Urogenital Schistosomiasis—History, Pathogenesis, and Bladder Cancer
Abstract
1. Introduction
2. Urogenital Schistosomiasis: Pathogenesis and Cancer
3. Evidence of Chemical Carcinogenesis as Initiator of Bladder Carcinogenesis Associated with Urogenital Schistosomiasis
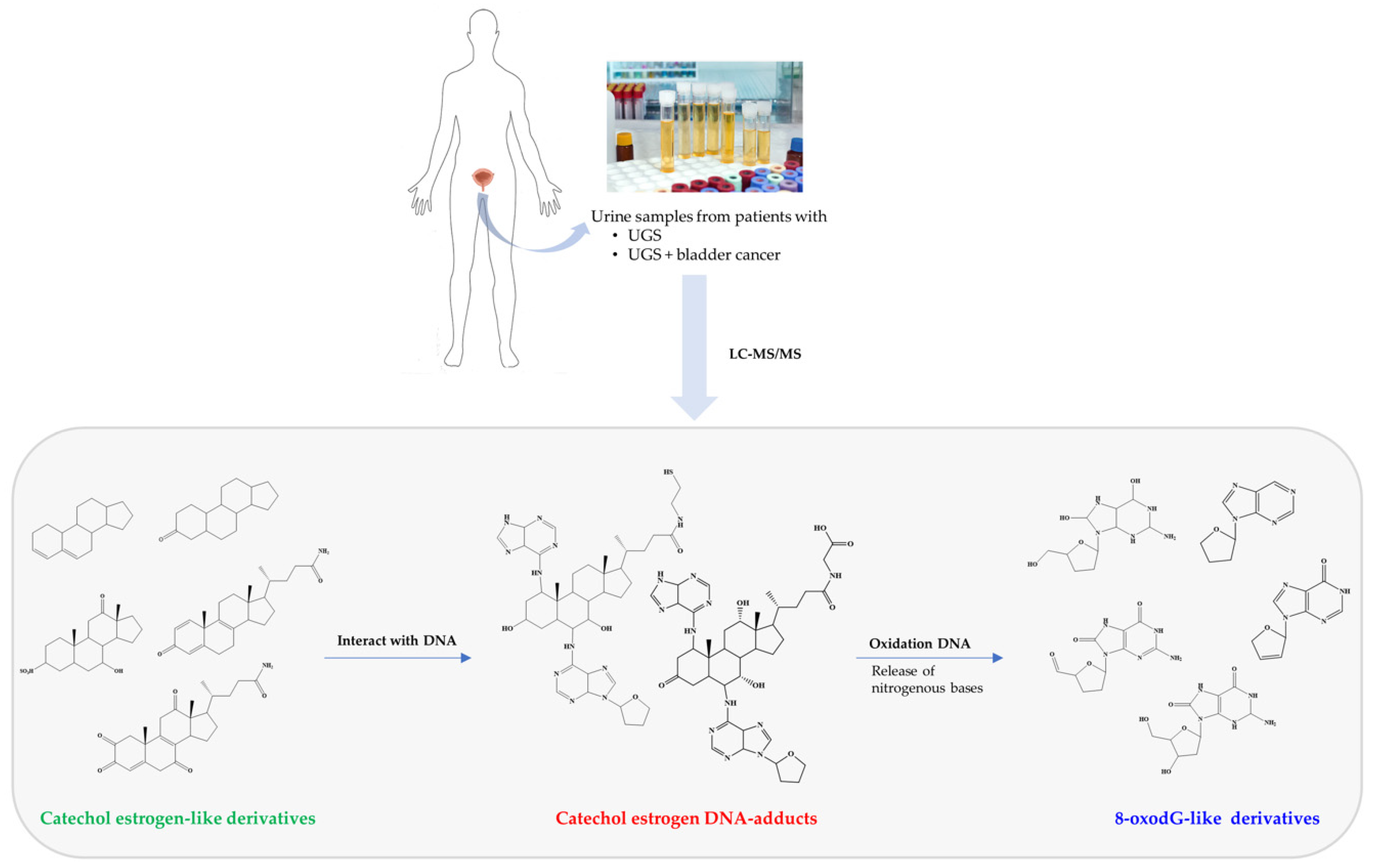
4. Urine Proteome in Urogenital Schistosomiasis
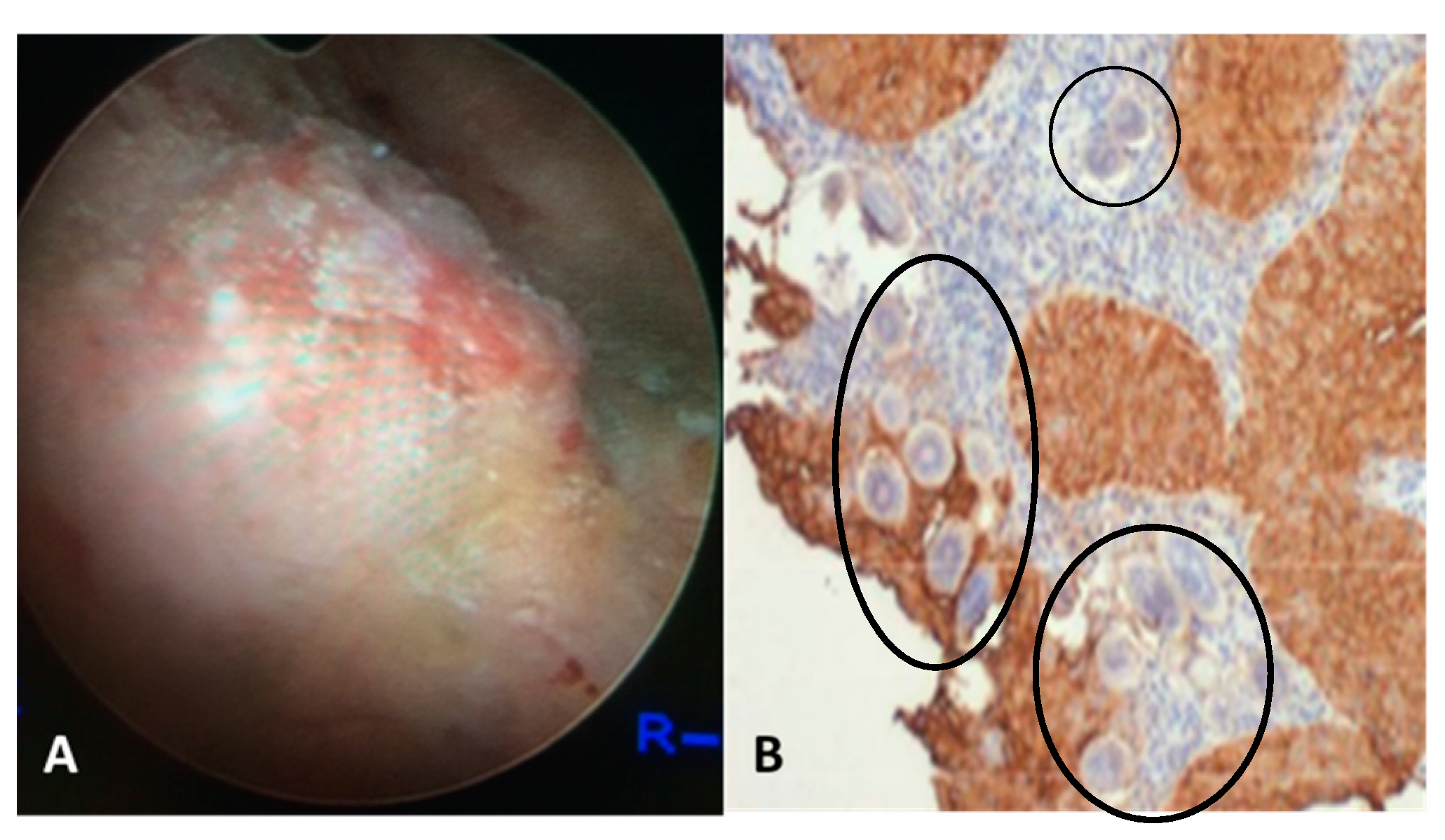
5. Alterations in the p53 Pathway Associated with UGS-Associated Bladder Cancer
6. Biomarker Candidates for UGS and SCC of the Bladder?
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bilharz, T. Fernere Mittheilungen uber Distomum haematobium. Zeitsch Wiss. Zool 1852, 4, 454–456. [Google Scholar]
- Sambon, L.W. Remarks on Schistosoma mansoni. J. Trop. Med. Hyg. 1907, 10, 303–304. [Google Scholar]
- Katsurada, F. Schistosomum japonicum, ein neuer menshlicher Parasit durch welchen eine endemisch Krankheit in verschiedenen Genenden Japans erusacht wird. Annot Zool Jpn. 1904, 5, 147–160. [Google Scholar]
- Fisher, A.C. Study of the schistosomiasis of the Stanleyville district of the Belgian Congo. T Roy. Soc. Trop. Med. H 1934, 28, 277–306. [Google Scholar]
- Voge, M.; Bruckner, D.; Bruce, J.I. Schistosoma mekongi sp. n. from man and animals, compared with four geographic strains of Schistosoma japonicum. J. Parasitol. 1978, 64, 577–584. [Google Scholar] [CrossRef]
- Machado-Silva, J.R.; Pelajo-Machado, M.; Lenzi, H.L.; Gomes, D.C. Morphological study of adult male worms of Schistosoma mansoni sambon, 1907 by confocal laser scanning microscopy. Mem. Inst. Oswaldo Cruz 1998, 93, 303–307. [Google Scholar]
- Cobbold, T.S. On some new forms of entozoa. Trans. Linn. Soc. Lond. 1859, 22, 363–366. [Google Scholar] [CrossRef]
- Harley, J. On the endemic haematuria of the Cape of Good Hope. Med. Chir. Trans. 1864, 47, 55–74. [Google Scholar]
- Manson, P. (Ed.) Tropical Diseases: A Manual, New Andrev; Cassell: London, UK, 1903. [Google Scholar]
- Katz, N. The discovery of Schistosomiasis mansoni in Brazil. Acta Trop. 2008, 108, 69–71. [Google Scholar]
- Leiper, R.T. Report on the results of the Bilharzia mission in Egypt, 1915. BMJ Mil. Health 1915, 25, 147–192. [Google Scholar]
- Fujinami, A.; Nakamura, H. The route of infection, the development of the parasite of Katayama disease and its infection in animals. Kyoto Med. J. 1909, 6, 224–252. [Google Scholar]
- Cook, G.C.; Zumla, A.I. Manson’s Tropical Diseases, 22nd ed.; Saunders Elsevier: Philadelphia, PA, USA, 2009. [Google Scholar]
- Webster, B.L.; Diaw, O.T.; Seye, M.M.; Webster, J.P.; Rollinson, D. Introgressive hybridization of Schistosoma haematobium group species in Senegal: Species barrier break down between ruminant and human schistosomes. PLoS Negl. Trop. Dis. 2013, 7, e2110. [Google Scholar] [CrossRef] [PubMed]
- Oleaga, A.; Rey, O.; Polack, B.; Grech-Angelini, S.; Quilichini, Y.; Pérez-Sánchez, R.; Boireau, P.; Mulero, S.; Brunet, A.; Rognon, A.; et al. Epidemiological surveillance of schistosomiasis outbreak in Corsica (France): Are animal reservoir hosts implicated in local transmission? PLoS Negl. Trop. Dis. 2019, 13, e0007543. [Google Scholar] [CrossRef] [PubMed]
- Vieira, P.; Miranda, H.P.; Cerqueira, M.; Delgado, M.L.; Coelho, H.; Antunes, D.; Cross, J.H.; da Costa, J.M. Latent schistosomiasis in Portuguese soldiers. Mil. Med. 2007, 172, 144–146. [Google Scholar] [CrossRef]
- Andrews, P.; Thomas, H.; Pohlke, R.; Seubert, J. Praziquantel. Med. Res. Rev. 1983, 3, 147. [Google Scholar] [CrossRef]
- Seubert, J.; Pohlke, R.; Loebich, F. Synthesis and properties of praziquantel, a novel broad spectrum anthelmintic with excellent activity against Schistosomes and Cestodes. Experientia 1977, 33, 1036. [Google Scholar]
- Lo, N.C.; Addiss, D.G.; Hotez, P.J.; King, C.H.; Stothard, J.R.; Evans, D.S.; Colley, D.G.; Lin, W.; Coulibaly, J.T.; Bustinduy, A.L.; et al. A call to strengthen the global strategy against schistosomiasis and soil-transmitted helminthiasis: The time is now. Lancet Infect. Dis. 2017, 17, e64–e69. [Google Scholar] [CrossRef]
- Lustigman, S.; Prichard, R.K.; Gazzinelli, A.; Grant, W.N.; Boatin, B.A.; McCarthy, J.S.; Basáñez, M.-G. A research agenda for helminth diseases of humans: The problem of helminthiases. PLoS Negl. Trop. Dis. 2012, 6, e1582. [Google Scholar] [CrossRef]
- Vale, N.; Gouveia, M.J.; Rinaldi, G.; Brindley, P.J.; Gärtner, F.; Correia da Costa, J.M. Praziquantel for schistosomiasis: Single-drug metabolism revisited, mode of action, and resistance. Antimicrob. Agents Chemother. 2017, 61, e02582-16. [Google Scholar]
- Crellen, T.; Walker, M.; Lamberton, P.H.; Kabatereine, N.B.; Tukahebwa, E.M.; Cotton, J.A.; Webster, J.P. Reduced efficacy of praziquantel against Schistosoma mansoni is associated with multiple rounds of mass drug administration. Clin. Infect. Dis. 2016, 63, 1151–1159. [Google Scholar]
- Van der Werf, M.J.; de Vlas, S.J.; Brooker, S.; Looman, C.W.; Nagelkerke, N.J.; Habbema, J.D. Engels D quantification of clinical morbidity associated with schistosome infection in Sub-Saharan Africa. Acta Trop. 2003, 86, 125–139. [Google Scholar] [PubMed]
- Brindley, P.J.; Hotez, P.J. Break out: Urogenital schistosomiasis and Schistosoma haematobium infection in the post-genomic era. PLoS Negl. Trop. Dis. 2013, 7, e1961. [Google Scholar] [CrossRef] [PubMed]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef]
- Kjetland, E.F.; Kurewa, E.N.; Ndhlovu, P.D.; Midzi, N.; Gwanzura, L.; Mason, P.R.; Gomo, E.; Sandvik, L.; Mduluza, T.; Friis, H.; et al. Female genital schistosomiasis—A differential diagnosis to sexually transmitted disease: Genital itch and vaginal discharge as indicators of genital Schistosoma haematobium morbidity in a crosssectional study in endemic rural Zimbabwe. Trop. Med. Int. Health 2008, 13, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Kjetland, E.F.; Leutscher, P.D.; Ndhlovu, P.D. A review of female genital schistosomiasis. Trends Parasitol. 2012, 28, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Kleppa, E.; Ramsuran, V.; Zulu, S.; Karlsen, G.H.; Bere, A.; Passmore, J.A.; Ndhlovu, P.; Lillebø, K.; Holmen, S.D.; Onsrud, M.; et al. Effect of female genital schistosomiasis and anti-schistosomal treatment on monocytes, CD4+ T-cells and CCR5 expression in the female genital tract. PLoS ONE 2014, 9, e98593. [Google Scholar] [CrossRef]
- Holtfreter, M.C.; Moné, H.; Müller-Stöver, I.; Mouahid, G.; Richter, J. Schistosoma haematobium infections acquired in Corsica, France. Euro Surveill. 2013, 19, 1. [Google Scholar]
- De Laval, F.; Savini, H.; Biance-Valero, E.; Simon, F. Human schistosomiasis: An emerging threat for Europe. Lancet 2014, 384, 1094–1095. [Google Scholar] [CrossRef]
- IARC. Biological Agents. A Review of Human Carcinogens. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organization/International Agency for Research on Cancer: Lyon, France, 2012; Volume 100, pp. 1–441.
- Berriman, M.; Haas, B.J.; LoVerde, P.T.; Wilson, R.A.; Dillon, G.P.; Cerqueira, G.C.; Mashiyama, S.T.; Al-Lazikani, B.; Andrade, L.F.; Ashton, P.D.; et al. The genome of the blood fluke Schistosoma Mansoni. Nature 2009, 460, 352–358. [Google Scholar] [CrossRef]
- Schistosoma Japonicum Genome Sequencing and Functional Analysis Consortium. The Schistosoma japonicum genome reveals features of host-parasite interplay. Nature 2009, 460, 345–351. [Google Scholar]
- Young, N.D.; Jex, A.R.; Li, B.; Liu, S.; Yang, L.; Xiong, Z.; Chen, F.; Wu, X.; Zerlotini, A.; Oliveira, G.; et al. Whole-genome sequence of Schistosoma haematobium. Nat. Genet. 2012, 44, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Mann, V.H.; Morales, M.E.; Rinaldi, G.; Brindley, P.J. Culture for genetic manipulation of developmental stages of Schistosoma mansoni. Parasitology 2010, 137, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, R.; Collins, J.J., III. Systematically improved in vitro culture conditions reveal new insights into the reproductive biology of the human parasite Schistosoma mansoni. PLoS Biol. 2019, 17, e3000254. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, G.; Eckert, S.E.; Tsai, I.J.; Suttiprapa, S.; Kines, K.; Tort, J.F.; Mann, V.H.; Turner, D.J.; Berriman, M.; Brindley, P. Germline transgenesis and insertional mutagenesis in Schistosoma mansoni mediated by murine leukemia vírus. PLoS Pathog. 2012, 8, e1002820. [Google Scholar] [CrossRef]
- Ittiprasert, W.; Mann, V.H.; Karinshak, S.E.; Coghlan, A.; Rinaldi, G.; Sankaranarayanan, G.; Chaidee, A.; Tanno, T.; Kumkhaek, C.; Prangtaworn, P.; et al. Programmed genome editing of the omega-1 ribonuclease of the blood fluke, Schistosoma mansoni. Elife 2019, 8, 41337. [Google Scholar] [CrossRef]
- Sankaranarayanan, G.; Coghlan, A.; Driguez, P.; Lotkowska, M.E.; Sanders, M.; Holroyd, N.; Tracey, A.; Berriman, M.; Rinaldi, G. Large CRISPR-Cas-induced deletions in the oxamniquine resistance locus of the human parasite Schistosoma mansoni. Wellcome Open Res. 2020, 5, 178. [Google Scholar] [CrossRef]
- Wang, J.; Paz, C.; Padalino, G.; Coghlan, A.; Lu, Z.; Gradinaru, I.; Collins, J.N.R.; Berriman, M.; Hoffmann, K.F.; Collins, J.J., III. Large-scale RNAi screening uncovers therapeutic targets in the parasite Schistosoma mansoni. Science 2020, 369, 1649–1653. [Google Scholar]
- Wendt, G.; Zhao, L.; Chen, R.; Liu, C.; O’Donoghue, A.J.; Caffrey, C.R.; Reese, M.L.; Collins, J.J., III. A single-cell RNA-seq atlas of Schistosoma mansoni identifies a key regulator of blood feeding. Science 2020, 369, 1644–1649. [Google Scholar]
- Chaves, J.; Araújo, H.; Valley, N.; Costa, J.M.; Brindley, P.J.; Lopes, C.; Naples, J.; Shiff, C.; Dupret, J.; Santos, L. Comparison of findings using ultrasonography and cystoscopy in urogenital schistosomiasis in a public health center in rural Angola. S Afr. Med. J. 2015, 105, 312–315. [Google Scholar]
- Shiff, C.; Veltri, R.; Naples, J.; Quartey, J.; Otchere, J.; Anyan, W.; Marlow, C.; Wiredu, E.; Adjei, A.; Brakohiapa, E.; et al. Ultrasound verification of bladder damage is associated with known biomarkers of bladder cancer in adults chronically infected with Schistosoma haematobium in Ghana. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 847–854. [Google Scholar]
- El-Bolkainy, M.N.; Mokhtar, N.M.; Ghoneim, M.A.; Hussein, M.H. The impact of schistosomiasis on the pathology of bladder carcinoma. Cancer 1981, 48, 2643–2648. [Google Scholar] [CrossRef]
- Koraitim, M.M.; Marzouk, M.E.; Atta, M.A.; Orabi, S.S. Risk factors and mechanism of urethral injury in pelvic fractures. Br. J. Urol. 1996, 77, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, M.H.; Sheweita, S.A.; O’Connor, P.J. Relationship between schistosomiasis and bladder cancer. Clin. Microbiol. Rev. 1999, 12, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Herrera, L.A.; Benitez-Bribiesca, L.; Mohar, A.; Ostrosky-Wegman, P. Role of infectious diseases in human carcinogenesis. Environ. Mol. Mutagen. 2005, 45, 284–303. [Google Scholar] [CrossRef] [PubMed]
- Porta, C.; Riboldi, E.; Sica, A. Mechanisms linking pathogens-associated inflammation and cancer. Cancer Lett. 2011, 305, 250–262. [Google Scholar] [PubMed]
- Salim, E.I.; Mourimura, K.; Menesi, A.; El-Lity, M.; Fukushima, S.; Wanibuchi, H. Elevated oxidative stress and DNA damage and repair levels in urinary bladder carcinomas associated with Schistosoma. Int. J. Cancer 2008, 123, 601–608. [Google Scholar]
- Mostafa, M.H.; Helmim, S.; Badawi, A.F.; Tricker, A.R.; Spiegelhalder, B.; Preussmann, R. Nitrate, nitrite and volatile N-nitroso compounds in the urine of Schistosoma haematobium and Schistosoma mansoni infected patients. Carcinogenesis 1994, 4, 619–625. [Google Scholar] [CrossRef]
- Dedon, P.C.; Tannenbaum, S.R. Reactive nitrogen species in the chemical biology inflammation. Arch. Biochem. Biophys. 2004, 423, 12–22. [Google Scholar] [CrossRef]
- Botelho, M.C.; Vale, N.; Gouveia, M.J.; Rinaldi, G.; Santos, J.; Santos, L.L.; Gomes, P.; Brindley, P.J.; Correia da Costa, J.M. Tumor-like phenotypes in urothelial cells after exposure to antigens from eggs of Schistosoma haematobium: An oestrogen-DNA adducts mediated pathway? Int. J. Parasitol. 2013, 43, 17–26. [Google Scholar] [CrossRef]
- Ohnishi, S.; Ma, N.; Thanan, R.; Pinlaor, S.; Hammam, O.; Murata, M.; Kawanishi, S. DNA damage in inflammation-related carcinogenesis and cancer stem cells. Oxid Med. Cell Longev. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Pimenta, R.; da Silva Orfanó, A.; Mosley, I.A.; Karinshak, S.E.; Ishida, K.; Mann, V.H.; Coelho, P.M.Z.; da Costa, J.M.C.; Hsieh, M.H.; Brindley, P.J.; et al. Differential responses of epithelial cells from urinary and biliary tract to eggs of Schistosoma haematobium and S. mansoni. Sci. Rep. 2019, 9, 10731. [Google Scholar] [CrossRef] [PubMed]
- Correia da Costa, J.M.; Vale, N.; Gouveia, M.J.; Botelho, M.C.; Sripa, B.; Santos, L.L.; Santos, J.H.; Rinaldi, G.; Brindley, P.J. Schistosome and liver fluke derived catechol-estrogens and helminth associated cancers. Front. Genet. 2014, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, M.J.; Santos, J.; Brindley, P.J.; Rinaldi, G.; Lopes, C.; Santos, L.L.; da Costa, J.M.; Vale, N. Estrogen-like metabolites and DNA-adducts in urogenital schistosomiasis-associated bladder cancer. Cancer Lett. 2015, 359, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Brindley, P.J.; da Costa, J.M.; Sripa, B. Why does infection with some helminths cause cancer? Trends Cancer 2015, 1, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Botelho, M.C.; Crespo, M.; Almeida, A.; Vieira, P.; Delgado, M.L.; Araújo, L.; Machado, J.C.; Correia da Costa, J.M. Schistosoma haematobium and Schistosomiasis mansoni: Production of an estradiol-related compound detected by ELISA. Exp. Parasitol. 2009, 122, 250–253. [Google Scholar] [CrossRef]
- Botelho, M.C.; Soares, R.; Vale, N.; Ribeiro, R.; Camilo, V.; Almeida, R.; Medeiros, R.; Gomes, P.; Machado, J.C.; Correia da Costa, J.M. Schistosoma haematobium: Identification of new estrogenic molecules with estradiol antagonistic activity and ability to inactive estrogen receptor in mammalian cells. Exp. Parasitol. 2010, 126, 526–535. [Google Scholar]
- Cavalieri, E.; Chakravarti, D.; Guttenplan, J.; Hart, E.; Ingle, J.; Jankowiak, R.; Muti, P.; Rogan, E.; Russo, J.; Santen, R.; et al. Catechol estrogen quinones as initiators of breast and other human cancers: Implications for biomarkers of susceptibility and cancer prevention. Biochim. Biophys. Acta 2006, 1766, 63–78. [Google Scholar]
- Santos, J.; Fernandes, E.; Ferreira, J.A.; Lima, L.; Tavares, A.; Peixoto, A.; Parreira, B.; Correia da Costa, J.M.; Brindley, P.J.; Lopes, C.; et al. p53 and cancer-associated sialylated glycans are surrogate markers of cancerization of the bladder associated with Schistosoma haematobium infection. PLoS Negl. Trop. Dis. 2014, 8, e3329. [Google Scholar] [CrossRef]
- Clemons, M.; Goss, P. Estrogen and the risk of breast cancer. N. Engl. J. Med. 2001, 344, 276–285. [Google Scholar]
- Yager, J.D.; Davidson, N.E. Estrogen carcinogenesis in breast cancer. N. Engl. J. Med. 2006, 354, 270–282. [Google Scholar]
- Ma, N.; Thanan, R.; Kobayashi, H.; Hammam, O.; Wishahi, M.; El Leithy, T.; Hiraku, Y.; Amro, E.-K.; Oikawa, S.; Ohnishi, S.; et al. Nitritative DNA damage and Oct3/4 expression in urinary bladder cancer with Schistosoma haematobium infection. Biochem. Biophys. Res. Commu. 2011, 414, 344–349. [Google Scholar] [CrossRef]
- Bruner, S.D.; Norman, D.P.G.; Verdine, G.L. Structural basis for recognition and repair of the endogenous mutagen 8-oxoguanine in DNA. Nature 2000, 403, 859–866. [Google Scholar] [PubMed]
- Bouatra, S.; Aziat, F.; Mandal, R.; Guo, A.C.; Wilson, M.R.; Knox, C.; Bjorndahl, T.C.; Krishnamurthy, R.; Saleem, F.; Liu, P.; et al. The human urine metabolome. PLoS ONE 2013, 8, e73076. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, C.; Cunha, M.C.; Santos, J.H.; da Costa, J.M.; Brindley, P.J.; Lopes, C.; Amado, F.; Ferreira, R.; Vitorino, R.; Santos, L.L. Insight into the molecular basis of Schistosoma haematobium-induced bladder cancer through urine proteomics. Tumor Biol. 2016, 37, 11279–11287. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Brindley, P.J.; McManus, D.P.; Feng, Z.; Han, Z.G. Schistosome transcriptomes: New insights into the parasite and schistosomiasis. Trends Mol. Med. 2004, 10, 217–225. [Google Scholar]
- Stroehlein, A.J.; Korhonen, P.; Chong, T.M.; Lim, Y.L.; Chan, K.G.; Webster, B.; Rollinson, D.; Brindley, P.J.; Gasser, R.B.; Young, N.D. High-quality Schistosoma haematobium genome achieved by single-molecule and long-range sequencing. GigaScience 2019, 8, 1–12. [Google Scholar] [CrossRef]
- El-Aal, A.A.; Emran, A.M.; Al-Antably, A.S.; El Saftawy, E.A.; Bayoumy, I.R.; Hassan, N.S.; Badawi, M. Immunohistochemical pattern of T lymphocytes population within bilharzial-associated bladder neoplasm microenvironment. Int. J. Immunopathol. Pharm. 2015, 28, 209–217. [Google Scholar] [CrossRef]
- Warren, W.; Biggs, P.J.; el-Baz, M.; Ghoneim, M.A.; Stratton, M.R.; Venitt, S. Mutations in the p53 gene in schistosomal bladder cancer: A study of 92 tumours from Egyptian patients and a comparison between mutational spectra from schistosomal and non-schistosomal urothelial tumours. Carcinogenesis 1995, 16, 1181–1189. [Google Scholar] [CrossRef]
- Brücher, B.L.; Jamall, I.S. Epistemology of the origin of cancer: A new paradigm. BMC Cancer 2014, 14, 331. [Google Scholar] [CrossRef]
- Baas, I.O.; Mulder, J.W.; Offerhaus, G.J.; Vogelstein, B.; Hamilton, S.R. An evaluation of six antibodies for immunohistochemistry of mutant p53 gene product in archival colorectal neoplasms. J. Pathol. 1994, 172, 5–12. [Google Scholar] [CrossRef]
- Mallofré, C.; Castillo, M.; Morente, V.; Solé, M. Immunohistochemical expression of CK20, p53, and Ki-67 as objective markers of urothelial dysplasia. Mod. Pathol. 2003, 16, 187–191. [Google Scholar] [CrossRef]
- Harney, J.; Murphy, D.M.; Jones, M.; Mothersill, C. Expression of p53 in urothelial cell cultures from tumour-bearing and tumour-free patients. Br. J. Cancer 1995, 71, 25–29. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gao, J.P.; Uchida, T.; Wang, C.; Jiang, S.X.; Matsumoto, K.; Satoh, T.; Minei, S.; Soh, S.; Kameya, T.; Baba, S. Relationship between p53 gene mutation and protein expression: Clinical significance in transitional cell carcinoma of the bladder. Int. J. Oncol. 2000, 16, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Vet, J.A.; Bringuier, P.P.; Schaafsma, H.E.; Witjes, J.A.; Debruyne, F.M.; Schalken, J.A. Comparison of p53 protein overexpression with p53 mutation in bladder cancer: Clinical and biologic aspects. Lab. Invest. 1995, 73, 837–843. [Google Scholar] [PubMed]
- Khaled, H.M.; Bahnassi, A.A.; Zekri, A.R.; Kassem, H.A.; Mokhtar, N. Correlation between p53 mutations and HPV in bilharzial bladder cancer. Urol. Oncol. 2003, 21, 334–341. [Google Scholar] [CrossRef]
- Costa, A.; Marasca, R.; Valentinis, B.; Savarino, M.; Faranda, A.; Silvestrini, R.; Torelli, G. p53 gene point mutations in relation to p53 nuclear protein accumulation in colorectal cancers. J. Pathol. 1995, 176, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Soong, R.; Robbins, P.D.; Dix, B.R.; Grieu, F.; Lim, B.; Knowles, S.; Williams, K.E.; Turbett, G.R.; House, A.K.; Iacopetta, B.J. Concordance between p53 protein overexpression and gene mutation in a large series of common human carcinomas. Hum. Pathol. 1996, 27, 1050–1055. [Google Scholar] [CrossRef]
- Stoehr, R.; Knuechel, R.; Boecker, J.; Blaszyk, H.; Schmitt, R.; Filbeck, T.; Hofstaedter, F.; Hartmann, A. Histologic-genetic mapping by allele-specific PCR reveals intraurothelial spread of p53 mutant tumor clones. Lab. Invest. 2002, 82, 1553–1561. [Google Scholar]
- Badawi, A.F. Molecular and genetic events in schistosomiasis-associated human bladder cancer: Role of oncogenes and tumor suppressor genes. Cancer Lett. 1996, 105, 123–138. [Google Scholar] [CrossRef]
- Abdulamir, A.S.; Hafidh, R.R.; Kadhim, H.S.; Abubakar, F. Tumor markers of bladder cancer: The schistosomal bladder tumors versus non-schistosomal bladder tumors. J. Exp. Clin. Cancer Res. 2009, 28, 27. [Google Scholar] [CrossRef]
- Kamel, D.; Soini, Y.; Nuorva, K.; Pääkkö, P.; Khalifa, A.; Mangoud, A.; Vähäkangas, K. p53 and c-erbB-2 expression in schistosomal urinary bladder carcinomas and schistosomal cystitis with premalignant lesions. Virchows Arch. 1994, 424, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.-L.; Odegaard, J.I.; Herbert, D.R.; Hsieh, M.H. A novel mouse of Schistosoma haematobium egg-induced immunopathology. PLoS Pathog. 2012, 8, e1002605. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.-L.; Knudsen, G.; Thathireddy, A.; Caffrey, C.C.; McKerrow, J.; Herbert, D.; Thai, K.; Xie, M.; Hsieh, M. A novel mouse model of Schistosoma haematobium infection to study inflammatory mechanisms of bladder fibrosis. J. Immunol. 2011, 186, 56.5. [Google Scholar]
- Honeycutt, J.; Hammam, O.; Hsieh, M.H. Schistosoma haematobium egg-induced bladder urothelial abnormalities dependente on p53 are modulated by host sex. Exp. Parasitol. 2015, 158, 55–60. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, L.L.; Santos, J.; Gouveia, M.J.; Bernardo, C.; Lopes, C.; Rinaldi, G.; Brindley, P.J.; Costa, J.M.C.d. Urogenital Schistosomiasis—History, Pathogenesis, and Bladder Cancer. J. Clin. Med. 2021, 10, 205. https://doi.org/10.3390/jcm10020205
Santos LL, Santos J, Gouveia MJ, Bernardo C, Lopes C, Rinaldi G, Brindley PJ, Costa JMCd. Urogenital Schistosomiasis—History, Pathogenesis, and Bladder Cancer. Journal of Clinical Medicine. 2021; 10(2):205. https://doi.org/10.3390/jcm10020205
Chicago/Turabian StyleSantos, Lúcio Lara, Júlio Santos, Maria João Gouveia, Carina Bernardo, Carlos Lopes, Gabriel Rinaldi, Paul J. Brindley, and José M. Correia da Costa. 2021. "Urogenital Schistosomiasis—History, Pathogenesis, and Bladder Cancer" Journal of Clinical Medicine 10, no. 2: 205. https://doi.org/10.3390/jcm10020205
APA StyleSantos, L. L., Santos, J., Gouveia, M. J., Bernardo, C., Lopes, C., Rinaldi, G., Brindley, P. J., & Costa, J. M. C. d. (2021). Urogenital Schistosomiasis—History, Pathogenesis, and Bladder Cancer. Journal of Clinical Medicine, 10(2), 205. https://doi.org/10.3390/jcm10020205






