Serum Albumin Concentrations in Stable Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Experimental Section
2.1. Search Strategy, Eligibility Criteria, and Study Selection
2.2. Statistical Analysis
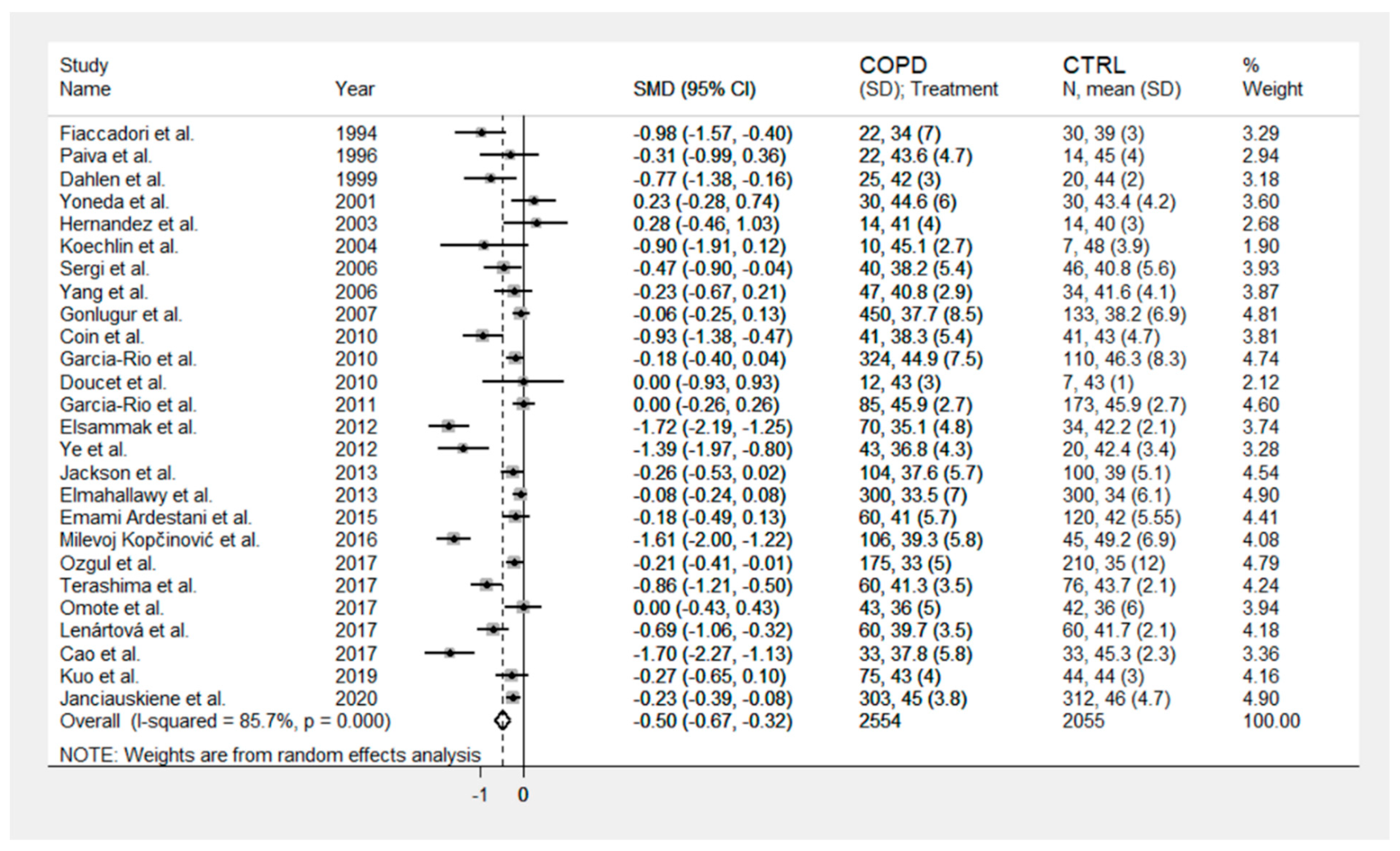
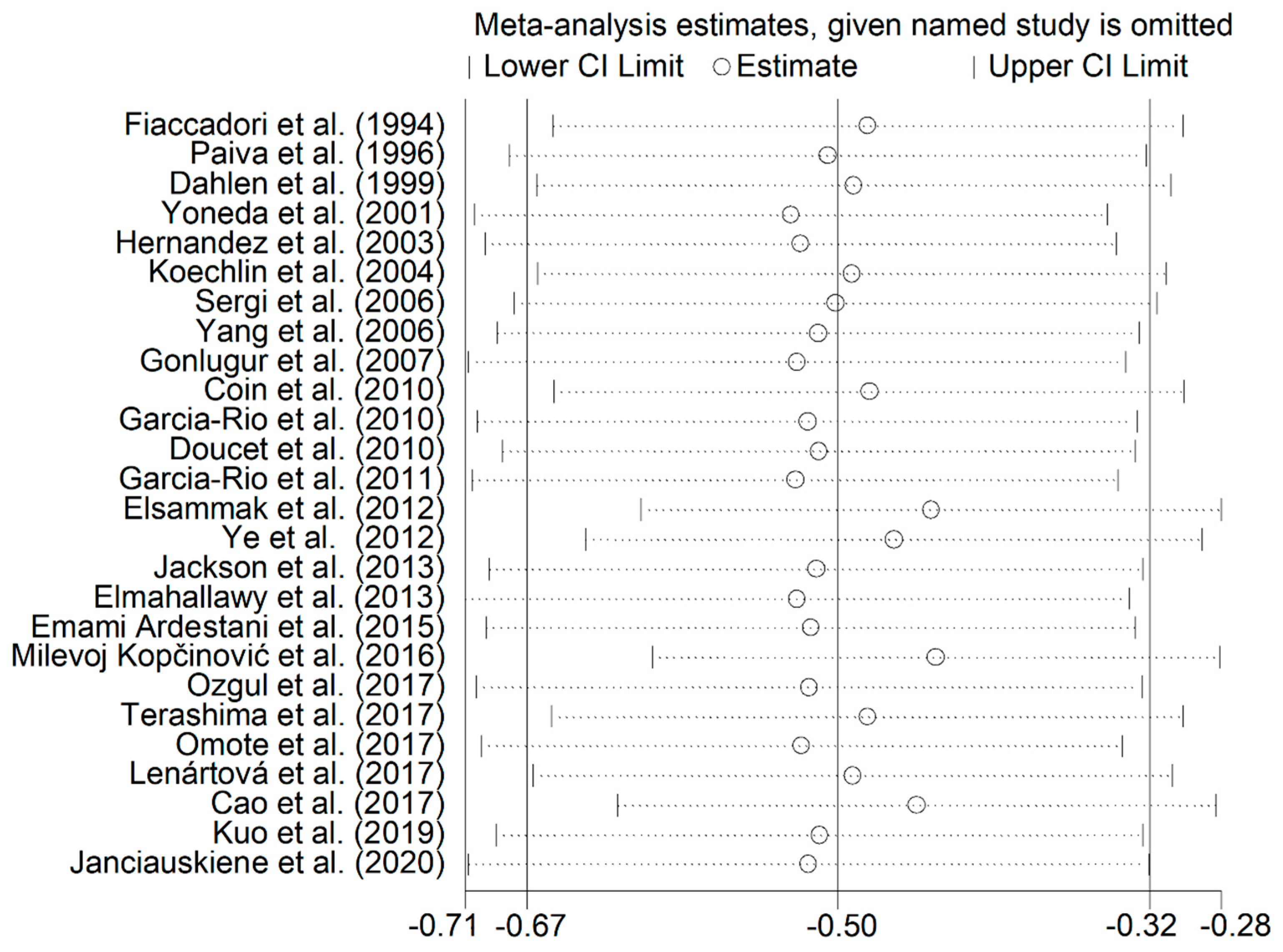
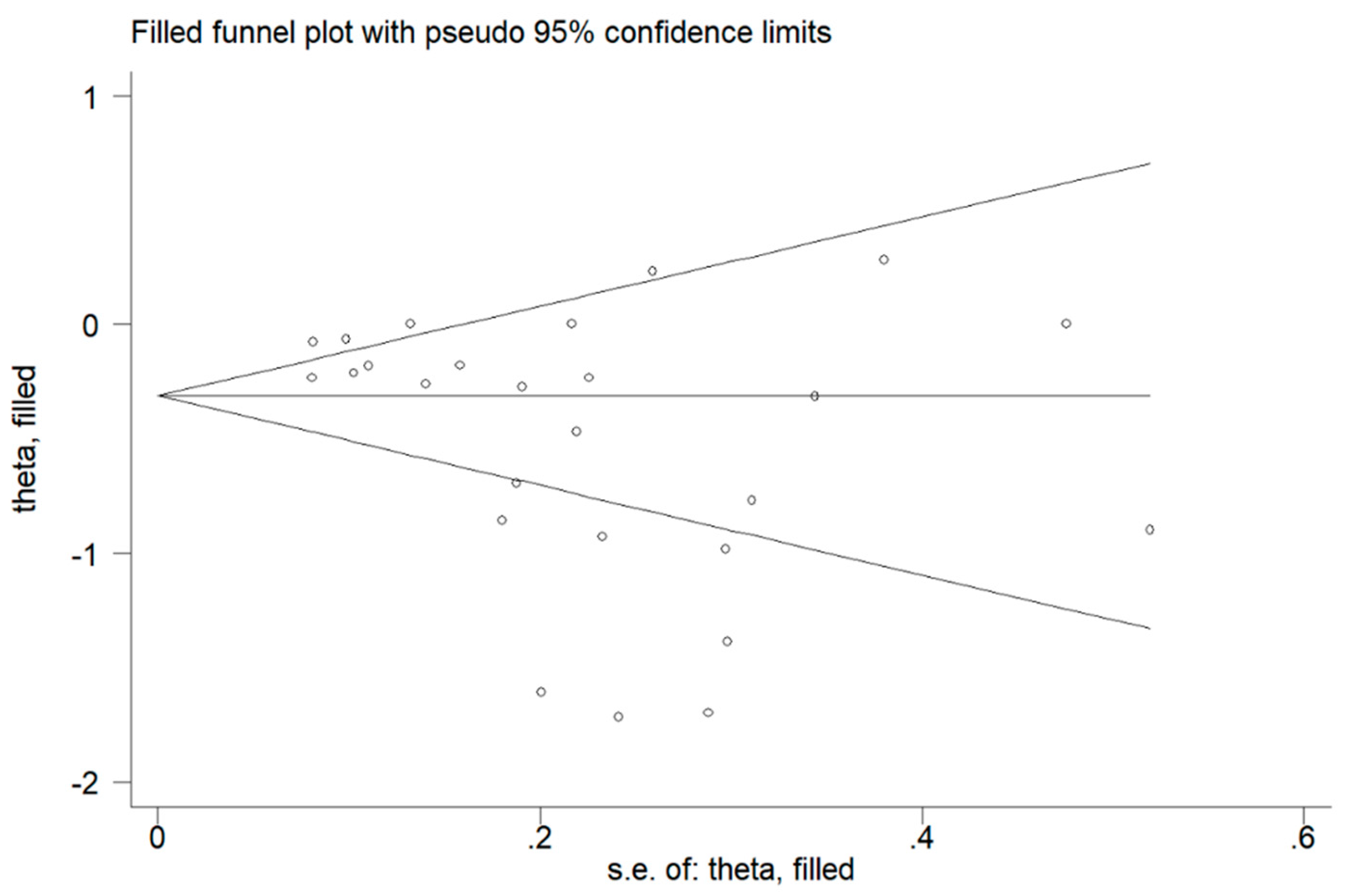
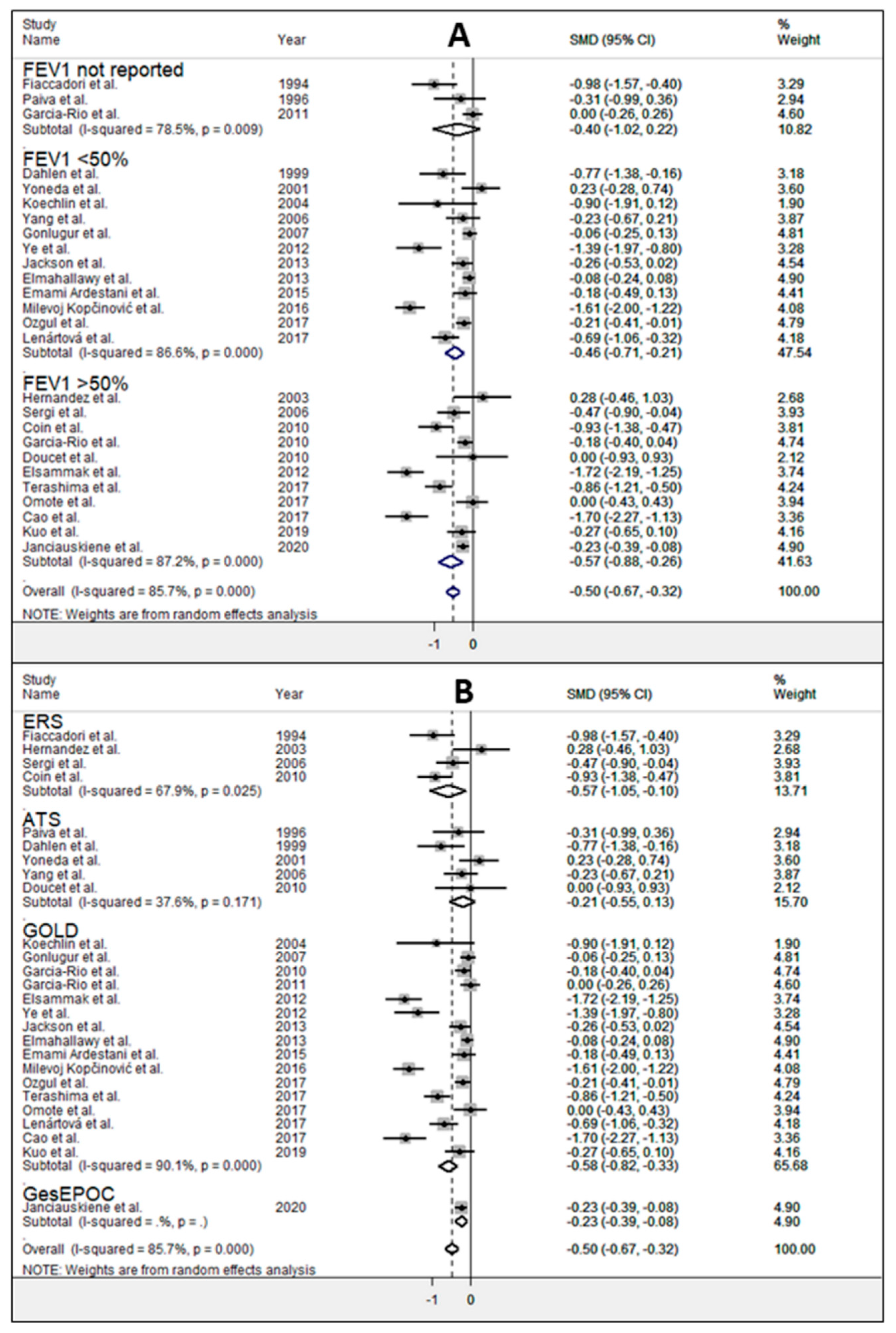
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am. J. Respir Crit Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef]
- Adeloye, D.; Chua, S.; Lee, C.; Basquill, C.; Papana, A.; Theodoratou, E.; Nair, H.; Gasevic, D.; Sridhar, D.; Campbell, H.; et al. Global and regional estimates of COPD prevalence: Systematic review and meta–analysis. J. Glob Health 2015, 5, 020415. [Google Scholar] [CrossRef]
- Lozano, R.M.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Postma, D.S.; Bush, A.; van den Berge, M. Risk factors and early origins of chronic obstructive pulmonary disease. Lancet 2015, 385, 899–909. [Google Scholar] [CrossRef]
- Salvi, S.S.; Barnes, P.J. Chronic obstructive pulmonary disease in non-smokers. Lancet 2009, 374, 733–743. [Google Scholar] [CrossRef]
- Barnes, P.J. Inflammatory Mechanisms in Patients with Chronic Obstructive Pulmonary Disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, P.A.; Barnes, P.J. Oxidative stress in COPD. Chest 2013, 144, 266–273. [Google Scholar] [CrossRef]
- Quinlan, G.J.; Martin, G.S.; Evans, T.W. Albumin: Biochemical properties and therapeutic potential. Hepatology 2005, 41, 1211–1219. [Google Scholar] [CrossRef]
- Oettl, K.; Stauber, R.E. Physiological and pathological changes in the redox state of human serum albumin critically influence its binding properties. Br. J. Pharmacol. 2007, 151, 580–590. [Google Scholar] [CrossRef]
- Roche, M.; Rondeau, P.; Singh, N.R.; Tarnus, E.; Bourdon, E. The antioxidant properties of serum albumin. FEBS Lett. 2008, 582, 1783–1787. [Google Scholar] [CrossRef]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef]
- Zhang, Z.; Pereira, S.L.; Luo, M.; Matheson, E.M. Evaluation of Blood Biomarkers Associated with Risk of Malnutrition in Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 829. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Collins, P.F.; Pavey, T.G.; Nguyen, N.V.; Pham, T.D.; Gallegos, D.L. Nutritional status, dietary intake, and health-related quality of life in outpatients with COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 215–226. [Google Scholar] [CrossRef]
- Wang, Y.; Stavem, K.; Dahl, F.A.; Humerfelt, S.; Haugen, T. Factors associated with a prolonged length of stay after acute exacerbation of chronic obstructive pulmonary disease (AECOPD). Int. J. Chron. Obstruct. Pulmon. Dis. 2014, 9, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Chen, Y.Y.; Lu, C.L.; Chen, S.C.; Chen, Y.J.; Lin, M.S.; Chen, W. Severe hypoalbuminemia is a strong independent risk factor for acute respiratory failure in COPD: A nationwide cohort study. Int. J. Chron. Obstruct. Pulmon. Dis. 2015, 10, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, W.; Yamauchi, Y.; Yasunaga, H.; Sunohara, M.; Jo, T.; Matsui, H.; Fushimi, K.; Takami, K.; Nagase, T. Factors affecting mortality following emergency admission for chronic obstructive pulmonary disease. BMC Pulm. Med. 2014, 14, 151. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2013. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 12 January 2021).
- Garcia-Rio, F.; Miravitlles, M.; Soriano, J.B.; Muñoz, L.; Duran-Tauleria, E.; Sánchez, G.; Sobradillo, V.; Ancochea, J.; EPI-SCAN Steering Committee. Systemic inflammation in chronic obstructive pulmonary disease: A population-based study. Respir. Res. 2010, 11, 63. [Google Scholar]
- Lenártová, P.; Kopčeková, J.; Gažarová, M.; Mrázová, J.; Wyka, J. Biochemical parameters as monitoring markers of the inflammatory reaction by patients with chronic obstructive pulmonary disease (COPD). Rocz Panstw Zakl Hig. 2017, 68, 185–190. [Google Scholar]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Bowden, J.; Tierney, J.F.; Copas, A.J.; Burdett, S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med. Res. Methodol. 2011, 11, 41. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Tobias, A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech. Bull. 1999, 47, 15–17. [Google Scholar]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Fiaccadori, E.; Coffrini, E.; Fracchia, C.; Rampulla, C.; Montagna, T.; Borghetti, A. Hypophosphatemia and phosphorus depletion in respiratory and peripheral muscles of patients with respiratory failure due to COPD. Chest 1994, 105, 1392–1398. [Google Scholar] [CrossRef]
- Paiva, S.A.; Godoy, I.; Vannucchi, H.; Fávaro, R.M.; Geraldo, R.R.; Campana, A.O. Assessment of vitamin A status in chronic obstructive pulmonary disease patients and healthy smokers. Am. J. Clin. Nutr. 1996, 64, 928–934. [Google Scholar] [CrossRef]
- Dahlén, I.; Lindberg, E.; Janson, C.; Stâlenheim, G. Delayed type of hypersensitivity and late allergic reactions in patients with stable COPD. Chest 1999, 116, 1625–1631. [Google Scholar] [CrossRef]
- Yoneda, T.; Yoshikawa, M.; Fu, A.; Tsukaguchi, K.; Okamoto, Y.; Takenaka, H. Plasma levels of amino acids and hypermetabolism in patients with chronic obstructive pulmonary disease. Nutrition 2001, 17, 95–99. [Google Scholar] [CrossRef]
- Hernández, N.; Orozco-Levi, M.; Belalcázar, V.; Pastó, M.; Minguella, J.; Broquetas, J.M.; Gea, J. Dual morphometrical changes of the deltoid muscle in patients with COPD. Respir. Physiol. Neurobiol. 2003, 134, 219–229. [Google Scholar] [CrossRef]
- Koechlin, C.; Couillard, A.; Cristol, J.P.; Chanez, P.; Hayot, M.; Le Gallais, D.; Préfaut, C. Does systemic inflammation trigger local exercise-induced oxidative stress in COPD? Eur. Respir. J. 2004, 23, 538–544. [Google Scholar] [CrossRef]
- Yang, Y.M.; Sun, T.Y.; Liu, X.M. The role of serum leptin and tumor necrosis factor-alpha in malnutrition of male chronic obstructive pulmonary disease patients. Chin. Med. J. (Engl.) 2006, 119, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Sergi, G.; Coin, A.; Marin, S.; Vianello, A.; Manzan, A.; Peruzza, S.; Inelmen, E.M.; Busetto, L.; Mulone, S.; Enzi, G. Body composition and resting energy expenditure in elderly male patients with chronic obstructive pulmonary disease. Respir. Med. 2006, 100, 1918–1924. [Google Scholar] [CrossRef] [PubMed]
- Gonlugur, U.; Gonlugur, T.A. Retrospective Analysis of Nutritional Parameters in Chronic Obstructive Pulmonary Disease between Sexes. J. Clin. Biochem. Nutr. 2007, 41, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Coin, A.; Sergi, G.; Marin, S.; Vianello, A.; Perissinotto, E.; Sarti, S.; Rinaldi, G.; Mosele, M.; Inelmen, E.M.; Enzi, G.; et al. Predictors of low bone mineral density in elderly males with chronic obstructive pulmonary disease: The role of body mass index. Aging Male 2010, 13, 142–147. [Google Scholar] [CrossRef]
- García-Rio, F.; Soriano, J.B.; Miravitlles, M.; Muñoz, L.; Duran-Tauleria, E.; Sánchez, G.; Sobradillo, V.; Ancochea, J. Overdiagnosing subjects with COPD using the 0.7 fixed ratio: Correlation with a poor health-related quality of life. Chest 2011, 139, 1072–1080. [Google Scholar] [CrossRef]
- Doucet, M.; Dubé, A.; Joanisse, D.R.; Debigaré, R.; Michaud, A.; Paré, M.È.; Vaillancourt, R.; Fréchette, E.; Maltais, F. Atrophy and hypertrophy signalling of the quadriceps and diaphragm in COPD. Thorax 2010, 65, 963–970. [Google Scholar] [CrossRef][Green Version]
- Elsammak, M.Y.; Attia, A.; Suleman, M. Fibroblast growth factor 23 (FGF23) and hypophosphatemia in patients with COPD. J. Med. Biochem. 2012, 31, 12–18. [Google Scholar] [CrossRef]
- Ye, M.; Yu, H.; Yu, W.; Zhang, G.; Xiao, L.; Zheng, X.; Wu, J. Evaluation of the significance of circulating insulin-like growth factor-1 and C-reactive protein in patients with chronic obstructive pulmonary disease. J. Int. Med. Res. 2012, 40, 1025–1035. [Google Scholar] [CrossRef]
- Jackson, A.S.; Shrikrishna, D.; Kelly, J.L.; Kemp, S.V.; Hart, N.; Moxham, J.; Polkey, M.I.; Kemp, P.; Hopkinson, N.S. Vitamin D and skeletal muscle strength and endurance in COPD. Eur. Respir J. 2013, 41, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Elmahallawy, I.I.; Qora, M.A. Prevalence of chronic renal failure in COPD patients. Egypt J. Chest Dis. Tuberc. 2013, 62, 221–227. [Google Scholar] [CrossRef]
- Emami Ardestani, M.; Zaerin, O. Role of Serum Interleukin 6, Albumin and C-Reactive Protein in COPD Patients. Tanaffos 2015, 14, 134–140. [Google Scholar] [PubMed]
- Milevoj Kopčinović, L.; Domijan, A.M.; Posavac, K.; Čepelak, I.; Žanić Grubišić, T.; Rumora, L. Systemic redox imbalance in stable chronic obstructive pulmonary disease. Biomarkers 2016, 21, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Ozgul, G.; Seyhan, E.C.; Özgül, M.A.; Günlüoğlu, M.Z. Red Blood Cell Distribution Width in Patients With Chronic Obstructive Pulmonary Disease and Healthy Subjects. Arch Bronconeumol. 2017, 53, 107–113. [Google Scholar] [CrossRef]
- Terashima, T.; Chubachi, S.; Matsuzaki, T.; Nakajima, T.; Satoh, M.; Iwami, E.; Yoshida, K.; Katakura, A.; Betsuyaku, T. The association between dental health and nutritional status in chronic obstructive pulmonary disease. Chron. Respir. Dis. 2017, 14, 334–341. [Google Scholar] [CrossRef]
- Omote, N.; Hashimoto, N.; Morise, M.; Sakamoto, K.; Miyazaki, S.; Ando, A.; Nakahara, Y.; Hasegawa, Y. Impact of mild to moderate COPD on feasibility and prognosis in non-small cell lung cancer patients who received chemotherapy. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 3541–3547. [Google Scholar] [CrossRef][Green Version]
- Cao, T.; Xu, N.; Wang, Z.; Liu, H. Effects of Glutathione S-Transferase Gene Polymorphisms and Antioxidant Capacity per Unit Albumin on the Pathogenesis of Chronic Obstructive Pulmonary Disease. Oxid Med. Cell Longev. 2017, 2017, 6232397. [Google Scholar] [CrossRef]
- Kuo, W.K.; Liu, Y.C.; Chu, C.M.; Hua, C.C.; Huang, C.Y.; Liu, M.H.; Wang, C.H. Amino Acid-Based Metabolic Indexes Identify Patients With Chronic Obstructive Pulmonary Disease And Further Discriminates Patients In Advanced BODE Stages. Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 2257–2266. [Google Scholar] [CrossRef]
- Janciauskiene, S.; DeLuca, D.S.; Barrecheguren, M.; Welte, T.; Miravitlles, M.; Scientific Committee; Participating sites and coordinators. Serum Levels of Alpha1-antitrypsin and Their Relationship with COPD in the General Spanish Population. Arch. Bronconeumol. 2020, 56, 76–83. [Google Scholar] [CrossRef]
- Mercado, N.; Thimmulappa, R.; Thomas, C.M.R.; Fenwick, P.S.; Chana, K.K.; Donnelly, L.E.; Biswal, S.; Ito, K.; Barnes, P.J. Decreased histone deacetylase 2 impairs Nrf2 activation by oxidative stress. Biochem. Biophys. Res. Commun. 2011, 406, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Liu, T.; Fan, H.; Chen, F.; Ding, H.; Wu, Z.; Wang, H.; Hou, S. Inflammatory Markers and the Risk of Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0150586. [Google Scholar] [CrossRef] [PubMed]
- Zinellu, E.; Zinellu, A.; Fois, A.G.; Fois, S.S.; Piras, B.; Carru, C.; Pirina, P. Reliability and Usefulness of Different Biomarkers of Oxidative Stress in Chronic Obstructive Pulmonary Disease. Oxid. Med. Cell Longev. 2020, 2020, 4982324. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Giustarini, D.; Milzani, A.; Dalle-Donne, I. Cysteinylation andhomocysteinylation of plasma protein thiols during ageing ofhealthy human beings. J. Cell Mol. Med. 2009, 13, 3131–3140. [Google Scholar] [CrossRef]
- Zinellu, A.; Fois, A.G.; Sotgia, S.; Zinellu, E.; Bifulco, F.; Pintus, G.; Mangoni, A.A.; Carru, C.; Pirina, P. Plasma protein thiols: An early marker of oxidative stress in asthma and chronic obstructive pulmonary disease. Eur. J. Clin. Invest. 2016, 46, 181–188. [Google Scholar] [CrossRef]
- Cabrerizo, S.; Cuadras, D.; Gomez-Busto, F.; Artaza-Artabe, I.; Marín-Ciancas, F.; Malafarina, V. Serum albumin and health in older people: Review and meta-analysis. Maturitas 2015, 81, 17–27. [Google Scholar] [CrossRef]
- Baty, F.; Putora, P.M.; Isenring, B.; Blum, T.; Brutsche, M. Comorbidities and Burden of COPD: A Population Based Case-Control Study. PLoS ONE 2013, 8, e63285. [Google Scholar] [CrossRef]
- Divo, M.J.; Cote, C.; de Torres, J.P.; Casanova, C.; Marin, J.M.; Pinto-Plata, V.; Zulueta, J.; Cabrera, C.; Zagaceta, J.; Hunninghake, G.; et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am. J. Respir Crit. Care Med. 2012, 186, 155–161. [Google Scholar] [CrossRef]
- Collins, P.F.; Yang, I.A.; Chang, Y.C.; Vaughan, A. Nutritional support in chronic obstructive pulmonary disease (COPD): An evidence update. J. Thorac. Dis. 2019, 11, S2230–S2237. [Google Scholar] [CrossRef]
- Pirabbasi, E.; Najafiyan, M.; Cheraghi, M.; Shahar, S.; Abdul Manaf, Z.; Rajab, N.; Abdul Manap, R. Predictors’ factors of nutritional status of male chronic obstructive pulmonary disease patients. ISRN Nurs. 2012, 2012, 782626. [Google Scholar] [CrossRef]

| Control Group | COPD Group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| First Author, Year Country | Study Design | Diagnosis | NOS (Stars) | n | Age (Years) | Gender (M/F) | Albumin Mean ±SD | n | Age (Years) | Gender (M/F) | Albumin Mean ± SD |
| Fiaccadori, 1994 Italy, [28] | P | ERS | 7 | 30 | 60 | 25/5 | 39 ± 3 | 22 | 63 | 19/3 | 34 ± 7 |
| Paiva, 1996 Brazil, [29] | P | ATS | 7 | 14 | 52.8 | 14/0 | 45 ± 4 | 22 | 59.4 | 22/0 | 43.6 ± 4.7 |
| Dahlen, 1999 Sweden, [30] | P | ATS | 6 | 20 | 66 | 4/16 | 44 ± 2 | 25 | 42 | 15/10 | 42 ± 3 |
| Yoneda, 2001 Japan, [31] | P | ATS | 7 | 30 | 64 | 29/1 | 44.6 ± 6.0 | 30 | 64 | 29/1 | 43.4 ± 4.2 |
| Hernandez, 2003 Spain, [32] | P | ERS | 6 | 14 | 59 | 14/0 | 40 ± 3 | 14 | 64 | 14/0 | 41 ± 4 |
| Koechlin, 2004 France, [33] | P | GOLD | 7 | 7 | 60 | 7/0 | 48 ± 3.9 | 10 | 58 | 10/0 | 45.1 ± 2.7 |
| Sergi, 2006 Italy, [35] | P | ERS | 7 | 46 | 77.7 | 46/0 | 40.8 ± 5.6 | 40 | 75.7 | 40/0 | 38.2 ± 5.4 |
| Yang, 2006 China, [34] | P | ATS | 7 | 34 | 64.8 | 34/0 | 41.6 ± 4.1 | 47 | 67.6 | 47/0 | 40.8 ± 2.9 |
| Gonlugur, 2007 Turkey, [36] | R | GOLD | 6 | 133 | 60.9 | 103/30 | 38.2 ± 6.9 | 450 | 61.9 | 348/102 | 37.7 ± 8.5 |
| Coin, 2010 Italy, [37] | P | ERS | 7 | 41 | 76 | 41/0 | 43 ± 4.7 | 41 | 75.7 | 41/0 | 38.3 ± 5.4 |
| Garcia-Rio, 2010 Spain, [18] | P | GOLD | 7 | 110 | 55 | 51/59 | 46.3 ± 8.3 §,* | 324 | 64 | 241/83 | 44.9 ± 7.5 §,* |
| Doucet, 2010 Canada, [39] | P | ATS | 6 | 7 | 60 | 2/5 | 43 ± 1 | 12 | 60 | 8/4 | 43 ± 3 |
| Garcia-Rio, 2011 Spain, [38] | P | GOLD | 7 | 85 | -- | -- | 45.9 ± 2.7 | 173 | -- | -- | 45.9 ± 2.7 |
| Elsammak, 2012 Saudi Arabian, [40] | P | GOLD | 7 | 34 | 63.0 | 20/14 | 42.2 ± 2.1 | 70 | 62.6 | 47/23 | 35.1 ± 4.8 |
| Ye, 2012 China, [41] | P | GOLD | 8 | 20 | 67.5 | 15/5 | 42.4 ± 3.4 | 43 | 68.4 | 40/3 | 36.8 ± 4.3 |
| Jackson, 2013 England, [42] | P | GOLD | 9 | 100 | 63 | 59/41 | 39.0 ± 5.1 | 104 | 65 | 60/44 | 37.6 ± 5.7 |
| Elmahallawy, 2013 Egypt, [43] | P | GOLD | 7 | 300 | 64.7 | 138/162 | 34.0 ± 6.1 | 300 | 65 | 152/148 | 33.5 ± 7 |
| Emami Ardestani, 2015 Iran, [44] | P | GOLD | 6 | 120 | 43.7 | 120/0 | 42 ± 5.55 | 60 | 59.1 | 60/0 | 41 ± 5.7 |
| Milevoj Kopčinović, 2016 Croatia, [45] | P | GOLD | 7 | 45 | 58.0 | 20/25 | 49.2 ± 6.9 | 106 | 64.3 | 80/26 | 39.3 ± 5.8 |
| Ozgul, 2017 Turkey, [46] | P | GOLD | 9 | 210 | 57.4 | 119/91 | 35 ± 12 | 175 | 61 | 110/65 | 33 ± 5 |
| Terashima, 2017 Japan, [47] | P | GOLD | 6 | 76 | 65.1 | 49/27 | 43.7 ± 2.1 | 60 | 73.9 | 55/5 | 41.3 ± 3.5 |
| Lenártová, 2017 Slovakia, [19] | P | GOLD | 7 | 60 | -- | -- | 41.7 ± 2.1 §,* | 60 | -- | -- | 39.7 ± 3.5 §,* |
| Omote, 2017 Japan, [48] | R | GOLD | 7 | 42 | 63 | 32/10 | 36 ± 6 | 43 | 67 | 37/6 | 36 ± 5 |
| Cao, 2017 China, [49] | P | GOLD | 6 | 33 | 70 | 26/7 | 45.3 ± 2.3 | 33 | 73 | 26/7 | 37.8 ± 5.8 |
| Kuo, 2019 Taiwan, [50] | P | GOLD | 6 | 44 | 53.3 | 36/8 | 44 ± 3 | 75 | 71.5 | 67/8 | 43 ± 4 |
| Janciauskiene, 2020 Spain, [51] | P | GesEPOC | 7 | 312 | 55 | 138/174 | 46 ± 4.7 | 303 | 64 | 223/80 | 45 ± 3.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zinellu, E.; Fois, A.G.; Sotgiu, E.; Mellino, S.; Mangoni, A.A.; Carru, C.; Zinellu, A.; Pirina, P. Serum Albumin Concentrations in Stable Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 269. https://doi.org/10.3390/jcm10020269
Zinellu E, Fois AG, Sotgiu E, Mellino S, Mangoni AA, Carru C, Zinellu A, Pirina P. Serum Albumin Concentrations in Stable Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2021; 10(2):269. https://doi.org/10.3390/jcm10020269
Chicago/Turabian StyleZinellu, Elisabetta, Alessandro G. Fois, Elisabetta Sotgiu, Sabrina Mellino, Arduino A. Mangoni, Ciriaco Carru, Angelo Zinellu, and Pietro Pirina. 2021. "Serum Albumin Concentrations in Stable Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 10, no. 2: 269. https://doi.org/10.3390/jcm10020269
APA StyleZinellu, E., Fois, A. G., Sotgiu, E., Mellino, S., Mangoni, A. A., Carru, C., Zinellu, A., & Pirina, P. (2021). Serum Albumin Concentrations in Stable Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 10(2), 269. https://doi.org/10.3390/jcm10020269








