Could Chronic Idiopatic Intestinal Pseudo-Obstruction Be Related to Viral Infections?
Abstract
1. Introduction
2. JC Virus
3. Herpes Virus
4. VZV
5. CMV
6. EBV
7. Flavivirus
8. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- De Giorgio, R.; Ricciardiello, L.; Naponelli, V.; Selgrad, M.; Piazzi, G.; Felicani, C.; Serra, M.; Fronzoni, L.; Antonucci, A.; Cogliandro, R.F.; et al. Chronicintestinal pseudo-obstructionrelated to viralinfections. Transpl. Proc. 2010, 42, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, A.; Fronzoni, L.; Cogliandro, L.; Cogliandro, R.F.; Caputo, C.; De Giorgio, R.; Pallotti, F.; Barbara, G.; Corinaldesi, R.; Stanghellini, V. Chronic intestinal pseudo-obstruction. World J. Gastroenterol. 2008, 14, 2953–2961. [Google Scholar] [CrossRef] [PubMed]
- Stanghellini, V.; Camilleri, M.; Malagelada, J.R. Chronic idiopathic intestinal pseudo-obstruction: Clinical and intestinal mano-metric findings. Gut 1987, 28, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Iida, H.; Ohkubo, H.; Inamori, M.; Nakajima, A.; Sato, H. Epidemiology and Clinical Experience of Chronic Intestinal Pseudo-Obstruction in Japan: A Nationwide Epidemiologic Survey. J. Epidemiol. 2013, 23, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Stanghellini, V.; Cogliandro, R.F.; De Giorgio, R.; Barbara, G.; Morselli-Labate, A.M.; Cogliandro, L.; Corinaldesi, R. Natural History of Chronic Idiopathic Intestinal Pseudo-Obstruction in Adults: A Single Center Study. Clin. Gastroenterol. Hepatol. 2005, 3, 449–458. [Google Scholar] [CrossRef]
- Cogliandro, R.F.; De Giorgio, R.; Barbara, G.; Cogliandro, L.; Concordia, A.; Corinaldesi, R.; Stanghellini, V. Chronicintestinal pseudo-obstruction. Best Pract. Res. Clin. Gastroenterol. 2007, 21, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Stanghellini, V.; Cogliandro, R.F.; De Giorgio, R.; Barbara, G.; Salvioli, B.; Corinaldesi, R. Chronic intestinal pseudo-obstruction: Manifestations, natural history and management. Neurogastroenterol. Motil. 2007, 19, 440–452. [Google Scholar] [CrossRef]
- Di Lorenzo, C. Pseudo-obstruction: Current approaches. Gastroenterology 1999, 116, 980–987. [Google Scholar] [CrossRef]
- Hall, K.E.; Wiley, J.W.I. New insights into neuronal injury: A cautionary tale. Am. J. Physiol. Liver Physiol. 1998, 274, G978–G983. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, N.; Camilleri, M.; Camoriano, J.K.; Low, P.A.; Fealey, R.D.; Perry, M.C. Autonomic function and motility in intestinal pseudoobstruction caused by paraneoplastic syndrome. Dig. Dis. Sci. 1989, 34, 1937–1942. [Google Scholar] [CrossRef]
- Kidher, E.S.; Briceno, N.; Taghi, A.; Chukwuemeka, A. An interesting collection of paraneoplastic syndromes in a patient with a malignant thymoma. BMJ Case Rep. 2012, 2012. [Google Scholar] [CrossRef]
- Cerra-Franco, J.A.; Fernandez-Cruz, C.; Estremera-Marcial, R.; Pagan-Torres, H.; Martinez-Souss, J.; Toro, D.H. Anti–Hu-Mediated Paraneoplastic Chronic Intestinal Pseudo-Obstruction Arising from Small Cell Prostate Cancer. ACG Case Rep. J. 2019, 6, e00105-3. [Google Scholar] [CrossRef] [PubMed]
- Khairullah, S.; Jasmin, R.; Yahya, F.; Cheah, T.; Ng, C.-T.; Sockalingam, S. Chronic intestinal pseudo-obstruction: A rare first manifestation of systemic lupus erythematosus. Lupus 2013, 22, 957–960. [Google Scholar] [CrossRef] [PubMed]
- Jäkel, J.; Heise, J.W.; Gässler, N.; Dietrich, C.G. Raising Awareness about Chronic Intestinal Pseudo-Obstruction (CIPO): A Case Report Showing CIPO as Initial Manifestation of Atypical Seronegative Systemic Sclerosis. Z. Gastroenterol. 2012, 50, 1100–1103. [Google Scholar] [CrossRef] [PubMed]
- De Giorgio, R.; Camilleri, M. Human enteric neuropathies: Morphology and molecular pathology. Neurogastroenterol. Motil. 2004, 16, 515–531. [Google Scholar] [CrossRef] [PubMed]
- De Giorgio, R.; Sarnelli, G.; Corinaldesi, R.; Stanghellini, V. Advances in our understanding of the pathology of chronic intestinal pseudo-obstruction. Gut 2004, 53, 1549–1552. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, H.; Kessoku, T.; Fuyuki, A.; Iida, H.; Inamori, M.; Fujii, T.; Kawamura, H.; Hata, Y.; Manabe, N.; Chiba, T.; et al. Assessment of Small Bowel Motility in Patients With Chronic Intestinal Pseudo-Obstruction Using Cine-MRI. Am. J. Gastroenterol. 2013, 108, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Fuyuki, A.; Ohkubo, H.; Higurashi, T.; Iida, H.; Inoh, Y.; Inamori, M.; Nakajima, A. Clinical importance of cine-MRI assessment of small bowel motility in patients with chronic intestinal pseudo-obstruction: A retrospective study of 33 patients. J. Gastroenterol. 2016, 52, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, Z.; McCallum, R.W. Small Bowel Dysmotility, Pseudoobstruction, and Functional Correlation with Histopathology: Lessons Learned. Curr. Gastroenterol. Rep. 2020, 22, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kuo, B.; Maneerattanaporn, M.; Lee, A.A.; Baker, J.R.; Wiener, S.M.; Chey, W.D.; Wilding, G.E.; Hasler, W.L. Generalized Transit Delay on Wireless Motility Capsule Testing in Patients with Clinical Suspicion of Gastroparesis, Small Intestinal Dysmotility, or Slow Transit Constipation. Dig. Dis. Sci. 2011, 56, 2928–2938. [Google Scholar] [CrossRef]
- Pinto, M.; Dobson, S. BK and JC virus: A review. J. Infect. 2014, 68, S2–S8. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.S.; Koralnik, I.J. JC, BK, and other polyomaviruses: Progressive multifocal leukoencephalopathy. In Principles and Practices of Infectious Diseases, 10th ed.; Mandell, G.L., Ed.; Churchill Livingstone: Philadelphia, PA, USA, 2009. [Google Scholar]
- Padgett, B.L.; Walker, D.L.; ZuRhein, G.M.; Eckroade, R.J.; Dessel, B.H. Cultivation of papova-like virus from human brain with pro-gressive multifocal leucoencephalopathy. Lancet 1971, 1, 1257–1260. [Google Scholar] [CrossRef]
- Bofill-Mas, S.; Formiga-Cruz, M.; Clemente-Casares, P.; Calafell, F.; Girones, R. Potential Transmission of Human Polyomaviruses through the Gastrointestinal Tract after Exposure to Virions or Viral DNA. J. Virol. 2001, 75, 10290–10299. [Google Scholar] [CrossRef] [PubMed]
- Monaco, M.C.G.; Jensen, P.N.; Hou, J.; Durham, L.C.; Major, E.O. Detection of JC Virus DNA in Human Tonsil Tissue: Evidence for Site of Initial Viral Infection. J. Virol. 1998, 72, 9918–9923. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, F.; Nagaki, D.; Saito, M.; Haruyama, C.; Iwasaki, K. Trans- plancental transmission of BK virus in human. Jpn. J. Microbiol. 1975, 19, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Bohl, D.L.; Storch, G.A.; Ryschkewitsch, C.; Gaudreault-Keener, M.; Schnitzler, M.A.; Major, E.O.; Brennan, D.C. Donor Origin of BK Virus in Renal Transplantation and Role of HLA C7 in Susceptibility to Sustained BK Viremia. Arab. Archaeol. Epigr. 2005, 5, 2213–2221. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.S.; Dezube, B.J.; Bhargava, P.; Autissier, P.; Wüthrich, C.; Miller, J.; Koralnik, I.J. Detection of JC Virus DNA and Proteins in the Bone Marrow of HIV-Positive and HIV-Negative Patients: Implications for Viral Latency and Neurotropic Transformation. J. Infect. Dis. 2009, 199, 881–888. [Google Scholar] [CrossRef]
- Power, C.; Gladden, J.G.B.; Halliday, W.; Del Bigio, M.R.; Nath, A.; Ni, W.; Major, E.O.; Blanchard, J.; Mowat, M. AIDS- and non-AIDS-related PML association with distinct p53 polymorphism. Neurology 2000, 54, 743. [Google Scholar] [CrossRef]
- Berger, J.R.; Kaszovitz, B.; Post, M.J.; Dickenson, G. Progressive multifocal leukoencephalopathy associated with human immuno-deficiency virus infection. A review of the literature with report of sixteen cases. Ann. Intern. Med. 1987, 107, 78–87. [Google Scholar] [CrossRef]
- Sinagra, E.; Raimondo, D.; Gallo, E.; Stella, M.; Cottone, M.; Rossi, F.; Messina, M.; Spada, M.; Tomasello, G.; Ferrara, G.; et al. JC Virus and Lung Adenocarcinoma: Fact or Myth? Anticancer Res. 2017, 37, 3311–3314. [Google Scholar] [CrossRef][Green Version]
- White, M.K.; Khalili, K. Polyomaviruses and human cancer: Molecular mechanisms underlying patterns of tumorigenesis. Virology 2004, 324, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sinagra, E.; Raimondo, D.; Gallo, E.; Stella, M.; Cottone, M.; Orlando, A.; Rossi, F.; Orlando, E.; Messina, M.; Tomasello, G.; et al. Could JC virus provoke metastasis in colon cancer? World J. Gastroenterol. 2014, 20, 15745–15749. [Google Scholar] [CrossRef][Green Version]
- Maginnis, M.S.; Atwood, W.J. JC Virus: An oncogenic virus in animals and humans? Semin. Cancer Biol. 2009, 19, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Niv, Y.; Goel, A.; Boland, C.R. JC virus and colorectal cancer: A possible trigger in the chromosomal instability pathways. Curr. Opin. Gastroenterol. 2005, 21, 85–89. [Google Scholar]
- Shavaleh, R.; Kamandi, M.; Disfani, H.F.; Mansori, K.; Naseri, S.N.; Rahmani, K.; Kanrash, F.A. Association between JC virus and colorectal cancer: Systematic review and meta-analysis. Infect. Dis. 2019, 52, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Ricciardiello, L.; De Giorgio, R.; Fini, L.; Cogliandro, R.F.; Williams, S.; Stanghellini, V.; Barbara, G.; Tonini, M.; Corinaldesi, R.; Genta, R.M.; et al. JC virus infects the enteric glia of patients with chronic idiopathic intestinal pseudo-obstruction. Gut 2008, 58, 25–32. [Google Scholar] [CrossRef]
- Sinagra, E.; Raimondo, D.; Gallo, E.; Calvaruso, M.; Lentini, V.L.; Cannizzaro, A.; Linea, C.; Giunta, M.; Montalbano, L.M.; D’Amico, G.; et al. Could JC virus be linked to chronic idiopathic intestinal pseudo-obstruction? Clin. J. Gastroenterol. 2019, 13, 377–381. [Google Scholar] [CrossRef]
- Wagner, E.K.; Hewlett, M.J. Basic Virology, 2nd ed.; Wiley-Blackwell: Malden, MA, USA, 2004; p. 311. [Google Scholar]
- Mettenleiter, T.C. Pathogenesis of neurotropic herpesviruses: Role of viral glycoproteins in neuroinvasion and transneuronal spread. Virus Res. 2003, 92, 197–206. [Google Scholar] [CrossRef]
- Chen, J.J.; Gershon, A.A.; Li, Z.; Cowles, R.A.; Gershon, M.D. Varicella zoster virus (VZV) infects and establishes latency in enteric neurons. J. Neurovirol. 2011, 17, 578–589. [Google Scholar] [CrossRef]
- Gershon, A.A.; Chen, J.; Davis, L.; Krinsky, C.; Cowles, R.; Reichard, R.; Gershon, M. Latency of Varicella Zoster Virus in Dorsal Root, Cranial, and Enteric Ganglia in Vaccinated Children. Trans. Am. Clin. Clim. Assoc. 2012, 123, 17–35. [Google Scholar]
- Gupta, S.K.; Helal, B.H.; Kiely, P. THE PROGNOSIS IN ZOSTER PARALYSIS. J. Bone Jt. Surg. Br. Vol. 1969, 593–603. [Google Scholar] [CrossRef]
- Yamagami, H.; Yuda, Y.; Shioya, M. Motor paralysis of abdominal muscles due to herpes zoster. Pain Clin. 1987, 8, 637–642. [Google Scholar]
- Bahadir, C.; Kalpakcioglu, A.B.; Kurtulus, D. Unilateral diaphragmatic paralysis and segmental motor paresis following herpes zoster. Muscle Nerve 2008, 38, 1070–1073. [Google Scholar] [CrossRef]
- Carrascosa, M.F.; Salcines-Caviedes, J.R.; Román, J.G.; Cano-Hoz, M.; Fernández-Ayala, M.; Casuso-Sáenz, E.; Abascal-Carrera, I.; Campo-Ruiz, A.; Martín, M.C.; Díaz-Pérez, A.; et al. Varicella-zoster virus (VZV) infection as a possible cause of Ogilvie’s syndrome in an immunocompromised host. J. Clin. Microbiol. 2014, 52, 2718–2721. [Google Scholar] [CrossRef]
- Tanida, E.; Izumi, M.; Abe, T.; Tsuchiya, I.; Okuma, K.; Uchida, E.; Hidaka, A.; Hayashi, E.; Noguchi, M.; Masui, Y.; et al. [Disseminated varicella-zoster virus infection complicated with severe abdominal pain and colonic pseudo-obstruction]. Nihon Shokakibyo Gakkai Zasshi 2013, 110, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Jucgla, A.; Badell, A.; Ballesta, C.; Arbizu, T. Colonic pseudo-obstruction: A complication of herpes zoster. Br. J. Dermatol. 1996, 134, 788–790. [Google Scholar] [CrossRef]
- Batke, M.; Cappell, M.S. Adynamic Ileus and Acute Colonic Pseudo-Obstruction. Med. Clin. N. Am. 2008, 92, 649–670. [Google Scholar] [CrossRef] [PubMed]
- Menuck, L.; Brahme, F.; Amberg, J.; Sherr, H. Colonic changes of herpes zoster. Am. J. Roentgenol. 1976, 127, 273–276. [Google Scholar] [CrossRef]
- Walsh, T.N.; Lane, D. Pseudo obstruction of the colon associated with varicella- zoster infection. Ir. J. Med. Sci. 1982, 151, 318–319. [Google Scholar] [CrossRef]
- Maeda, K.; Furukawa, K.; Sanada, M.; Kawai, H.; Yasuda, H. Constipation and Segmental Abdominal Paresis Followed by Herpes Zoster. Intern. Med. 2007, 46, 1487–1488. [Google Scholar] [CrossRef]
- Caccese, W.J.; Bronzo, R.L.; Wadler, G.; McKinley, M.J. Ogilvie’s Syndrome Associated with Herpes Zoster Infection. J. Clin. Gastroenterol. 1985, 7, 309–313. [Google Scholar] [CrossRef]
- Pai, N.B.; Murthy, R.S.; Kumar, H.T.; Gerst, P.H. Association of acute colonic pseudo-obstruction (Ogilvie’s syndrome) with herpes zoster. Am. Surg. 1990, 56, 691–694. [Google Scholar] [PubMed]
- Alpay, K.; Yandt, M. Herpes zoster and Ogilvie’s syndrome. Dermatology 1994, 189, 312. [Google Scholar] [CrossRef] [PubMed]
- Nomdedéu, J.F.; Nomdedéu, J.; Martino, R.; Bordes, R.; Portorreal, R.; Sureda, A.; Domingo-Albós, A.; Rutllant, M.; Soler, J. Ogilvie’s syndrome from disseminated varicella-zoster infection and infarcted celiac ganglia. J. Clin. Gastroenterol. 1995, 20, 157–159. [Google Scholar] [CrossRef]
- Wright, F.J. Glossopharyngeal zoster followed by varicella in two contacts. Lancet 1947, 2, 946. [Google Scholar] [CrossRef]
- Herath, P.; Gunawardana, S. Acute colonic pseudo-obstruction associated with varicella zoster infection and acyclovir therapy. Ceylon Med. J. 1997, 42, 36–37. [Google Scholar] [PubMed]
- Giunta, R.; Marfella, M.; Maffei, A.; Lucivero, G. Herpes zoster infection and Ogilvie’s syndrome in non-Hodgkin’s lymphoma with hypogammaglobulinemia. Ann. Ital. Med. Int. 2001, 16, 50–53. [Google Scholar]
- Braude, M.R.; Trubiano, J.A.; Heriot, A.; Dickinson, M.; Carney, D.; Seymour, J.F.; Tam, C.S. Disseminated visceral varicella zoster virus presenting with the constellation of colonic pseudo-obstruction, acalculous cholecystitis and syndrome of inappropriate ADH secretion. Intern. Med. J. 2016, 46, 238–239. [Google Scholar] [CrossRef]
- Masood, I.; Majid, Z.; Rind, W.; Zia, A.; Riaz, H.; Raza, S. Herpes Zoster-Induced Ogilvie’s Syndrome. Case Rep. Surg. 2015, 2015, 563659. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Johnson, J.N.; Sells, R.A. Herpes zoster and paralytic ileus: A case report. BJS 1977, 64, 143–144. [Google Scholar] [CrossRef]
- Cane, H. Herpes Zoster and Paralytic Ileus. Br. Med. J. 1959, 2, 887. [Google Scholar] [CrossRef][Green Version]
- Hiramatsu, S.; Nebiki, H.; Ueno, A.; Wakahara, Y.; Maruyama, H.; Suekane, T.; Yamasaki, T.; Sasaki, E.; Sano, K.; Sato, H.; et al. [A case of paralytic ileus associated with varicella zoster virus infection]. Nihon Shokakibyo Gakkai Zasshi 2013, 110, 1007–1013. [Google Scholar] [PubMed]
- Chang, A.; Young, N.; Reddick, R.L.; Orenstein, J.M.; Hosea, S.W.; Katz, P.; Brennan, M.F. Small bowel obstruction as a complication of disseminated varicella-zoster infection. Surgery 1978, 83, 371–374. [Google Scholar] [PubMed]
- Anaya-Prado, R.; Pérez-Navarro, J.V.; Corona-Nakamura, A.; Anaya-Fernández, M.M.; Anaya-Fernández, R.; Izaguirre-Pérez, M.E. Intestinal pseudo-obstruction caused by herpes zoster: Case report and pathophysiology. World J. Clin. Cases 2018, 6, 132–138. [Google Scholar] [CrossRef]
- Pui, J.C.; Furth, E.E.; Minda, J.; Montone, K.T. Demonstration of varicella-zoster virus infection in the muscularispropria and myentericplexi of the colon in an HIV-positive patient with herpes zoster and small bowel pseudo-obstruction (Ogilvie’s syn-drome). Am. J. Gastroenterol. 2001, 96, 1627–1630. [Google Scholar] [CrossRef]
- Tribble, D.R.; Church, P.; Frame, J.N. Gastrointestinal Visceral Motor Complications of Dermatomal Herpes Zoster: Report of Two Cases and Review. Clin. Infect. Dis. 1993, 17, 431–436. [Google Scholar] [CrossRef]
- Hosoe, N.; Sakakibara, R.; Yoshida, M.; Wakabayashi, T.; Kikuchi, H.; Yamada, T.; Kawahara, K.; Kishi, M. Acute, severe constipation in a 58-year-old Japanese patient. Gut 2010, 60, 1059. [Google Scholar] [CrossRef]
- Mathias, J.R.; Baskin, G.S.; Reeves-Darby, V.G.; Clench, M.H.; Smith, L.L.; Calhoon, J.H. Chronic intestinal pseudoobstruction in a patient with heart-lung transplant therapeutic effect of leuprolide acetate. Dig. Dis. Sci. 1992, 37, 1761–1768. [Google Scholar] [CrossRef]
- Press, M.F.; Riddell, R.H.; Ringus, J. Cytomegalovirus inclusion disease. Its occurrence in the myenteric plexus of a renal transplant patient. Arch. Pathol. Lab. Med. 1980, 104, 580–583. [Google Scholar]
- Levine, R.; Warnker, N.; Johnson, C. Cytomegalovirus inclusion disease in the gastrointestinal tract of adults. Ann. Surg. 1964, 159, 37–48. [Google Scholar] [CrossRef]
- Déchelotte, P.J.; Mulliez, N.M.; Bouvier, R.J.; Vanlieféringhen, P.C.; Lemery, D.J. Pseudo-Meconium Ileus Due to Cytomegalovirus Infection: A Report of Three Cases. Pediatr. Pathol. 1992, 12, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Foucaud, P.; Sonsino, E.; Mouy, R.; Aigrain, Y.; Cezard, J.P.; Navarro, J. Pseudo-obstruction intestinale et infection à cytomégalovirus des plexus myentériques [Intestinal pseudo-obstruction and cytomegalovirus infection of myenteric plexuses]. Arch. Fr. Pediatr. 1985, 42, 713–715. [Google Scholar] [PubMed]
- Ategbo, S.; Turck, D.; Gottrand, F.; Bonnevalle, M.; Wattre, P.; Lecomte-Houcke, M.; Farriaux, J.P. Chronic Intestinal Pseudo-Obstruction Associated with Cytomegalovirus Infection in an Infant. J. Pediatr. Gastroenterol. Nutr. 1996, 23, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Debinski, H.S.; Kamm, M.; Talbot, I.C.; Khan, G.; Kangro, H.; Jeffries, D.J. DNA viruses in the pathogenesis of sporadic chronic idiopathic intestinal pseudo-obstruction. Gut 1997, 41, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, A.; Steinberg, G.; Nathanson, M. Nervous System Involvement in Infectious Mononucleosis. Arch. Neurol. 1972, 26, 353–358. [Google Scholar] [CrossRef]
- Yahr, M.D.; Frontera, A.T. Acute autonomic neuropathy. Its occurrence in infectious mononucleosis. Arch. Neurol. 1975, 32, 132–133. [Google Scholar] [CrossRef]
- Fujii, N.; Tabira, T.; Shibasaki, H.; Kuroiwa, Y.; Ohnishi, A.; Nagaki, J. Acute autonomic and sensory neuropathy elevated Ep-stein-Barr virus antibody titre. Psychiatry 1982, 45, 656–658. [Google Scholar]
- Appenzeller, O.; Arnason, B.G.; Adams, R.D. Experimental autonomic neuropathy: An immunologically induced disorders of reflex vasomotor function. J. Neurol. Neurosurg. Psychiatry 1965, 8, 510–515. [Google Scholar] [CrossRef][Green Version]
- Vassallo, M.; Camilleri, M.; Caron, B.; Low, P.A. Gastrointestinal motor dysfunction in acquired selective cholinergic dysautonomia associated with infectious mononucleosis. Gastroenterology 1991, 100, 252–258. [Google Scholar] [CrossRef]
- Besnard, M.; Faure, C.; Fromont-Hankard, G.; Ansart-Pirenne, H.; Peuchmaur, M.; Cezard, J.; Navarro, J. Intestinal pseudo-obstruction and acute pandysautonomia associated with epstein-barr virus infection. Am. J. Gastroenterol. 2000, 95, 280–284. [Google Scholar] [CrossRef]
- Suthar, M.S.; Diamond, M.S.; Gale, M., Jr. West Nile virus infection and immunity. Nat. Rev. Genet. 2013, 11, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.A.; Wang, H.; Siddharthan, V.; Morrey, J.D.; Diamond, M.S. Axonal transport mediates West Nile virus entry into the central nervous system and induces acute flaccid paralysis. Proc. Natl. Acad. Sci. USA 2007, 104, 17140–17145. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.J.; Cao, B.; Govero, J.; Smith, A.M.; Fernandez, E.; Cabrera, O.H.; Garber, C.; Noll, M.; Klein, R.S.; Noguchi, K.K.; et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell 2016, 165, 1081–1091. [Google Scholar] [CrossRef]
- Kimura, T.; Sasaki, M.; Okumura, M.; Kim, E.; Sawa, H. Flavivirus encephalitis: Pathological aspects of mouse and other animal models. Vet. Pathol. 2010, 47, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Nagata, N.; Iwata-Yoshikawa, N.; Hayasaka, D.; Sato, Y.; Kojima, A.; Kariwa, H.; Takashima, I.; Takasaki, T.; Kurane, I.; Sata, T.; et al. The Pathogenesis of 3 Neurotropic Flaviviruses in a Mouse Model Depends on the Route of Neuroinvasion After Viremia. J. Neuropathol. Exp. Neurol. 2015, 74, 250–260. [Google Scholar] [CrossRef] [PubMed]
- White, J.P.; Xiong, S.; Malvin, N.P.; Khoury-Hanold, W.; Heuckeroth, R.O.; Stappenbeck, T.; Diamond, M.S. Intestinal Dysmotility Syndromes following Systemic Infection by Flaviviruses. Cell 2018, 175, 1198–1212.e12. [Google Scholar] [CrossRef]
- Chowers, Y.; Lang, R.; Nassar, F.; Ben-David, D.; Giladi, M.; Rubinshtein, E.; Itzhaki, A.; Al, M.Y.C.E.; Siegman-Igra, Y.; Kitzes, R.; et al. Clinical Characteristics of the West Nile Fever Outbreak, Israel, 2000. Emerg. Infect. Dis. 2001, 7, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Weiss, D.; Carr, D.; Kellachan, J.; Tan, C.; Phillips, M.S.; Bresnitz, E.; Layton, M.; West Nile Virus Outbreak Response Working Group. Clinical Findings of West Nile Virus Infection in Hospitalized Patients, New York and New Jersey, 2000. Emerg. Infect. Dis. 2001, 7, 654–658. [Google Scholar] [CrossRef]
- Armah, H.B.; Wang, G.; Omalu, B.I.; Tesh, R.B.; Gyure, K.A.; Chute, D.J.; Smith, R.D.; Dulai, P.; Vinters, H.V.; Kleinschmidt-DeMasters, B.K.; et al. Systemic Distribution of West Nile Virus Infection: Postmortem Immunohistochemical Study of Six Cases. Brain Pathol. 2007, 17, 354–362. [Google Scholar] [CrossRef]
- Pironi, L.; Sasdelli, A.S. Management of the Patient with Chronic Intestinal Pseudo-Obstruction and Intestinal Failure. Gastroenterol. Clin. N. Am. 2019, 48, 513–524. [Google Scholar] [CrossRef]
- Houldcroft, C.J.; Beale, M.A.; Breuer, J. Clinical and biological insights from viral genome sequencing. Nat. Rev. Genet. 2017, 15, 183–192. [Google Scholar] [CrossRef]
- Fleming, M.A.; Ehsan, L.; Moore, S.R.; Levin, D.E. The Enteric Nervous System and Its Emerging Role as a Therapeutic Target. Gastroenterol. Res. Pract. 2020, 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.C.; Parkman, H.; McCallum, R. Handbook of Gastrointestinal Motility and Functional Disorders; SLACK Incorporated: Thorofare, NJ, USA, 2015. [Google Scholar]
- Uchida, K.; Otake, K.; Inoue, M.; Koike, Y.; Matsushita, K.; Araki, T.; Okita, Y.; Tanaka, K.; Uchida, K.; Yodoya, N.; et al. Chronic intestinal pseudo-obstruction due to lymphocytic intestinal leiomyositis: Case report and literature review. Intractable Rare Dis. Res. 2012, 1, 35–39. [Google Scholar] [CrossRef][Green Version]
- Camilleri, M.; Cowen, T.; Koch, T.R. Enteric neurodegeneration in ageing. Neurogastroenterol. Motil. 2008, 20, 418–429. [Google Scholar] [CrossRef]
- Lindberg, G. Chronic intestinal pseudo-obstruction: How important is JC virus infection? Gut 2008, 58, 2–3. [Google Scholar] [CrossRef] [PubMed]
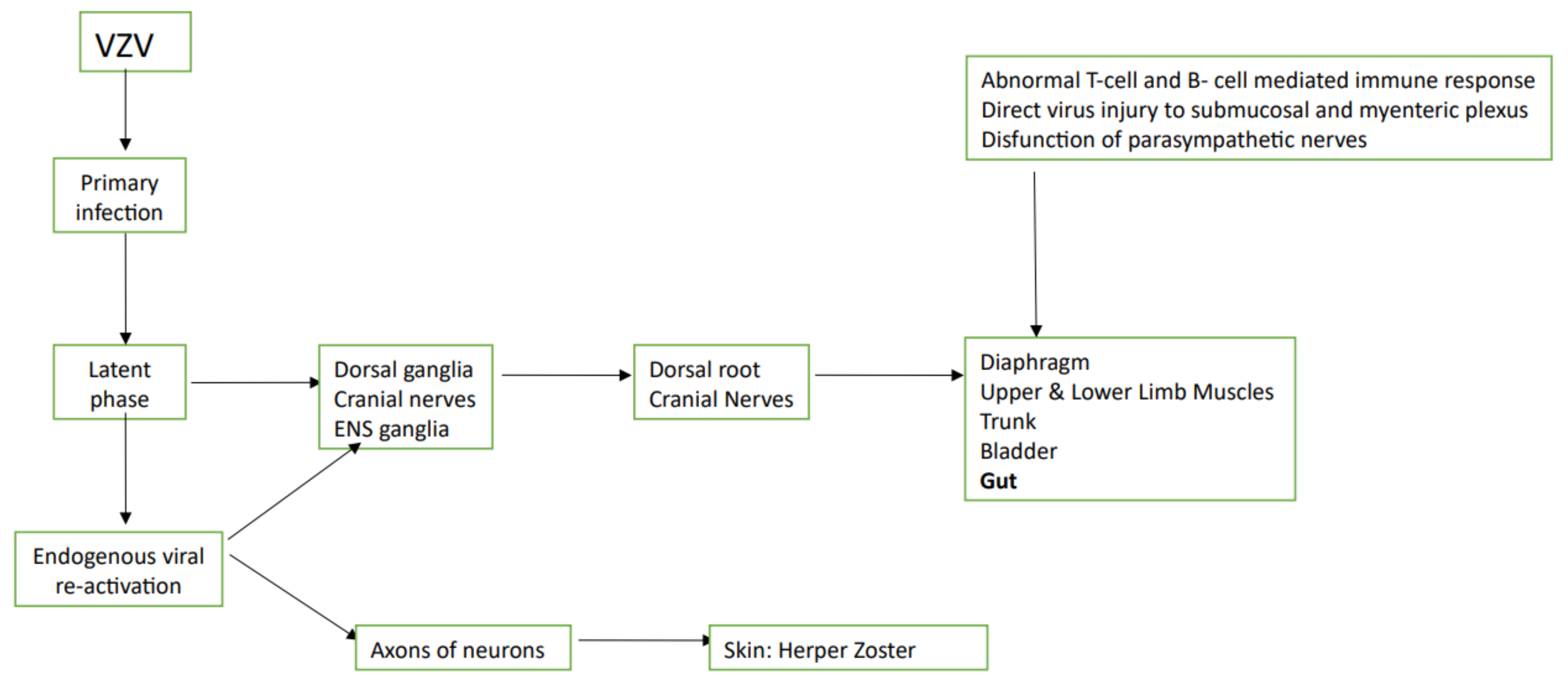
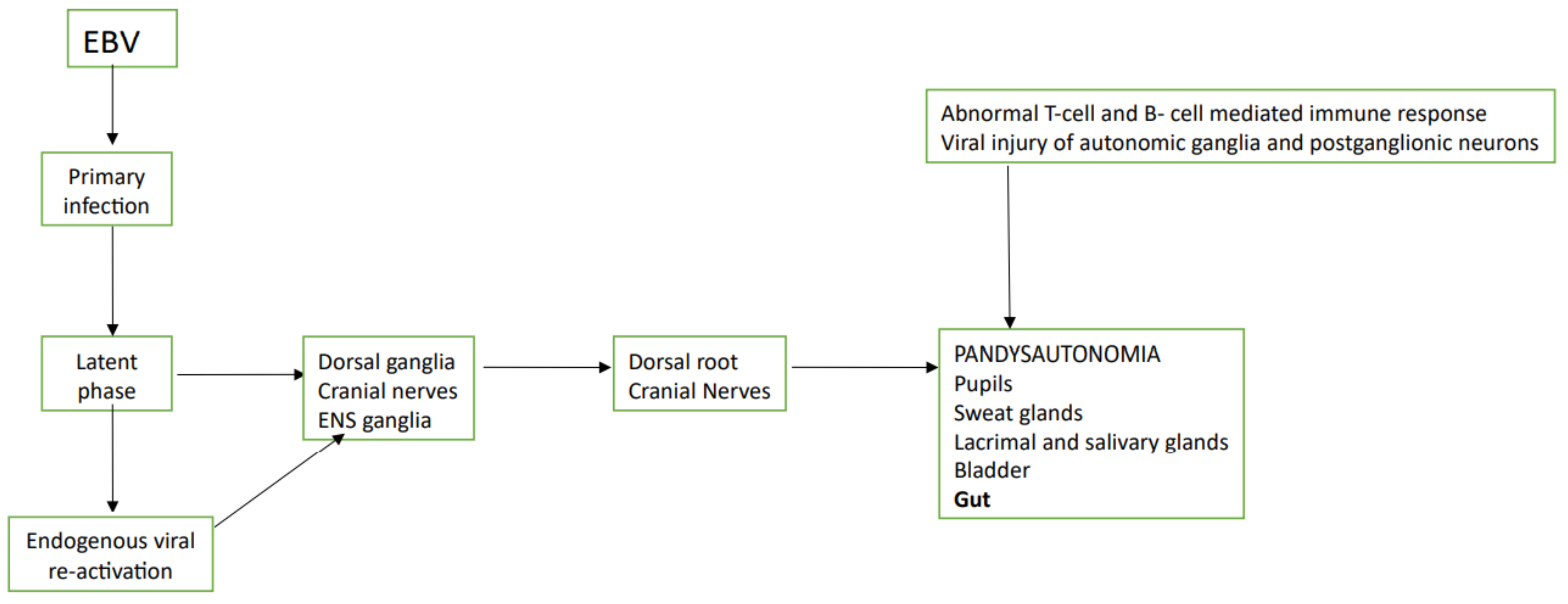
| Reference | Author | Year of Publication and Study Protocol | Virus Involved and Technique of Its Detection | Suspected Mechanism of Action |
|---|---|---|---|---|
| [37] | Selgrad, et al. | 2009, case-control study | John Cunningham Virus. DNA was extracted from the myenteric plexuses of colonic and ileal specimens, and JCV T antigen (TAg) DNA and the viral regulatory region were detected by PCR and sequencing | The John Cunningham Virus localization in enteroglial cells suggests a possible pathological role for this virus in enteric neuropathy |
| [38] | Sinagra, et al. | 2020, case report on 2 patients | John Cunningham Virus. PCR amplifications were performed using gene-specific primers for T antigen; PCR for the only JCV was positive in duodenal and jejunal samples in both the patients | The John Cunningham localization in small bowel suggested a possible pathological role for this virus in enteric neuropathy not otherwise classified |
| [62] | Johnson, et al. | 1977, case report | Varicella Zoster Virus. Detection through PCR in ileocolonic samples | Direct viral injury to colonic enteric nervous system and muscularispropria |
| [46] | Carrascosa, et al. | 2014, case report | Varicella Zoster Virus. Detection through PCR in colonic samples | Direct viral injury to colonic enteric nervous system and muscularispropria |
| [63] | Cane, et al. | 1959, case report | Varicella Zoster Virus. First detection in ileal samples | Direct viral injury to colonic enteric nervous system and muscularispropria |
| [64] | Hiramatsu, et al. | 2013, case report | Varicella Zoster Virus. Detection through PCR in ileal samples | Direct viral injury to colonic enteric nervous system and muscularispropria |
| [65] | Chang, et al. | 1978, case report | Varicella Zoster Virus. Detection through PCR in ileal samples | Direct viral injury to colonic enteric nervous system and muscularispropria |
| [66] | Anaya-Prado, et al. | 2018, case report | Varicella Zoster Virus. Detection through PCR in ileocolonic samples | Direct viral injury to colonic enteric nervous system and muscularispropria |
| [67] | Pui, et al. | 2001, case report | Varicella Zoster Virus. Detection through PCR in ileocolonic samples | Direct infection of celiac plexus ganglion and colonic autonomic nervous system (ANS) and hemorrhagic infarction of abdominal sympathetic celiac ganglia |
| [68] | Tribble, et al. | 1993, case report | Varicella Zoster Virus. Detection through PCR in ileocolonic samples | Viral involvement of the thoracolumbar or sacral lateral columns causing parasympathetic nerves disfunction and intestinal hypomotility |
| [56] | Nomdedéu, et al. | 1995, case report | Varicella Zoster Virus. Detection through PCR in ileocolonic samples | Peritoneal inflammation due to vescicular eruptions |
| [70] | Hosoe, et al. | 2010, case report | Varicella Zoster Virus. Detection through PCR in colonic samples | Injury of afferent C-fibers leading to intestinal pseudo-obstruction |
| [74] | Dèchelotte, et al. | 1992, case report on 3 patients | Cytomegalovirus. Virus had been identified through PCR in the ganglion cells or in the myenteric and submucosal plexuses of the small bowel and colon | Antenatal paralytic ileus caused by cytomegalovirus infection |
| [75] | Foucaud, et al. | 1985, case report | Cytomegalovirus. Viruria and specific IgM and IgG antibodies confirmed cytomegalovirus infection. The patient underwent also rectal biopsy with detection of hypoganglionosis, nerve thickening and cytomegalovirusintranuclear inclusions | Paralytic ileus caused by cytomegalovirus infection |
| [77] | Debinsky, et al. | 1992, case control study | Cytomegalovirus. One patient, who presented visceral neuropathy and myopathy, had small intestine samples positive for cytomegalovirus DNA. No control tissue was positive for any virus | Visceral neuropathy and myopathy caused by cytomegalovirus |
| [76] | Ategbo, et al. | 1996, case report | Cytomegalovirus. At the small bowel biopsy, cytomegalovirus was identified. Moreover, the patient’s trend of IgM and IgG and the absence of maternal IgG were indicative of infection within the first few weeks of life | Visceral neuropathy caused by cytomegalovirus |
| [82] | Vassallo, et al. | 1991, case report | Epstein–Barr virus. The ganglion cells of the myenteric plexus were normal at morphological and immunohistochemical evaluation and therefore the selective cholinergic dysautonomia was identified as the most likely pathophysiologic process responsible for the symptoms | Selective cholinergic dysautonomia that occurred following acute infectious mononucleosis |
| [83] | Besnard, et al. | 2000, case report | Epstein–Barr virus. The patient has undergone resection appendix and sigmoidoscopy and the tissue samples showed hypoganglionosis and a mononuclear inflammatory infiltrate in the myenteric neural plexus. EBV-PCR was positive in the blood and cerebrospinal fluid, and EBV-RNA was identified in myenteric appendix cells, in a mesenteric lymph node, and in gastric biopsies | Viral direct invasion of autonomic ganglia or postganglionic neurons |
| [1] | De Giorgio, et al. | 2010, case control study | Epstein–Barr virus. PCR-based study of 13 clinically and manometrically characterized patients with chronic idiopatic intestinal pseudo-obstruction (2 patients positive for Epstein–Barr virus infection) | Viral direct invasion of autonomic ganglia or postganglionic neurons |
| [92] | Armah, et al. | 2007, post-mortem study | West Nile Virus, Flavivirus acute infection affected by GI symptoms and located in the intestine | Viral antigens, RNA, lesions of the myenteric plexus and necrosis of enterocytes have been identified in the intestinal tissue isolated also in infected rodents |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinagra, E.; Pellegatta, G.; Maida, M.; Rossi, F.; Conoscenti, G.; Pallio, S.; Alloro, R.; Raimondo, D.; Anderloni, A. Could Chronic Idiopatic Intestinal Pseudo-Obstruction Be Related to Viral Infections? J. Clin. Med. 2021, 10, 268. https://doi.org/10.3390/jcm10020268
Sinagra E, Pellegatta G, Maida M, Rossi F, Conoscenti G, Pallio S, Alloro R, Raimondo D, Anderloni A. Could Chronic Idiopatic Intestinal Pseudo-Obstruction Be Related to Viral Infections? Journal of Clinical Medicine. 2021; 10(2):268. https://doi.org/10.3390/jcm10020268
Chicago/Turabian StyleSinagra, Emanuele, Gaia Pellegatta, Marcello Maida, Francesca Rossi, Giuseppe Conoscenti, Socrate Pallio, Rita Alloro, Dario Raimondo, and Andrea Anderloni. 2021. "Could Chronic Idiopatic Intestinal Pseudo-Obstruction Be Related to Viral Infections?" Journal of Clinical Medicine 10, no. 2: 268. https://doi.org/10.3390/jcm10020268
APA StyleSinagra, E., Pellegatta, G., Maida, M., Rossi, F., Conoscenti, G., Pallio, S., Alloro, R., Raimondo, D., & Anderloni, A. (2021). Could Chronic Idiopatic Intestinal Pseudo-Obstruction Be Related to Viral Infections? Journal of Clinical Medicine, 10(2), 268. https://doi.org/10.3390/jcm10020268






