Considerations in the Use of Gravitational Valves in the Management of Hydrocephalus. Some Lessons Learned with the Dual-Switch Valve
Abstract
1. Introduction
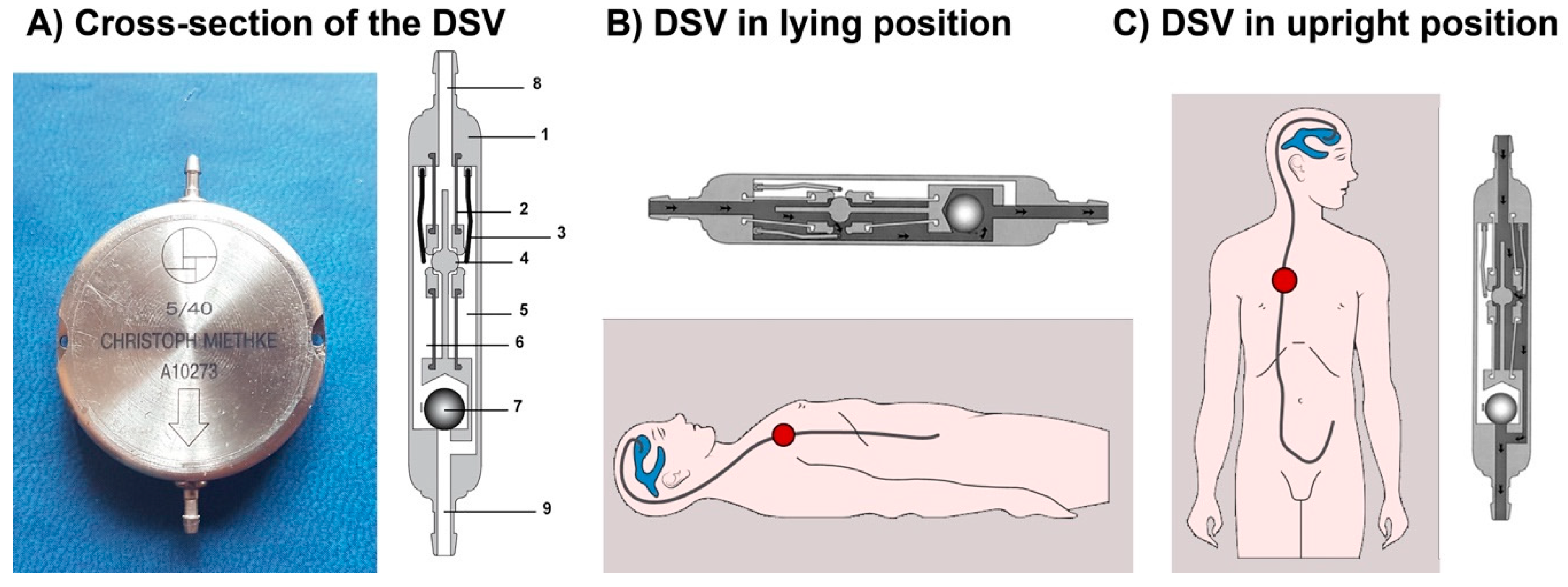
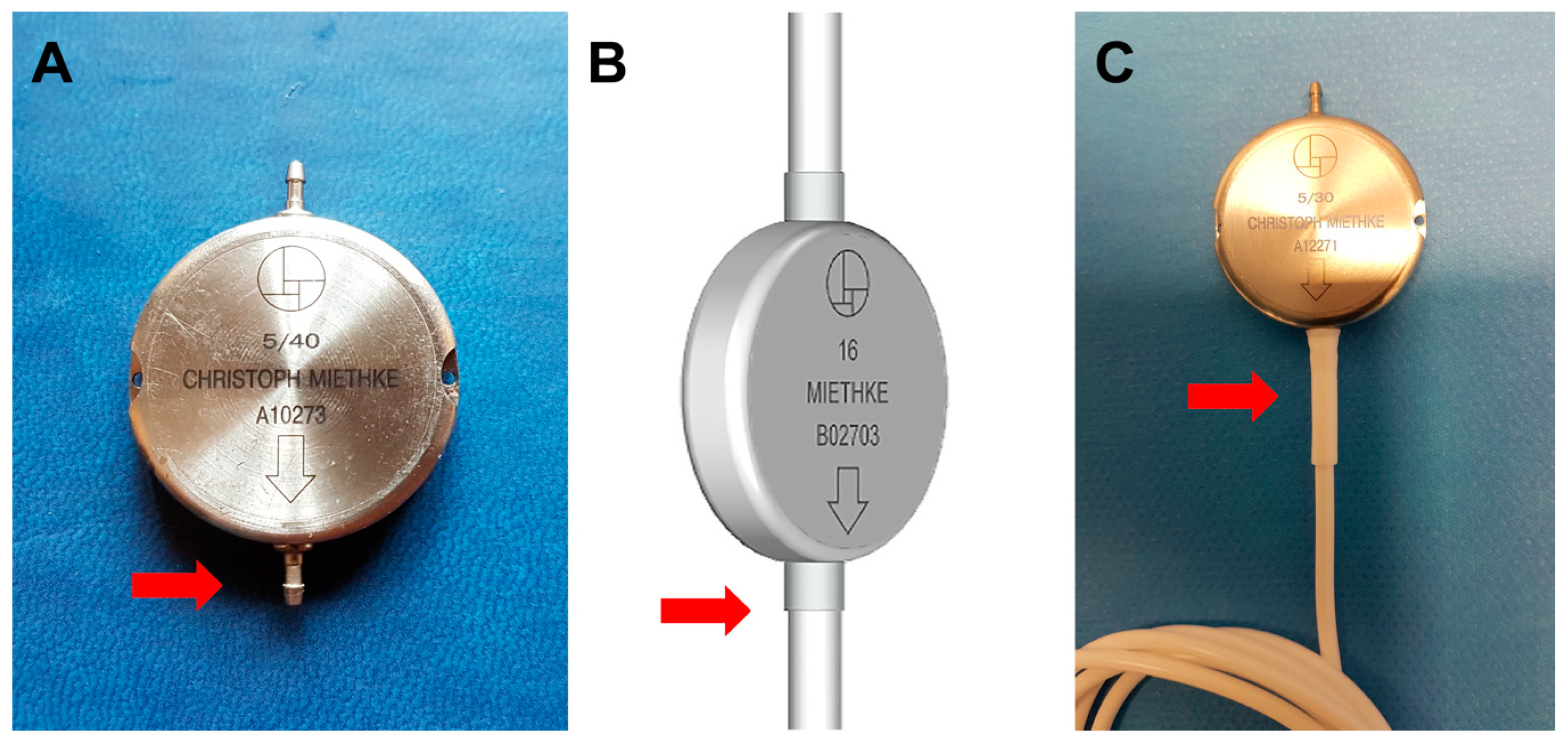
2. The Miethke® Dual-Switch Valve
3. Dual-Switch Valve Performance Reported by the Manufacturer
4. Independent Evaluation of the Performance of the Dual-Switch Valve
5. The Effect of MRI on the Function of M-DSV
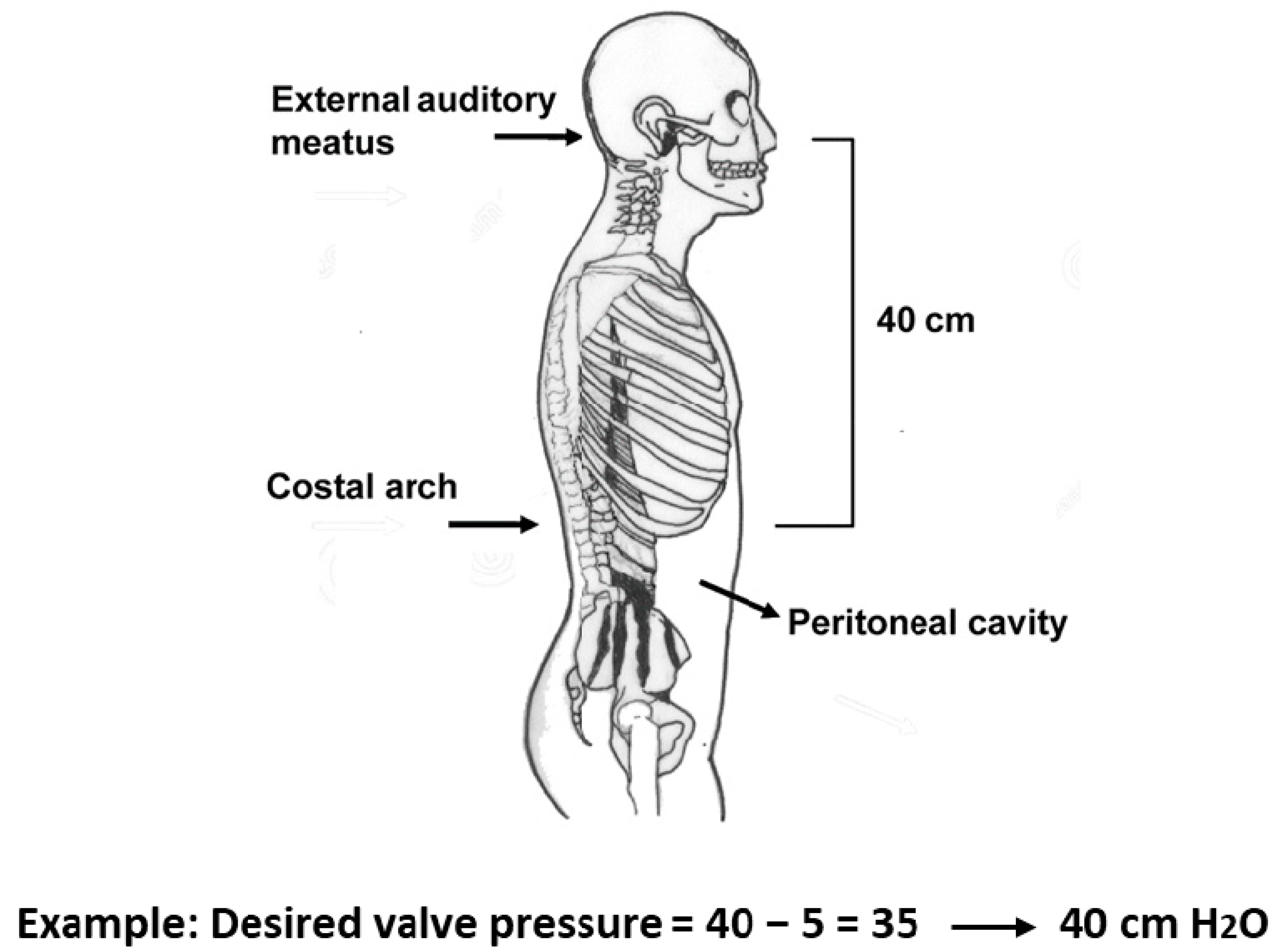
6. Pressure Selection of the Two Valves in the M-DSV
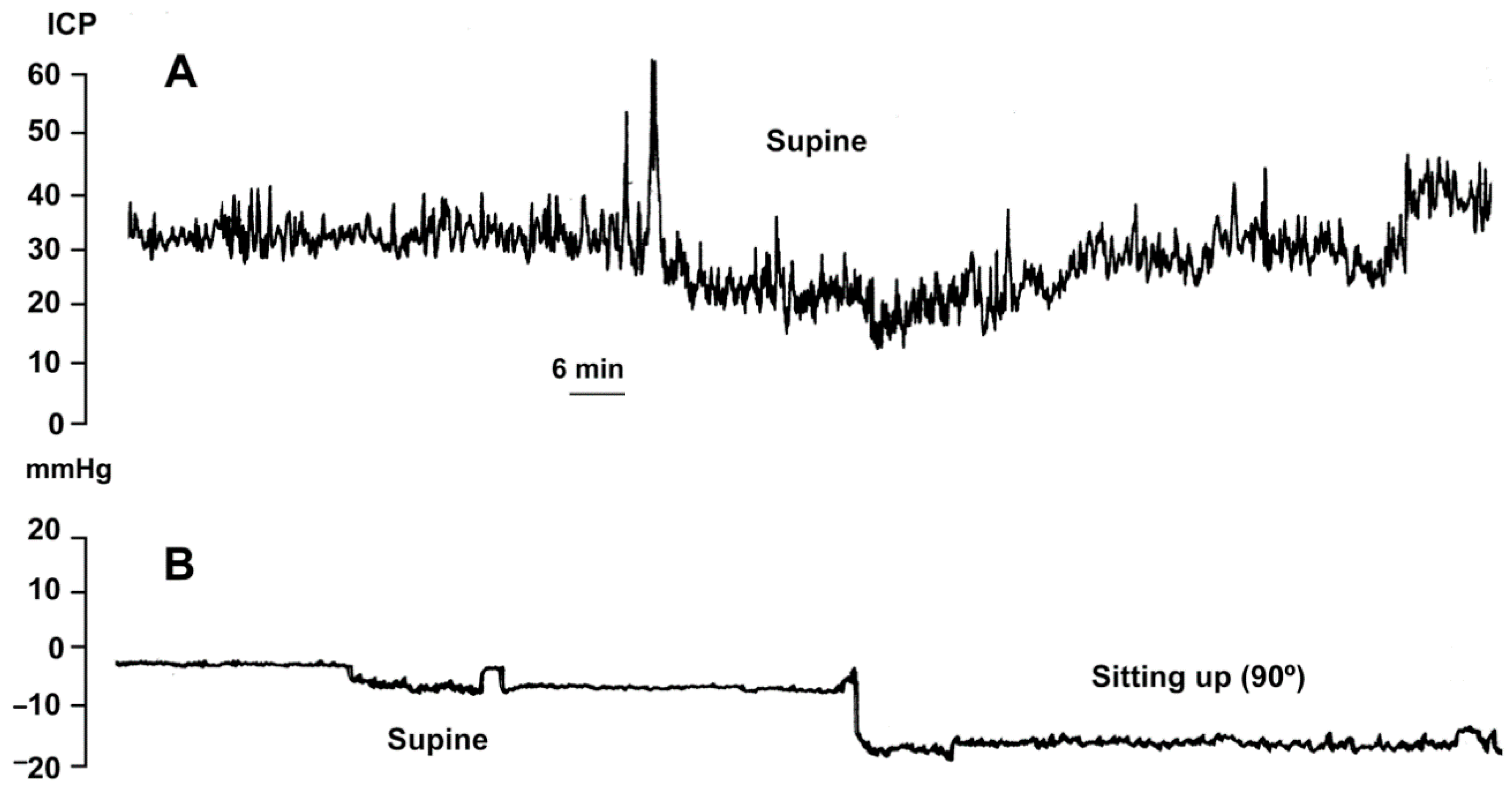
7. Intracranial Pressure Changes after M-DSV Implantation
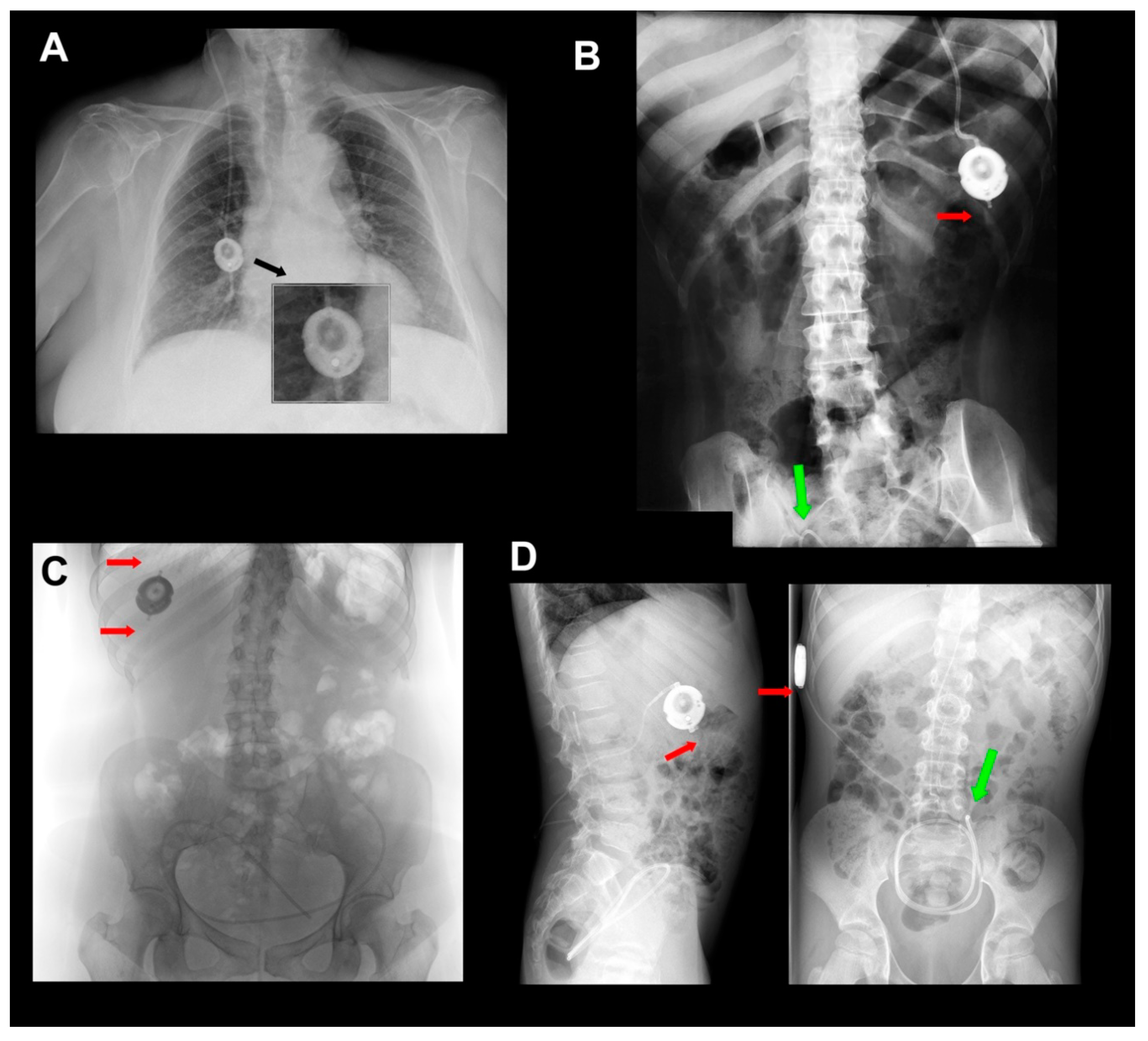
8. Surgical Aspects in the Implantation of the M-DSV
9. Clinical Experience with M-DSV Use
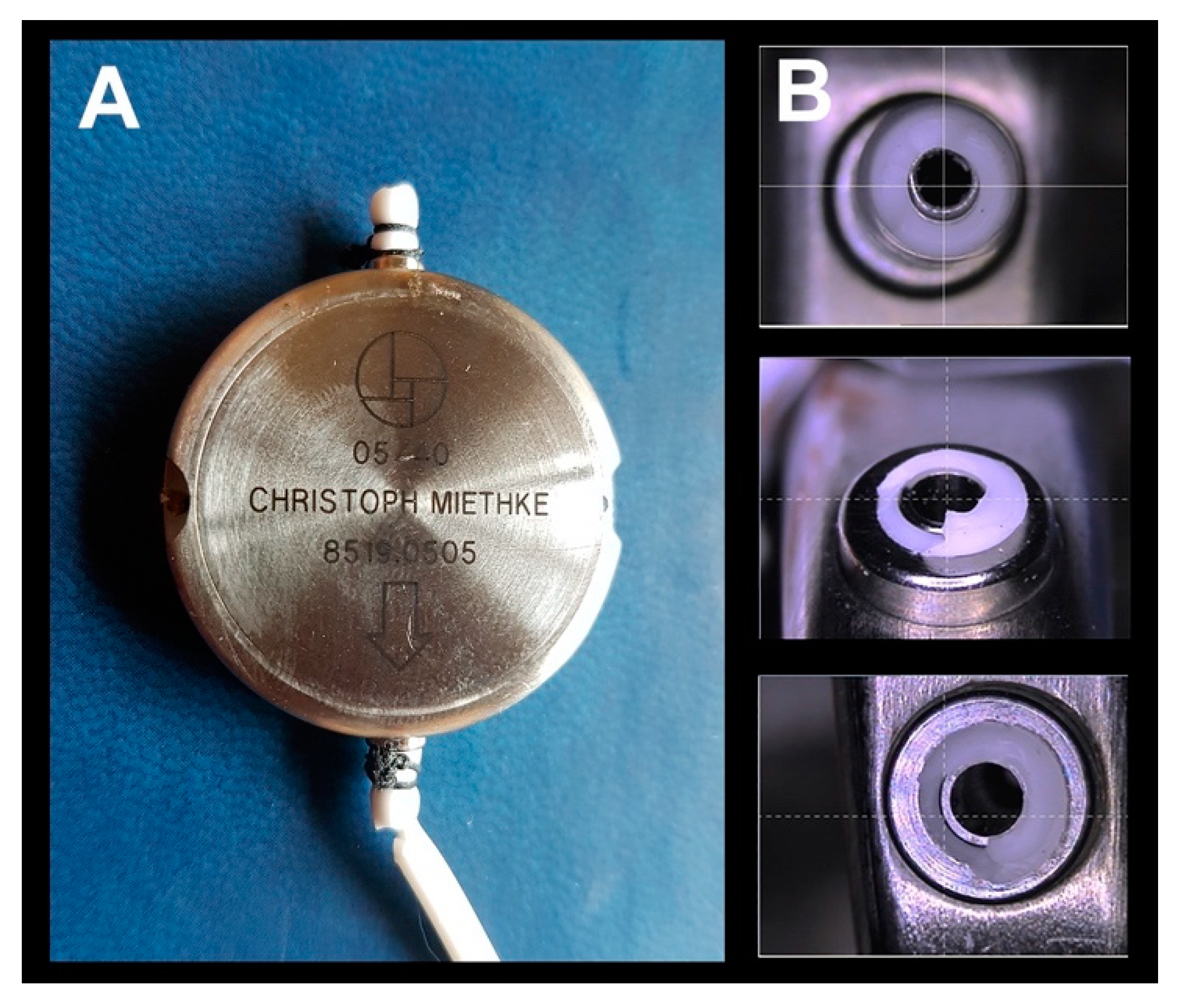
10. The Problem of Catheter Dislocation/Fracture in the M-DSV
11. Final Considerations and Recommendations
- The M-DSV is s hunt device that controls posture-related over-drainage.
- The M-DSV is designed for adults and should not be implanted in children, as they always have greater body mobility than adults.
- When implanting the M-DSV valve, the flattest part of the chest should be selected, and the device correctly fixed to the muscular plane so that the vertical direction of the valve is in line with the vertical axis of the patient’s body.
- Due to the risk of fracture in the catheters at the level of the proximal or distal connectors of the valve, it is advisable to include in the patient’s follow-up periodic radiological controls for ruling out this potential problem.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fox, J.L.; McCullough, D.C.; Green, R.C. Effect of cerebrospinal fluid shunts on intracranial pressure and on cerebrospinal fluid dynamics: 2. A new technique of pressure measurements: Results and concepts 3.A concept of hydrocephalus. J. Neurol. Neurosurg. Psychiatry 1973, 36, 302–312. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aschoff, A.; Kremer, P.; Hashemi, B.; Kunze, S. The scientific history of hydrocephalus and its treatment. Neurosurg. Rev. 1999, 22, 67–93. [Google Scholar] [CrossRef] [PubMed]
- Portnoy, H.D.; Schulte, R.R.; Fox, J.L.; Croissant, P.D.; Tripp, L. Anti-siphon and reversible occlusion valves for shunting in hydrocephalus and preventing post-shunt subdural hematomas. J. Neurosurg. 1973, 38, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Miyake, H. Shunt Devices for the Treatment of Adult Hydrocephalus: Recent Progress and Characteristics. Neurol. Medico-Chirurgica 2016, 56, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Lemcke, J.; Meier, U.; Müller, C.; Fritsch, M.J.; Kehler, U.; Langer, N.; Kiefer, M.; Eymann, R.; Schuhmann, M.U.; Speil, A.; et al. Safety and efficacy of gravitational shunt valves in patients with idiopathic normal pressure hydrocephalus: A pragmatic, randomised, open label, multicentre trial (SVASONA). J. Neurol. Neurosurg. Psychiatry 2013, 84, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Miethke, C.; Affeld, K.A. New Valve for the Treatment of Hydrocephalus—Ein neues Ventil zur Behandlung des Hydrocephalus. Biomed. Tech. Eng. 1994, 39, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Sprung, C.; Miethke, C.; A Trost, H.; Lanksch, W.R.; Stolke, D. The dual-switch valve. A new hydrostatic valve for the treatment of hydrocephalus. Child’s Nerv. Syst. 1996, 12, 573–581. [Google Scholar]
- Drake, J.M.; Da Silva, M.C.; Rutka, J.T. Functional Obstruction of an Antisiphon Device by Raised Tissue Capsule Pressure. Neurosurg. 1993, 32, 137–139. [Google Scholar] [CrossRef]
- Czosnyka, M.; Czosnyka, Z.H.; Pickard, J.D. Evaluation Report of Aesculap-Miethke Dual-Switch and Paedi-GAV Valves; Academic Neurosurgical Unit: Cambridge, UK, 2003; Volume 144, pp. 525–538. [Google Scholar]
- Czosnyka, Z.; Czosnyka, M.; Richards, H.K.; Pickard, J.D. Evaluation of three new models of hydrocephalus shunts. Oper. Neuromodul. 2005, 95, 223–227. [Google Scholar] [CrossRef]
- Chari, A.; Czosnyka, M.; Richards, H.K.; Pickard, J.D.; Czosnyka, Z.H. Hydrocephalus shunt technology: 20 years of experience from the Cambridge Shunt Evaluation Laboratory. J. Neurosurg. 2014, 120, 697–707. [Google Scholar] [CrossRef]
- Czosnyka, Z.; Czosnyka, M.; Pickard, J.D.; Chari, A. Who Needs a Revision? 20 Years of Cambridge Shunt Lab. Oper. Neuromodul. 2016, 122, 347–351. [Google Scholar] [CrossRef]
- Lindner, D.; Preul, C.; Trantakis, C.; Moeller, H.; Meixensberger, J. Effect of 3T MRI on the function of shunt valves—Evaluation of Paedi GAV, Dual Switch and proGAV. Eur. J. Radiol. 2005, 56, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Udayakumaran, S.; Roth, J.; Kesler, A.; Constantini, S. Miethke DualSwitch Valve in lumboperitoneal shunts. Acta Neurochir. 2010, 152, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Hertel, F.; Züchner, M.; Decker, C.; Schill, S.; Bosniak, I.; Bettag, M. The Miethke Dual Switch Valve: Experience in 169 Adult Patients with Different Kinds of Hydrocephalus: An Open Field Study. Minim. Invasive Neurosurg. 2008, 51, 147–153. [Google Scholar] [CrossRef]
- Sahuquillo, J.; Arikan, F.; Poca, M.A.; Noguer, M.; Martínez-Ricarte, F. Intra-abdominal pressure: The neglected variable in selecting the ventriculoperitoneal shunt for treating hydrocephalus. Neurosurgery 2008, 62, 143–150. [Google Scholar] [CrossRef]
- Sprung, C.; Miethke, C.; Schlosser, H.-G.; Brock, M. The enigma of underdrainage in shunting with hydrostatic valves and possible solutions. Oper. Neuromodul. 2005, 95, 229–235. [Google Scholar] [CrossRef]
- Meier, U.; Kiefer, M.; Sprung, C. Evaluation of the Miethke dual- switch valve in patients with normal pressure hydrocephalus. Surg. Neurol. 2004, 61, 119–127. [Google Scholar] [CrossRef]
- Sprung, C.; Miethke, C.; Shaken, K.; Lanksch, W. The Importance of the Dual-Switch Valve for the Treatment of Adult Normotensive or Hypertensive Hydrocephalus. Eur. J. Pediatr. Surg. 1997, 7, 38–40. [Google Scholar] [CrossRef]
- Trost, H.; Sprung, C.; Lanksch, W.; Stolke, D.; Miethke, C. Dual-switch valve: Clinical performance of a new hydrocephalus valve. Oper. Neuromodul. 1998, 71, 360–363. [Google Scholar] [CrossRef]
- Zeilinger, F.S.; Reyer, T.; Meier, U.; Kintzel, D. Clinical experiences with the dual-switch valve in patients with normal pressure hydrocephalus. In Brain Edema XI. Acta Neurochirurgica Supplements; Mendelow, A.D., Baethmann, A., Czernicki, Z., Hoff, J.T., Ito, U., James, H.E., Kuroiwa, T., Marmarou, A., Marshall, L.F., Reulen, H.-J., Eds.; Springer: Vienna, Austria, 2000; Volume 76, pp. 559–562. [Google Scholar]
- Meier, U.; Kintzel, D. Clinical experiences with different valve systems in patients with normal-pressure hydrocephalus: Evaluation of the Miethke dual-switch valve. Childs Nerv. Syst. 2002, 18, 288–294. [Google Scholar] [CrossRef]
- Tsunoda, A.; Maruki, C. Clinical experience with a dual switch valve (Miethke) for the management of adult hydrocephalus. Neurol. Med. Chir. (Tokyo) 2007, 47, 403–408. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tsunoda, A.; Mitsuoka, H.; Sato, K.; Kanayama, S. A quantitative index of intracranial cerebrospinal fluid distribution in normal pressure hydrocephalus using an MRI-based processing technique. Neuroradiology 2000, 42, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, M.; Eymann, R. Gravitational shunt complications after a five-year follow-up. Acta Neurochir. Suppl. 2010, 106, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Boch, A.L.; Hermelin, E.; Sainte-Rose, C.; Sgouros, S. Mechanical dysfunction of ventriculoperitoneal shunts caused by calcification of the silicone rubber catheter. J. Neurosurg. 1998, 88, 975–982. [Google Scholar] [CrossRef]
- Aschoff, A.; Kremer, P.; Benesch, C.; Fruh, K.; Klank, A.; Kunze, S. Overdrainage and shunt technology. Child’s Nerv. Syst. 1995, 11, 193–202. [Google Scholar] [CrossRef]
| Pressure Settings for Ventriculoperitoneal Shunts | |||
|---|---|---|---|
| Low Pressure (Supine Position) mm H2O | High Pressure (Upright Position) mm H2O | Low Pressure (Supine Position) mm Hg | High Pressure (Upright Position) mm Hg |
| 5 * | 30 | 3.7 | 22.0 |
| 5 * | 40 | 3.7 | 29.4 |
| 5 * | 50 | 3.7 | 36.8 |
| 10 | 30 | 7.4/(7 ± 0.6) ** | 22.0/(22 ± 2.1) ** |
| 13 | 30 | 9.5/(9.5 ± 0.4) ** | 22.0 |
| 16 | 30 | 11.8/(12 ± 1.1) ** | 22.0 |
| 10 | 40 | 7.4 | 29.4/(28 ± 1.9) ** |
| 13 | 40 | 9.5 | 29.4 |
| 16 | 40 | 11.8 | 29.4 |
| 10 | 50 | 7.4 | 36.8/(38 ± 2.2) ** |
| 13 | 50 | 9.5 | 36.8 |
| 16 | 50 | 11.8 | 36.8 |
| Pressure Settings for Lumboperitoneal Shunts | |||
| 10 | 30 | 7.4 | 22.0 |
| 10 | 40 | 7.4 | 29.4 |
| 10 | 50 | 7.4 | 36.8 |
| Mechanical Durability | All the shunts showed mechanical durability over the period of testing (>48 days) and good stability of hydrodynamic performance over a 28-day period. |
| Opening and Closing Pressures | Very well defined and stable in time, linearly changing with performance level. The distal catheter did not change the opening and closing pressure significantly. |
| Pressure-Flow Performance Curves (PFPC) | The PFPC, operating, opening, and closing pressures were stable, with minimal scatter, and were in accordance with data reported by the manufacturer for valves working in both horizontal and vertical positions. |
| Hysteresis of the PFPC | There was no hysteresis of PFPC. Slight hysteresis (but very narrow, 1 mm Hg of width) appeared after connection of the distal catheter. |
| Pressure Changes Related to Body Posture | The valves showed increased stepwise operational pressure in vertical position when compared to horizontal position, according to its fixed parameters. |
| Hydrodynamic Resistance | The valves have rather low and stable hydrodynamic resistance (2.2 ± 0.8 mm Hg/mL/min) and therefore are able to stabilize ICP according to the fixed settings. The addition of a distal catheter increases the shunt resistance to 2.9 ± 1.1 mm Hg/mL/min. |
| Effect of Particles in Reagent | Small particles (10 microns diameter) temporarily increased the valve’s hydrodynamic resistance to 7 mm Hg/mL/min. Large microspheres (with a diameter greater than erythrocytes—25 µm) were found to open the shunt temporarily (closing pressure decreased from around 9 to 3 mm Hg). In both cases, these effects were only temporary when the particles were washed out spontaneously and on the next day the valve returned to normal performance. |
| ISO/DIS 7197 Compliances | The M-DSV complied with this international standard for the testing of hydrocephalus valves. |
| Resistance to Breakage and Leakage of Preassembled Junctions | Preassembled junctions did not break when a test specimen was subjected to a load of 1 Kg of force for 1 min. All junctions remained free from leakage when the water pressure was increased to 3 kPa (about 25 mm Hg). |
| Reflux | The valves did not show any reflux when tested according to the ISO standard. Valves did not exhibit flow reversal for an outlet-inlet differential pressure of up to 100 mm Hg. |
| Magnetic Influence | Magnetic field 3T did not have any influence on the shunt’s performance. |
| External Pressure Influence | No change in operating pressure occurred when an environmental pressure of 7 mm Hg was applied. |
| Temperature Influence | None of the parameters (opening, closing pressure, and resistance) were altered by a temperature change of 30 °C to 40 °C. |
| Abbreviation | Complete Name | Distributed by: |
|---|---|---|
| CSV | Cordis-Standard differential-pressure valve | INTEGRA LIFESCIENCES CORPORATION, Ratingen, Germany |
| DPV | Differential-pressure valve | |
| GAV | MIETHKE® gravitational valve | Aesculap, AG, Tuttlingen, Germany |
| PaediGAV * | Pediatric MIETHKE® gravitational valve | Aesculap, AG, Tuttlingen, Germany |
| M-DSV | Miethke® Dual-Switch valve | Aesculap, AG, Tuttlingen, Germany |
| OSV | Orbis Sigma valve | INTEGRA LIFESCIENCES CORPORATION, Ratingen, Germany |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poca, M.A.; Gándara, D.F.; Rosas, K.; Alcina, A.; López-Bermeo, D.; Sahuquillo, J. Considerations in the Use of Gravitational Valves in the Management of Hydrocephalus. Some Lessons Learned with the Dual-Switch Valve. J. Clin. Med. 2021, 10, 246. https://doi.org/10.3390/jcm10020246
Poca MA, Gándara DF, Rosas K, Alcina A, López-Bermeo D, Sahuquillo J. Considerations in the Use of Gravitational Valves in the Management of Hydrocephalus. Some Lessons Learned with the Dual-Switch Valve. Journal of Clinical Medicine. 2021; 10(2):246. https://doi.org/10.3390/jcm10020246
Chicago/Turabian StylePoca, Maria A., Dario F. Gándara, Katiuska Rosas, Aloma Alcina, Diego López-Bermeo, and Juan Sahuquillo. 2021. "Considerations in the Use of Gravitational Valves in the Management of Hydrocephalus. Some Lessons Learned with the Dual-Switch Valve" Journal of Clinical Medicine 10, no. 2: 246. https://doi.org/10.3390/jcm10020246
APA StylePoca, M. A., Gándara, D. F., Rosas, K., Alcina, A., López-Bermeo, D., & Sahuquillo, J. (2021). Considerations in the Use of Gravitational Valves in the Management of Hydrocephalus. Some Lessons Learned with the Dual-Switch Valve. Journal of Clinical Medicine, 10(2), 246. https://doi.org/10.3390/jcm10020246







