40 |
| 6/7 | Complete | Complete | 40 |
| 7/8 | Complete | Incomplete–complete occlusion after reintervention | 25 |
| 8/9 | Complete | Complete | 24 |
| 9/10 | Complete | Complete | 21 |
| 10/11 | Complete | Complete | 21 |
| 11/12 | Complete | Complete | 20 |
| 12/13 | Complete | Complete | 15 |
| 13/14 | Complete | Complete | 93 |
| 14/15 | Complete | Complete | 31 |
| 15/16 | Complete | Complete | 36 |
| 16/17 | Complete | Complete | 42 |
| 17/18 | Complete | - | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Secco, G.; Chevallier, O.; Falvo, N.; Guillen, K.; Comby, P.-O.; Mousson, C.; Majbri, N.; Midulla, M.; Loffroy, R. Packing Technique with or without Remodeling for Endovascular Coil Embolization of Renal Artery Aneurysms: Safety, Efficacy and Mid-Term Outcomes. J. Clin. Med. 2021, 10, 326. https://doi.org/10.3390/jcm10020326
Secco G, Chevallier O, Falvo N, Guillen K, Comby P-O, Mousson C, Majbri N, Midulla M, Loffroy R. Packing Technique with or without Remodeling for Endovascular Coil Embolization of Renal Artery Aneurysms: Safety, Efficacy and Mid-Term Outcomes. Journal of Clinical Medicine. 2021; 10(2):326. https://doi.org/10.3390/jcm10020326
Chicago/Turabian StyleSecco, Grégory, Olivier Chevallier, Nicolas Falvo, Kévin Guillen, Pierre-Olivier Comby, Christiane Mousson, Nabil Majbri, Marco Midulla, and Romaric Loffroy. 2021. "Packing Technique with or without Remodeling for Endovascular Coil Embolization of Renal Artery Aneurysms: Safety, Efficacy and Mid-Term Outcomes" Journal of Clinical Medicine 10, no. 2: 326. https://doi.org/10.3390/jcm10020326
APA StyleSecco, G., Chevallier, O., Falvo, N., Guillen, K., Comby, P.-O., Mousson, C., Majbri, N., Midulla, M., & Loffroy, R. (2021). Packing Technique with or without Remodeling for Endovascular Coil Embolization of Renal Artery Aneurysms: Safety, Efficacy and Mid-Term Outcomes. Journal of Clinical Medicine, 10(2), 326. https://doi.org/10.3390/jcm10020326





