Structural and Functional Characteristics of Mitral Paravalvular Leakage Identified by Multimodal Imaging and Their Implication on Clinical Presentation
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Echocardiography
2.3. Cardiac CT Acquisition
2.4. Cardiac CT Analysis
2.5. Clinical Presentation
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
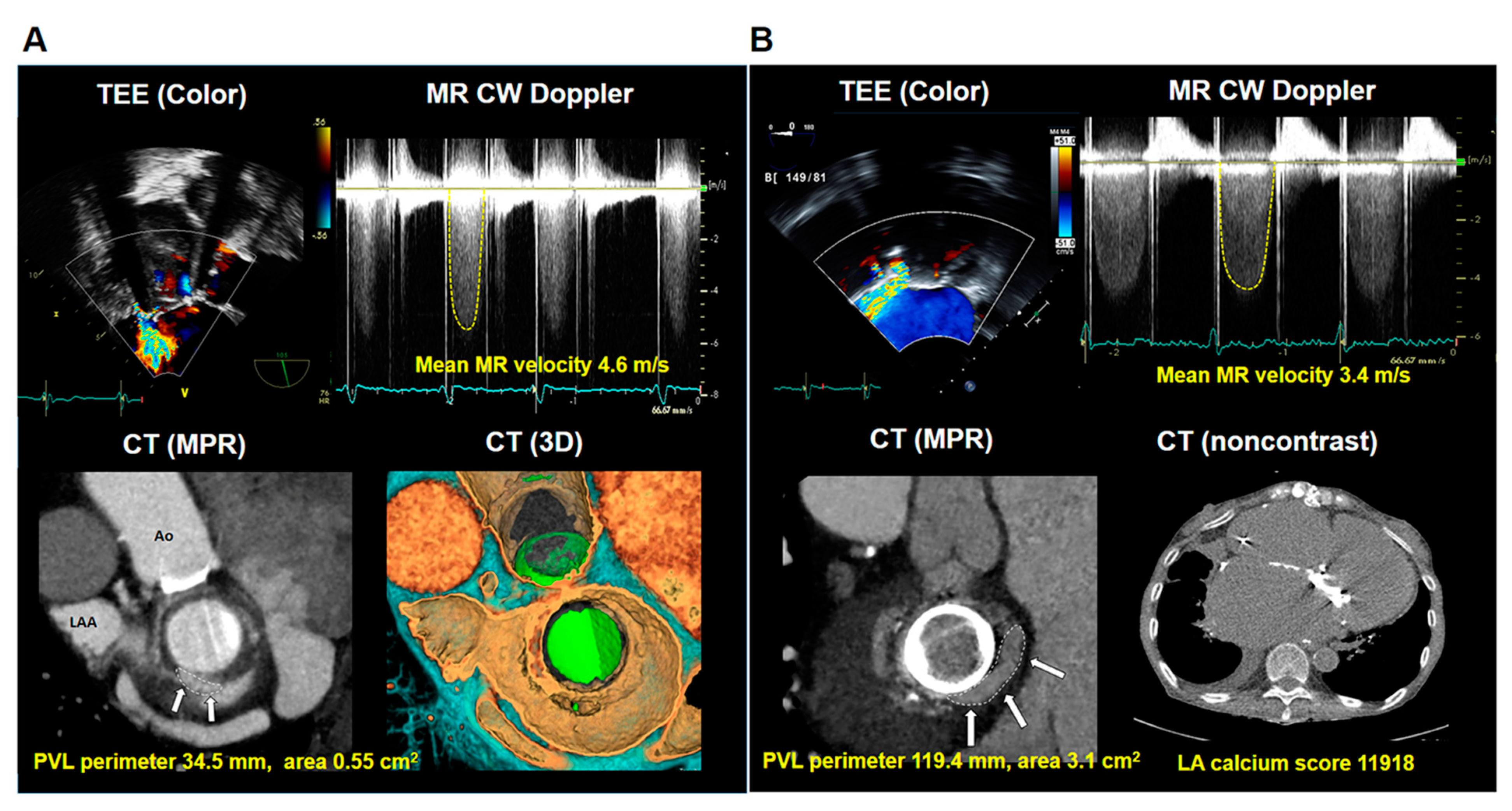
3.2. Echocardiographic and Cardiac CT Characteristics
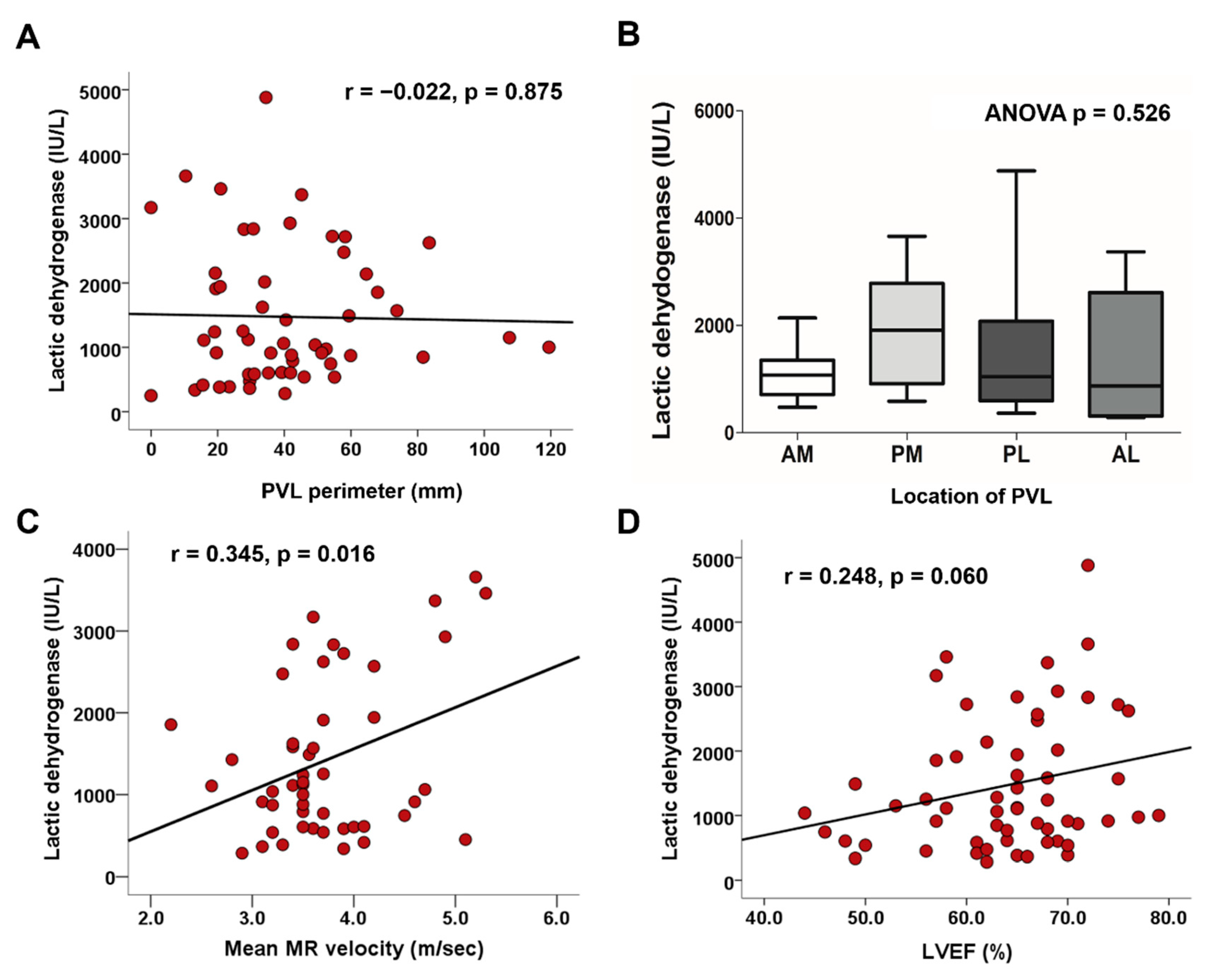
3.3. Factors Associated with Hemolysis
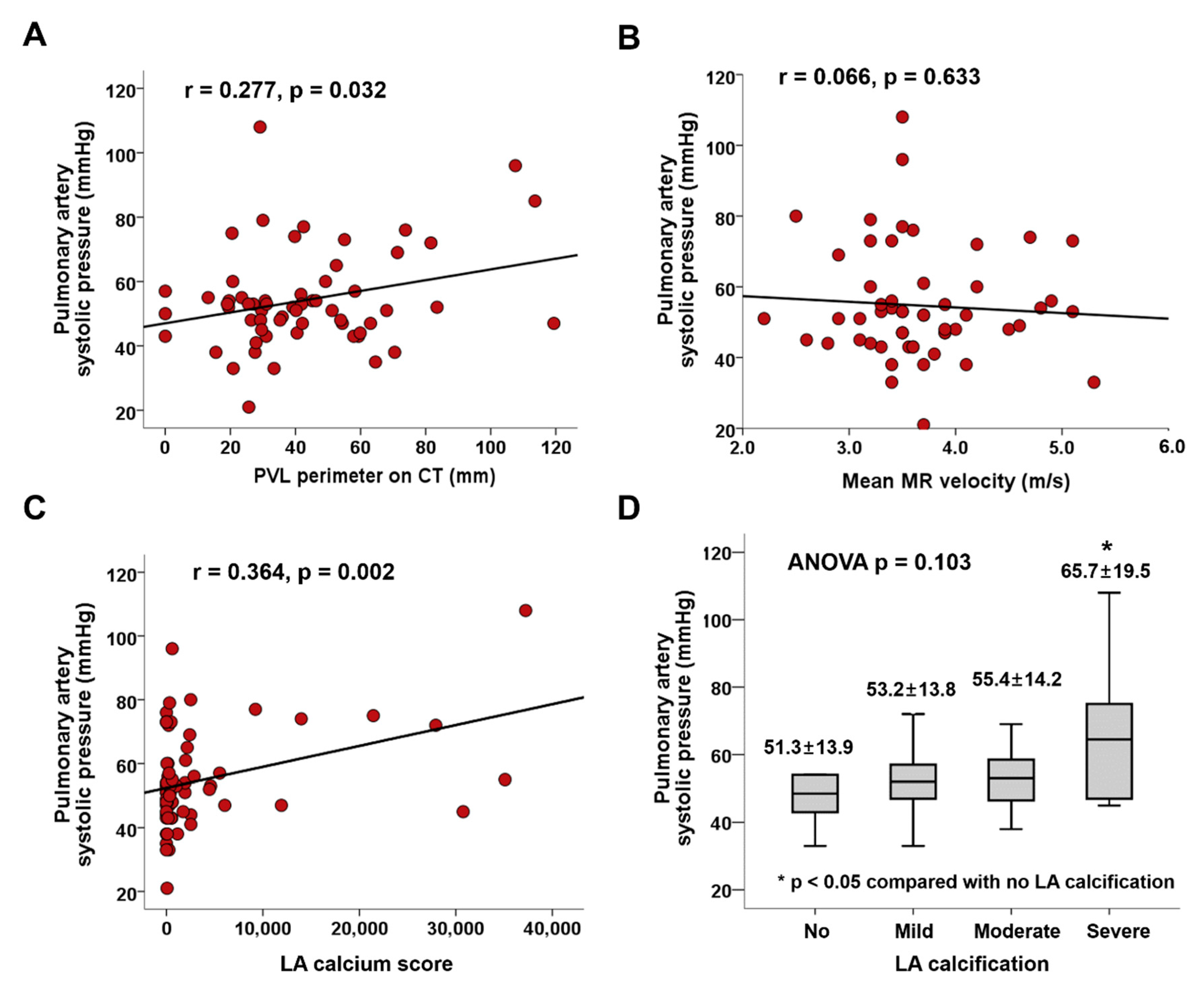
3.4. Factors Associated with Pulmonary Hypertension
4. Discussion
4.1. Study Limitations
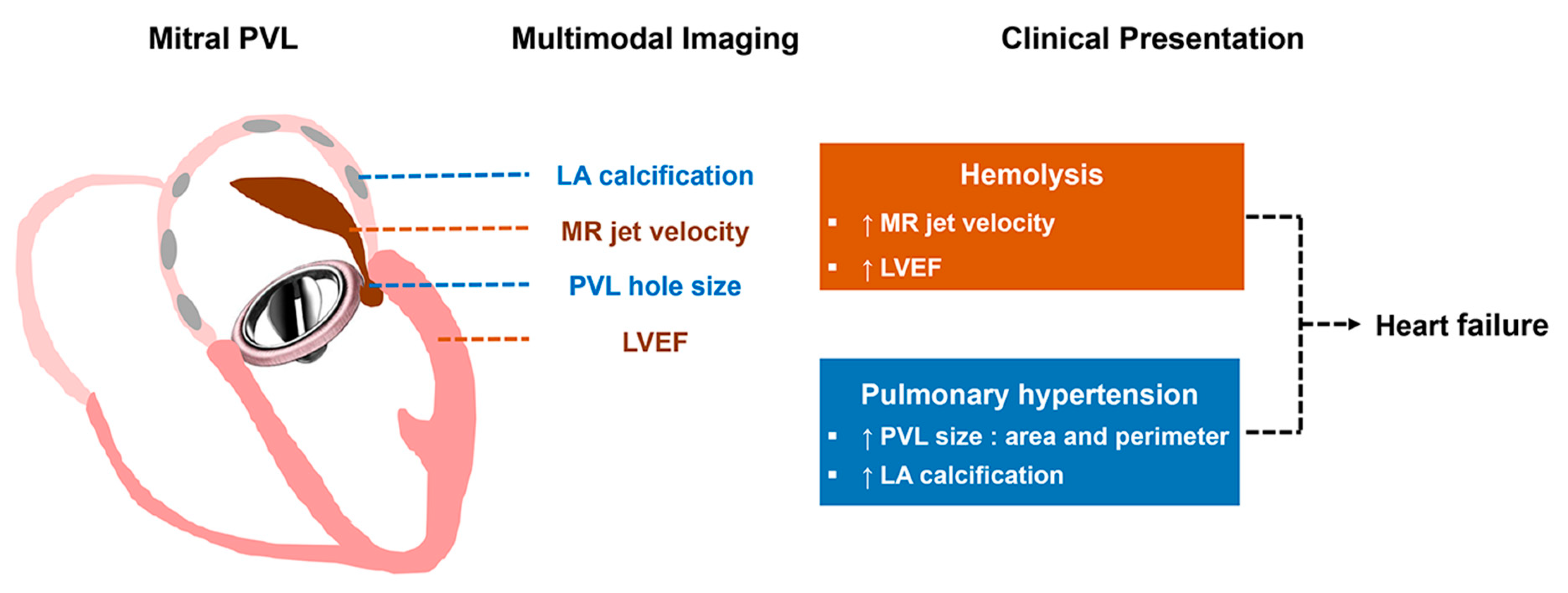
4.2. Clinical Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kliger, C.; Eiros, R.; Isasti, G.; Einhorn, B.; Jelnin, V.; Cohen, H.; Kronzon, I.; Perk, G.; Fontana, G.P.; Ruiz, C.E. Review of surgical prosthetic paravalvular leaks: Diagnosis and catheter-based closure. Eur. Heart J. 2013, 34, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.J.; Moon, J.; Shim, C.Y.; Jang, Y.; Chung, N.; Chang, B.C.; Ha, J.W. Different clinical outcome of paravalvular leakage after aortic or mitral valve replacement. Am. J. Cardiol. 2011, 107, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Taramasso, M.; Maisano, F.; Latib, A.; Denti, P.; Guidotti, A.; Sticchi, A.; Panoulas, V.; Giustino, G.; Pozzoli, A.; Buzzatti, N.; et al. Conventional surgery and transcatheter closure via surgical transapical approach for paravalvular leak repair in high-risk patients: Results from a single-centre experience. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 1161–1167. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Akins, C.W.; Bitondo, J.M.; Hilgenberg, A.D.; Vlahakes, G.J.; Madsen, J.C.; MacGillivray, T.E. Early and late results of the surgical correction of cardiac prosthetic paravalvular leaks. J. Heart Valve Dis. 2005, 14, 792–799; discussion 799–800. [Google Scholar] [PubMed]
- Taramasso, M.; Maisano, F.; Denti, P.; Guidotti, A.; Sticchi, A.; Pozzoli, A.; Buzzatti, N.; De Bonis, M.; La Canna, G.; Alfieri, O. Surgical treatment of paravalvular leak: Long-term results in a single-center experience (up to 14 years). J. Thorac. Cardiovasc. Surg. 2015, 149, 1270–1275. [Google Scholar] [CrossRef]
- Kim, I.C.; Chang, S.; Hong, G.R.; Lee, S.H.; Lee, S.; Ha, J.W.; Chang, B.C.; Kim, Y.J.; Shim, C.Y. Comparison of Cardiac Computed Tomography with Transesophageal Echocardiography for Identifying Vegetation and Intracardiac Complications in Patients with Infective Endocarditis in the Era of 3-Dimensional Images. Circ. Cardiovasc. Imaging 2018, 11, e006986. [Google Scholar] [CrossRef]
- Suh, Y.J.; Hong, G.R.; Han, K.; Im, D.J.; Chang, S.; Hong, Y.J.; Lee, H.J.; Hur, J.; Choi, B.W.; Chang, B.C.; et al. Assessment of Mitral Paravalvular Leakage After Mitral Valve Replacement Using Cardiac Computed Tomography: Comparison With Surgical Findings. Circ. Cardiovasc. Imaging 2016, 9. [Google Scholar] [CrossRef]
- Eleid, M.F.; Cabalka, A.K.; Malouf, J.F.; Sanon, S.; Hagler, D.J.; Rihal, C.S. Techniques and Outcomes for the Treatment of Paravalvular Leak. Circ. Cardiovasc. Interv. 2015, 8, e001945. [Google Scholar] [CrossRef]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Munoz, D.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; Edvardsen, T.; Delgado, V.; Dulgheru, R.; Pepi, M.; Cosyns, B.; Dweck, M.R.; Garbi, M.; et al. Recommendations for the imaging assessment of prosthetic heart valves: A report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 589–590. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Chambers, J.B.; Dumesnil, J.G.; Foster, E.; Gottdiener, J.S.; Grayburn, P.A.; Khandheria, B.K.; Levine, R.A.; Marx, G.R.; Miller, F.A., Jr.; et al. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2009, 22, 975–1014; quiz 1082–1014. [Google Scholar] [CrossRef] [PubMed]
- Mauro, F.R.; Foa, R.; Cerretti, R.; Giannarelli, D.; Coluzzi, S.; Mandelli, F.; Girelli, G. Autoimmune hemolytic anemia in chronic lymphocytic leukemia: Clinical, therapeutic, and prognostic features. Blood 2000, 95, 2786–2792. [Google Scholar] [CrossRef] [PubMed]
- Sorajja, P.; Cabalka, A.K.; Hagler, D.J.; Rihal, C.S. Percutaneous repair of paravalvular prosthetic regurgitation: Acute and 30-day outcomes in 115 patients. Circ. Cardiovasc. Interv. 2011, 4, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013, 128, e240–e327. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., III; Guyton, R.A.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, e57–e185. [Google Scholar] [CrossRef] [PubMed]
- Said, S.M.; Schaff, H.V.; Greason, K.L.; Pochettino, A.; Daly, R.C.; Dearani, J.A. Reoperation for mitral paravalvular leak: A single-centre experience with 200 patients. Interact. Cardiovasc. Thorac. Surg. 2017, 25, 806–812. [Google Scholar] [CrossRef]
- Yang, C.; Liu, Y.; Tang, J.; Jin, P.; Li, L.; Yu, S.; Yang, J. Prognosis of Transcatheter Closure Compared with Surgical Repair of Paravalvular Leak after Prosthetic Valve Replacement: A Retrospective Comparison. Thorac. Cardiovasc. Surg. 2018. [Google Scholar] [CrossRef]
- Alkhouli, M.; Zack, C.J.; Sarraf, M.; Eleid, M.F.; Cabalka, A.K.; Reeder, G.S.; Hagler, D.J.; Maalouf, J.F.; Nkomo, V.T.; Rihal, C.S. Successful Percutaneous Mitral Paravalvular Leak Closure Is Associated With Improved Midterm Survival. Circ. Cardiovasc. Interv. 2017, 10. [Google Scholar] [CrossRef]
- Garcia, E.; Arzamendi, D.; Jimenez-Quevedo, P.; Sarnago, F.; Marti, G.; Sanchez-Recalde, A.; Lasa-Larraya, G.; Sancho, M.; Iniguez, A.; Goicolea, J.; et al. Outcomes and predictors of success and complications for paravalvular leak closure: An analysis of the SpanisH real-wOrld paravalvular LEaks closure (HOLE) registry. EuroIntervention 2017, 12, 1962–1968. [Google Scholar] [CrossRef] [PubMed]
- Giblett, J.P.; Rana, B.S.; Shapiro, L.M.; Calvert, P.A. Percutaneous management of paravalvular leaks. Nat. Rev. Cardiol. 2019, 16, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Sorajja, P.; Cabalka, A.K.; Hagler, D.J.; Rihal, C.S. Long-term follow-up of percutaneous repair of paravalvular prosthetic regurgitation. J. Am. Coll. Cardiol. 2011, 58, 2218–2224. [Google Scholar] [CrossRef] [PubMed]
- Hein, R.; Wunderlich, N.; Robertson, G.; Wilson, N.; Sievert, H. Catheter closure of paravalvular leak. EuroIntervention 2006, 2, 318–325. [Google Scholar] [PubMed]
- Pysz, P.; Kozlowski, M.; Malczewska, M.; Adamczyk-Filipek, E.; Wojakowski, W.; Smolka, G. Prospective registry validating the reproducibility of mitral paravalvular leak measurements in a standardized real-time three-dimensional transesophageal echocardiography algorithm for optimal choice of the closure device. Postepy Kardiol. Interwencyjnej 2019, 15, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Franco, E.; Almeria, C.; de Agustin, J.A.; Arreo Del Val, V.; Gomez de Diego, J.J.; Garcia Fernandez, M.A.; Macaya, C.; Perez de Isla, L.; Garcia, E. Three-dimensional color Doppler transesophageal echocardiography for mitral paravalvular leak quantification and evaluation of percutaneous closure success. J. Am. Soc. Echocardiogr. 2014, 27, 1153–1163. [Google Scholar] [CrossRef]
- Hascoet, S.; Smolka, G.; Bagate, F.; Guihaire, J.; Potier, A.; Hadeed, K.; Lavie-Badie, Y.; Bouvaist, H.; Dauphin, C.; Bauer, F.; et al. Multimodality imaging guidance for percutaneous paravalvular leak closure: Insights from the multi-centre FFPP register. Arch. Cardiovasc. Dis. 2018, 111, 421–431. [Google Scholar] [CrossRef]
- Ruiz, C.E.; Hahn, R.T.; Berrebi, A.; Borer, J.S.; Cutlip, D.E.; Fontana, G.; Gerosa, G.; Ibrahim, R.; Jelnin, V.; Jilaihawi, H.; et al. Clinical Trial Principles and Endpoint Definitions for Paravalvular Leaks in Surgical Prosthesis: An Expert Statement. J. Am. Coll. Cardiol. 2017, 69, 2067–2087. [Google Scholar] [CrossRef]
- Garcia, M.J.; Vandervoort, P.; Stewart, W.J.; Lytle, B.W.; Cosgrove, D.M.; Thomas, J.D.; Griffin, B.P. Mechanisms of hemolysis with mitral prosthetic regurgitation study using transesophageal echocardiography and fluid dynamic simulation. J. Am. Coll. Cardiol. 1996, 27, 399–406. [Google Scholar] [CrossRef]
- O’Neill, A.C.; Martos, R.; Murtagh, G.; Ryan, E.R.; McCreery, C.; Keane, D.; Quinn, M.; Dodd, J.D. Practical tips and tricks for assessing prosthetic valves and detecting paravalvular regurgitation using cardiac CT. J. Cardiovasc. Comput. Tomogr. 2014, 8, 323–327. [Google Scholar] [CrossRef]
- Koo, H.J.; Lee, J.Y.; Kim, G.H.; Kang, J.W.; Kim, Y.H.; Kim, D.H.; Song, J.M.; Kang, D.H.; Song, J.K.; Lim, T.H.; et al. Paravalvular leakage in patients with prosthetic heart valves: Cardiac computed tomography findings and clinical features. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.C.; Humphries, J.O.; Morrow, A.G. Giant right atrium in rheumatic mitral stenosis. Atrial enlargement restricted by mural calcification. Am. Heart J. 1970, 79, 28–35. [Google Scholar] [CrossRef]
- Lee, W.J.; Son, C.W.; Yoon, J.C.; Jo, H.S.; Son, J.W.; Park, K.H.; Lee, S.H.; Shin, D.G.; Hong, G.R.; Park, J.S.; et al. Massive left atrial calcification associated with mitral valve replacement. J. Cardiovasc. Ultrasound. 2010, 18, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Suh, Y.J.; Kim, Y.J.; Lee, S.H.; Lee, S.; Hong, G.R.; Ha, J.W.; Shim, C.Y. Characteristics and Implications of Left Atrial Calcium on Cardiac Computed Tomography in Patients with Earlier Mitral Valve Operation. Am. J. Cardiol. 2020, 128, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Horstkotte, D.; Aul, C.; Seipel, L.; Körfer, R.; Budde, T.; Schulte, H.D.; Bircks, W.; Loogen, F. Effect of valve type and valve function on chronic intravascular hemolysis after alloprosthetic mitral and aortic valve replacement. Z. Kardiol. 1983, 72, 119–131. [Google Scholar] [PubMed]
- Smolka, G.; Pysz, P.; Ochala, A.; Kozlowski, M.; Zasada, W.; Parma, Z.; Tendera, M.; Wojakowski, W. Transcatheter paravalvular leak closure and hemolysis—A prospective registry. Arch. Med. Sci. 2017, 13, 575–584. [Google Scholar] [CrossRef]
| Variables | n = 74 |
|---|---|
| Age, year | 62.9 ± 9.0 |
| Male, n (%) | 31 (41.9) |
| Hypertension, n (%) | 42 (56.8) |
| Diabetes mellitus, n (%) | 18 (24.3) |
| Dyslipidemia, n (%) | 23 (31.1) |
| Chronic kidney disease, n (%) | 13 (17.6) |
| End-stage renal disease, n (%) | 4 (5.4) |
| Atrial fibrillation, n (%) | 62 (83.8) |
| Warfarin use, n (%) | 74 (100.0) |
| Statin use, n (%) | 26 (35.1) |
| Type of MV surgery, n (%) | |
| MV repair | 1 (1.4) |
| MV replacement, bioprosthetic valve | 6 (8.1) |
| MV replacement, mechanical valve | 67 (90.5) |
| Number of MV surgery, n (%) | |
| Once | 47 (63.5) |
| Twice | 24 (32.4) |
| Three times or more | 3 (4.1) |
| Time to last MV surgery to diagnosis of PVL, year | 16.6 ± 8.2 |
| Concomitant surgery, n (%) | |
| Aortic valve replacement | 19 (25.7) |
| Tricuspid valve surgery | 15 (20.3) |
| Coronary artery bypass graft | 2 (2.7) |
| Symptoms of heart failure, n (%) | 50 (70.3) |
| NYHA I | 3 (4.1) |
| NYHA II | 28 (37.8) |
| NYHA III | 13 (17.6) |
| NYHA IV | 8 (10.8) |
| Hemoglobin (g/dL) | 8.4 ± 1.8 |
| LDH (IU/L) | 1450 ± 1022 |
| Total bilirubin (mg/dL) | 3.3 ± 2.9 |
| Transfusion requirement, n (%) | 31 (41.9) |
| Creatinine (mg/dL) | 1.08 ± 0.92 |
| NT-pro BNP (pg/mL) | 1465 ± 1799 |
| Variables | n = 74 |
|---|---|
| Echocardiographic characteristics | |
| LV end-diastolic dimension, mm | 52.6 ± 7.4 |
| LV end-systolic dimension, mm | 35.2 ± 6.3 |
| LV ejection fraction (%) | 64.2 ± 8.0 |
| LA volume index, mL/m2 | 128.6 ± 99.1 |
| MV MDPG, mmHg | 6.4 ± 2.5 |
| PVL severity, n (%) | |
| Mild | 40 (54.1) |
| Moderate | 24 (32.4) |
| Severe | 10 (13.5) |
| MR velocity through the PVL, m/s | |
| Peak | 5.4 ± 0.9 |
| Mean | 3.7 ± 0.7 |
| SPAP, mmHg | 55.2 ± 15.2 |
| CT characteristics | |
| PVL hole area, cm2 | 0.83 ± 0.87 |
| PVL perimeter, mm | 41.3 ± 24.8 |
| PVL location, n (%) | |
| Anteromedial | 13 (17.6) |
| Posteromedial | 15 (20.3) |
| Posterolateral | 33 (44.6) |
| Anterolateral | 5 (6.8) |
| Multiple PVL | 4 (5.4) |
| LA calcification | |
| No, n (%) | 20 (27.0) |
| Mild, n (%) | 25 (33.8) |
| Moderate, n (%) | 17 (23.0) |
| Severe, n (%) | 10 (13.5) |
| LA calcium score | 3507 ± 8057 |
| Hemolysis: LDH | Univariable | Multivariable * | ||||
|---|---|---|---|---|---|---|
| β | t | p Value | β | t | p Value | |
| Age, year | −0.086 | −0.660 | 0.512 | |||
| Female sex | −0.041 | −0.319 | 0.751 | |||
| Presence of atrial fibrillation | −0.081 | −0.623 | 0.536 | |||
| LV ejection fraction, % | 0.248 | 1.916 | 0.060 | 0.243 | 1.793 | 0.080 |
| Previous mechanical MV | 6.007 | 0.012 | 0.990 | |||
| Time to last MV surgery to diagnosis of PVL, year | −20.272 | −1.284 | 0.204 | |||
| Multiple PVL | 0.030 | 0.231 | 0.818 | |||
| PVL location (posterolateral) | 0.003 | 0.025 | 0.980 | |||
| PVL hole area, cm2 | 0.036 | 0.262 | 0.794 | |||
| PVL perimeter, mm | −0.022 | −0.158 | 0.875 | |||
| Peak MR velocity, m/s | 0.271 | 1.912 | 0.062 | |||
| Mean MR velocity, m/s | 0.345 | 2.495 | 0.016 | 0.345 | 2.495 | 0.016 |
| Mean diastolic pressure gradient, mmHg | −9.320 | −0.187 | 0.852 | |||
| LA volume index, ml/m2 | −0.094 | −0.709 | 0.482 | |||
| LA calcium score | −0.116 | −0.899 | 0.372 | |||
| Heart Failure: PASP | Univariable | Multivariable * | ||||
|---|---|---|---|---|---|---|
| β | t | p Value | β | t | p Value | |
| Age, year | 0.017 | 0.142 | 0.888 | |||
| Female sex | 0.122 | 1.001 | 0.321 | |||
| Presence of atrial fibrillation | 0.124 | 1.006 | 0.318 | |||
| LV ejection fraction, % | 0.136 | 1.106 | 0.273 | |||
| Previous mechanical MV | 5.199 | 0.853 | 0.396 | |||
| Time to last MV surgery to diagnosis of PVL, year | 0.071 | 0.305 | 0.761 | |||
| Multiple PVL | 0.153 | 1.235 | 0.221 | |||
| PVL location (posterolateral) | −0.202 | −1.675 | 0.099 | |||
| PVL hole area, cm2 | 0.272 | 2.156 | 0.035 | |||
| PVL perimeter, mm | 0.277 | 2.199 | 0.032 | 0.249 | 2.151 | 0.036 |
| Peak MR velocity, m/s | 0.034 | 0.249 | 0.804 | |||
| Mean MR velocity, m/s | −0.066 | −0.480 | 0.633 | |||
| Mean diastolic pressure gradient, mmHg | 0.100 | 0.150 | 0.881 | |||
| LA volume index, ml/m2 | −0.099 | −0.800 | 0.427 | |||
| LA calcium score | 0.364 | 3.147 | 0.002 | 0.467 | 3.992 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.Y.; Suh, Y.J.; Seo, J.; Choi, K.-U.; Hong, G.-R.; Lee, S.; Lee, S.-H.; Ha, J.-W.; Kim, Y.J.; Shim, C.Y. Structural and Functional Characteristics of Mitral Paravalvular Leakage Identified by Multimodal Imaging and Their Implication on Clinical Presentation. J. Clin. Med. 2021, 10, 222. https://doi.org/10.3390/jcm10020222
Choi JY, Suh YJ, Seo J, Choi K-U, Hong G-R, Lee S, Lee S-H, Ha J-W, Kim YJ, Shim CY. Structural and Functional Characteristics of Mitral Paravalvular Leakage Identified by Multimodal Imaging and Their Implication on Clinical Presentation. Journal of Clinical Medicine. 2021; 10(2):222. https://doi.org/10.3390/jcm10020222
Chicago/Turabian StyleChoi, Jah Yeon, Young Joo Suh, Jiwon Seo, Kang-Un Choi, Geu-Ru Hong, Sak Lee, Seung-Hyun Lee, Jong-Won Ha, Young Jin Kim, and Chi Young Shim. 2021. "Structural and Functional Characteristics of Mitral Paravalvular Leakage Identified by Multimodal Imaging and Their Implication on Clinical Presentation" Journal of Clinical Medicine 10, no. 2: 222. https://doi.org/10.3390/jcm10020222
APA StyleChoi, J. Y., Suh, Y. J., Seo, J., Choi, K.-U., Hong, G.-R., Lee, S., Lee, S.-H., Ha, J.-W., Kim, Y. J., & Shim, C. Y. (2021). Structural and Functional Characteristics of Mitral Paravalvular Leakage Identified by Multimodal Imaging and Their Implication on Clinical Presentation. Journal of Clinical Medicine, 10(2), 222. https://doi.org/10.3390/jcm10020222






