Unilateral versus Bilateral Endoscopic Nasobiliary Drainage and Subsequent Metal Stent Placement for Unresectable Malignant Hilar Obstruction: A Multicenter Randomized Controlled Trial
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Patients
2.3. Definitions of Outcome Variables
2.4. Sample Size Calculation
2.5. Randomization
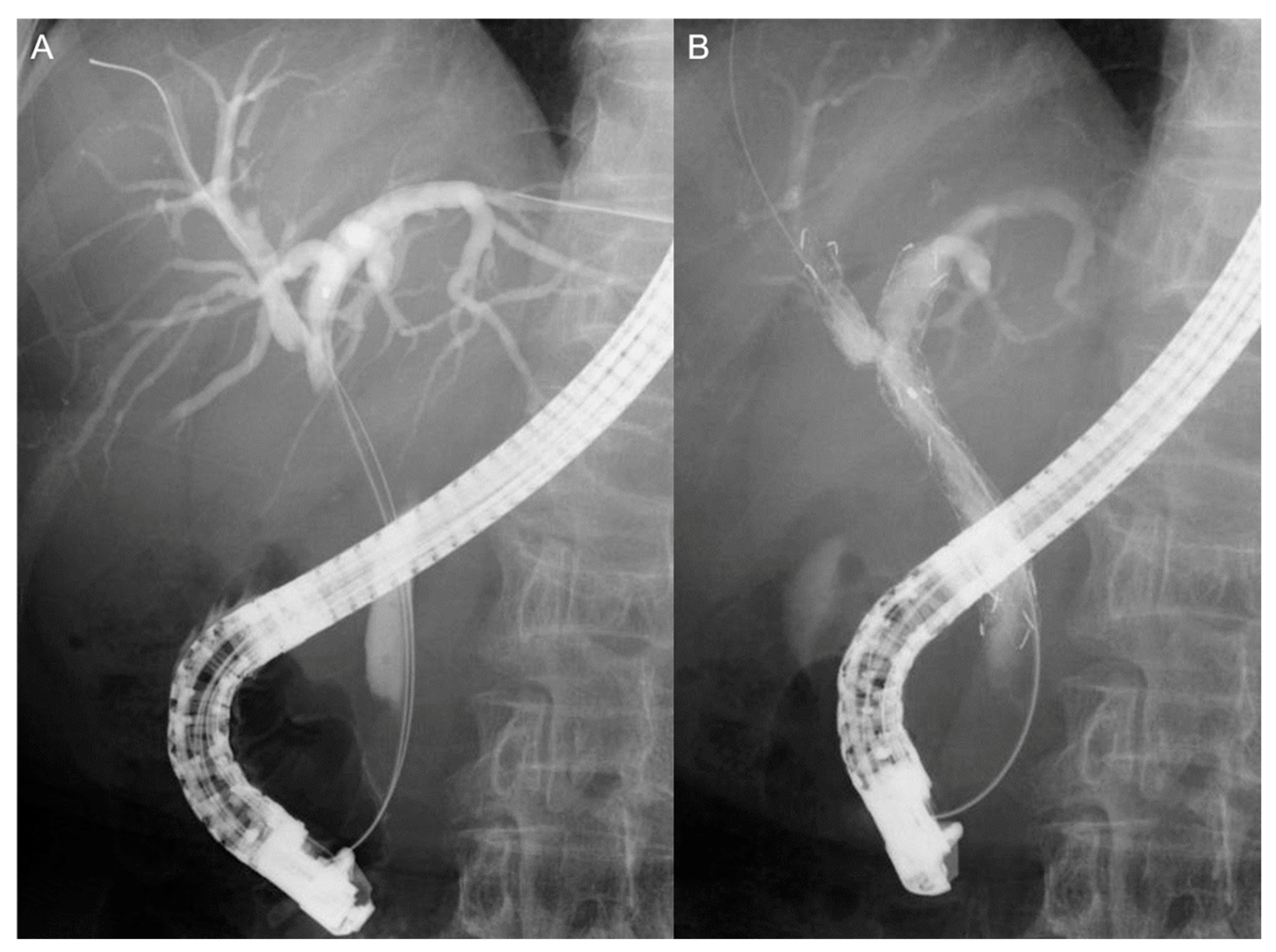
2.6. Endoscopic Procedures
2.7. Statistical Analysis
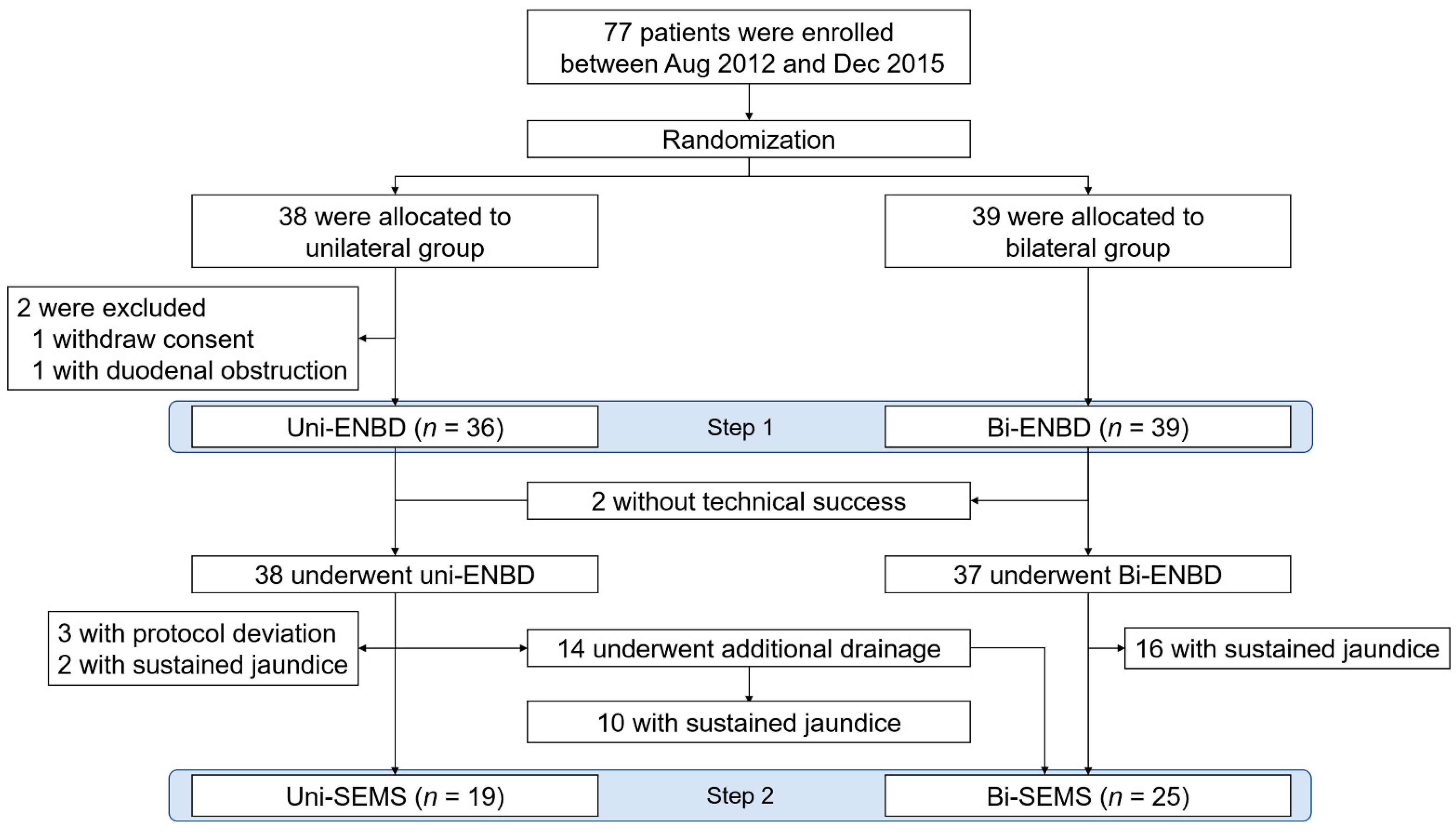
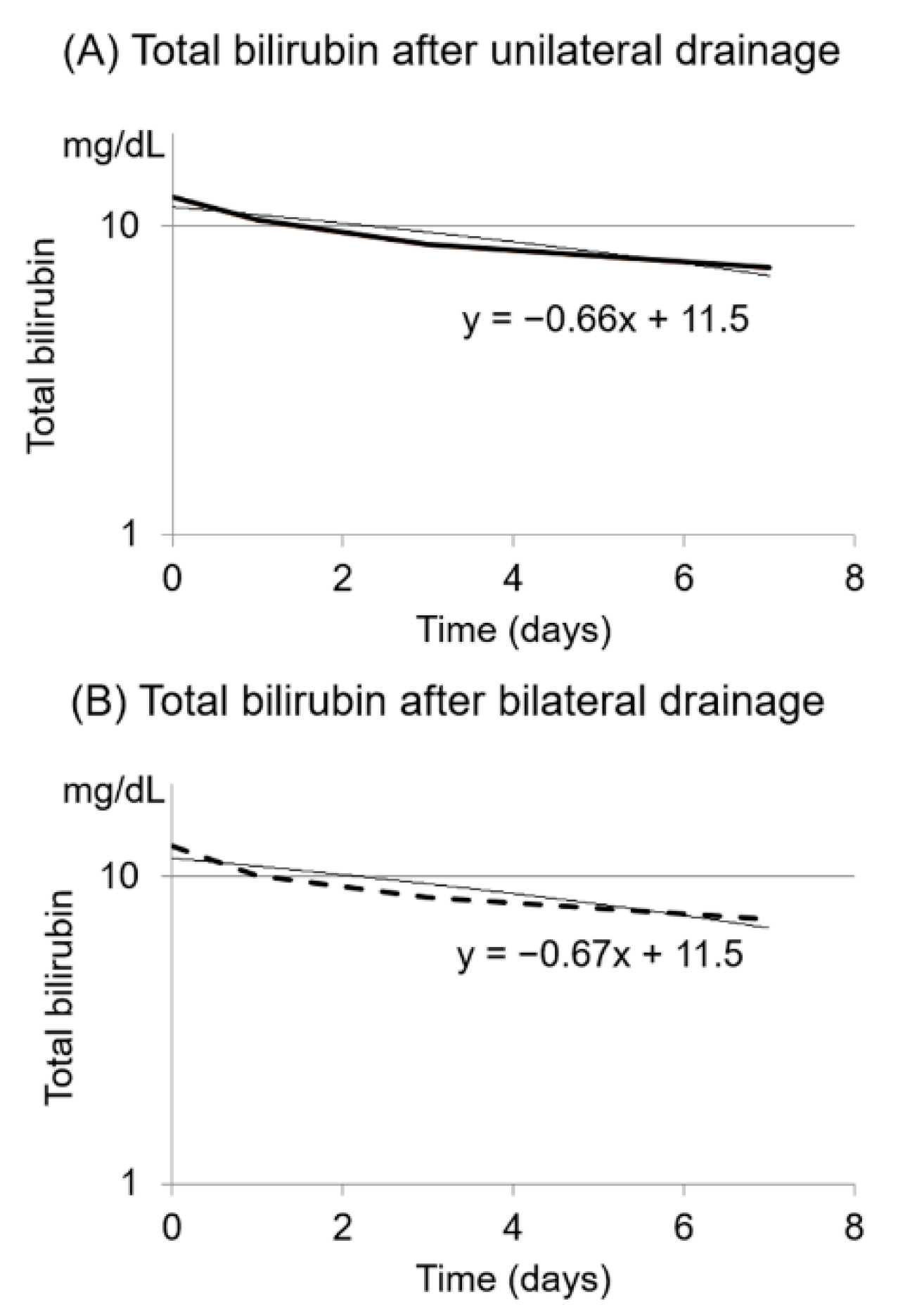
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adler, D.G.; Baron, T.H.; Davila, R.E.; Egan, J.; Hirota, W.K.; Leighton, J.A.; Qureshi, W.; Rajan, E.; Zuckerman, M.J.; Fanelli, R.; et al. ASGE guideline: The role of ERCP in diseases of the biliary tract and the pancreas. Gastrointest. Endosc. 2005, 62, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Moon, J.H.; Park, S. Biliary stenting for hilar malignant biliary obstruction. Dig. Endosc. 2020, 32, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Kim, T.H.; Moon, J.H.; Lee, S.H.; Choi, H.J.; Hwangbo, Y.; Hyun, J.J.; Choi, J.-H.; Jeong, S.; Kim, J.H.; et al. Bilateral versus unilateral placement of metal stents for inoperable high-grade malignant hilar biliary strictures: A multicenter, prospective, randomized study (with video). Gastrointest. Endosc. 2017, 86, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Ashat, M.; Arora, S.; Klair, J.S.; Childs, C.A.; Murali, A.R.; Johlin, F.C. Bilateral vs unilateral placement of metal stents for inoperable high-grade hilar biliary strictures: A systemic review and meta-analysis. World J. Gastroenterol. 2019, 25, 5210–5219. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, I.; Mukai, T.; Moriwaki, H. Unilateral versus bilateral endoscopic biliary stenting for malignant hilar biliary strictures. Dig. Endosc. 2013, 25 (Suppl. 2), 81–85. [Google Scholar] [CrossRef] [PubMed]
- Naitoh, I.; Ohara, H.; Nakazawa, T.; Ando, T.; Hayashi, K.; Okumura, F.; Okayama, Y.; Sano, H.; Kitajima, Y.; Hirai, M.; et al. Unilateral versus bilateral endoscopic metal stenting for malignant hilar biliary obstruction. J. Gastroenterol. Hepatol. 2009, 24, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Iwano, H.; Ryozawa, S.; Ishigaki, N.; Taba, K.; Senyo, M.; Yoshida, K.; Sakaida, I. Unilateral versus Bilateral Drainage Using Self-Expandable Metallic Stent for Unresectable Hilar Biliary Obstruction. Dig. Endosc. 2010, 23, 43–48. [Google Scholar] [CrossRef]
- Vienne, A.; Hobeika, E.; Gouya, H.; Lapidus, N.; Fritsch, J.; Choury, A.D.; Chryssostalis, A.; Gaudric, M.; Pelletier, G.; Buffet, C.; et al. Prediction of drainage effectiveness during endoscopic stenting of malignant hilar strictures: The role of liver volume assessment. Gastrointest. Endosc. 2010, 72, 728–735. [Google Scholar] [CrossRef]
- De Palma, G.D.; Galloro, G.; Siciliano, S.; Iovino, P.; Catanzano, C. Unilateral versus bilateral endoscopic hepatic duct drainage in patients with malignant hilar biliary obstruction: Results of a prospective, randomized, and controlled study. Gastrointest. Endosc. 2001, 53, 547–553. [Google Scholar] [CrossRef]
- Staub, J.; Siddiqui, A.; Murphy, M.; Lam, R.; Parikh, M.; Pleskow, D.; Papachristou, G.; Sharaiha, R.; Iqbal, U.; Loren, D.; et al. Unilateral versus bilateral hilar stents for the treatment of cholangiocarcinoma: A multicenter international study. Ann. Gastroenterol. 2020, 33, 202–209. [Google Scholar] [CrossRef]
- Cassani, L.S.; Chouhan, J.; Chan, C.; Lanke, G.; Chen, H.-C.; Wang, X.; Weston, B.; Ross, W.A.; Raju, G.S.; Lee, J.H. Biliary Decompression in Perihilar Cholangiocarcinoma Improves Survival: A Single-Center Retrospective Analysis. Dig. Dis. Sci. 2019, 64, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Mukai, T.; Yasuda, I.; Nakashima, M.; Doi, S.; Iwashita, T.; Iwata, K.; Kato, T.; Tomita, E.; Moriwaki, H. Metallic stents are more efficacious than plastic stents in unresectable malignant hilar biliary strictures: A randomized controlled trial. J. Hepato-Biliary-Pancreat. Sci. 2012, 20, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Hashimoto, S.; Tanoue, S.; Tsuneyoshi, K.; Nakamura, Y.; Hinokuchi, M.; Iwaya, H.; Arima, S.; Iwashita, Y.; Sasaki, F.; et al. Factors Associated with the Technical Success of Bilateral Endoscopic Metallic Stenting with Partial Stent-In-Stent Placement in Patients with Malignant Hilar Biliary Obstruction. Gastroenterol. Res. Pract. 2019, 2019, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Moon, J.H.; Kim, J.H.; Park, D.H.; Lee, S.S.; Choi, H.J.; Cho, Y.D.; Park, S.H.; Kim, S.J. Primary and revision efficacy of cross-wired metallic stents for endoscopic bilateral stent-in-stent placement in malignant hilar biliary strictures. Endoscopy 2012, 45, 106–113. [Google Scholar] [CrossRef]
- Kogure, H.; Isayama, H.; Nakai, Y.; Tsujino, T.; Matsubara, S.; Yashima, Y.; Ito, Y.; Tsuyoshi, H.; Takahara, N.; Miyabayashi, K.; et al. High single-session success rate of endoscopic bilateral stent-in-stent placement with modified large cell Niti-S stents for malignant hilar biliary obstruction. Dig. Endosc. 2014, 26, 93–99. [Google Scholar] [CrossRef]
- Kogure, H.; Isayama, H.; Kawakubo, K.; Sasaki, T.; Yamamoto, N.; Hirano, K.; Sasahira, N.; Tsujino, T.; Tada, M.; Koike, K. Endoscopic bilateral metallic stenting for malignant hilar obstruction using newly designed stents. J. Hepato-Biliary-Pancreat. Sci. 2011, 18, 653–657. [Google Scholar] [CrossRef]
- Kawakami, H.; Kuwatani, M.; Onodera, M.; Haba, S.; Eto, K.; Ehira, N.; Yamato, H.; Kudo, T.; Tanaka, E.; Hirano, S.; et al. Endoscopic nasobiliary drainage is the most suitable preoperative biliary drainage method in the management of patients with hilar cholangiocarcinoma. J. Gastroenterol. 2010, 46, 242–248. [Google Scholar] [CrossRef]
- Kawakubo, K.; Kawakami, H.; Kuwatani, M.; Haba, S.; Kudo, T.; A Taya, Y.; Kawahata, S.; Kubota, Y.; Kubo, K.; Eto, K.; et al. Lower incidence of complications in endoscopic nasobiliary drainage for hilar cholangiocarcinoma. World J. Gastrointest. Endosc. 2016, 8, 385–390. [Google Scholar] [CrossRef]
- Isayama, H.; Hamada, T.; Yasuda, I.; Itoi, T.; Ryozawa, S.; Nakai, Y.; Kogure, H.; Koike, K. TOKYO criteria 2014 for transpapillary biliary stenting. Dig. Endosc. 2015, 27, 259–264. [Google Scholar] [CrossRef]
- Cotton, P.B.; Eisen, G.M.; Aabakken, L.; Baron, T.H.; Hutter, M.M.; Jacobson, B.C.; Mergener, K.; Nemcek, A.; Petersen, B.T.; Petrini, J.L.; et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest. Endosc. 2010, 71, 446–454. [Google Scholar] [CrossRef]
- Suda, K.; Ohtsuka, M.; Ambiru, S.; Kimura, F.; Shimizu, H.; Yoshidome, H.; Miyazaki, M. Risk factors of liver dysfunction after extended hepatic resection in biliary tract malignancies. Am. J. Surg. 2009, 197, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2012, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.J.; Kim, J.H.; Hwang, J.C.; Yoo, B.M.; Lee, S.H.; Ryu, J.K.; Kim, Y.-T.; Woo, S.M.; Lee, W.J.; Jeong, S.; et al. Prospective Multicenter Study of the Challenges Inherent in Using Large Cell-Type Stents for Bilateral Stent-in-Stent Placement in Patients with Inoperable Malignant Hilar Biliary Obstruction. Gut Liver 2018, 12, 722–727. [Google Scholar] [CrossRef]
- Lee, T.H.; Moon, J.H.; Choi, J.-H.; Lee, S.H.; Lee, Y.N.; Paik, W.H.; Jang, D.K.; Cho, B.W.; Yang, J.K.; Hwangbo, Y.; et al. Prospective comparison of endoscopic bilateral stent-in-stent versus stent-by-stent deployment for inoperable advanced malignant hilar biliary stricture. Gastrointest. Endosc. 2019, 90, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Ishigaki, K.; Hamada, T.; Nakai, Y.; Isayama, H.; Sato, T.; Hakuta, R.; Saito, K.; Saito, T.; Takahara, N.; Mizuno, S.; et al. Retrospective Comparative Study of Side-by-Side and Stent-in-Stent Metal Stent Placement for Hilar Malignant Biliary Obstruction. Dig. Dis. Sci. 2020, 65, 3710–3718. [Google Scholar] [CrossRef]
- Miura, S.; Kanno, A.; Masamune, A.; Hamada, S.; Hongou, S.; Yoshida, N.; Nakano, E.; Takikawa, T.; Kume, K.; Kikuta, K.; et al. Risk factors for recurrent biliary obstruction following placement of self-expandable metallic stents in patients with malignant perihilar biliary stricture. Endoscopy 2016, 48, 536–545. [Google Scholar] [CrossRef]
- Inoue, T.; Ibusuki, M.; Kitano, R.; Kobayashi, Y.; Ohashi, T.; Nakade, Y.; Sumida, Y.; Ito, K.; Yoneda, M. Endobiliary radiofrequency ablation combined with bilateral metal stent placement for malignant hilar biliary obstruction. Endoscopy 2020, 52, 595–599. [Google Scholar] [CrossRef]
- Kongkam, P.; Tasneem, A.A.; Rerknimitr, R. Combination of endoscopic retrograde cholangiopancreatography and endoscopic ultrasonography-guided biliary drainage in malignant hilar biliary obstruction. Dig. Endosc. 2019, 31 (Suppl. 1), 50–54. [Google Scholar] [CrossRef]
| Characteristic * | Unilateral (n = 38) | Bilateral (n = 39) | p Value |
|---|---|---|---|
| Age | 78 (72–82) | 72 (69–81) | 0.15 |
| Gender | 0.24 | ||
| Male | 21 (55%) | 27 (69%) | |
| Female | 17 (45%) | 12 (31%) | |
| ASA-PS score †, 1/2/3 | 15/18/5 (39%/47%/13%) | 17/22/0 (44%/56%/0%) | 0.07 |
| Primary cancer | 0.89 | ||
| Hilar cholangiocarcinoma | 12 (32%) | 13 (33%) | |
| Gallbladder cancer | 10 (26%) | 12 (31%) | |
| ICC | 6 (16%) | 6 (15%) | |
| Others | 10 (26%) | 8 (21%) | |
| Bismuth type | 0.92 | ||
| II | 10 (26%) | 10 (26%) | |
| IIIa | 11 (29%) | 9 (23%) | |
| IIIb | 2 (5.3%) | 2 (5.1%) | |
| IV | 15 (40%) | 18 (46%) | |
| Liver metastasis | 12 (32%) | 16 (41%) | 0.48 |
| Ascites | 7 (18%) | 7 (18%) | 0.99 |
| Previous gastrectomy | 1 (2.6%) | 1 (2.6%) | 0.99 |
| Laboratory data | |||
| White blood cell, 104/μL | 6.1 (5.3–7.3) | 6.5 (5.0–8.2) | 0.33 |
| CRP, mg/dL | 1.4 (0.6–3.2) | 1.5 (0.8–3.4) | 0.68 |
| Albumin, mg/dL | 3.2 (2.8–3.7) | 3.3 (2.9–3.5) | 0.82 |
| Total bilirubin, mg/dL | 10 (5.1–15) | 9.7 (5.7–17) | 0.88 |
| Prothrombin time, s | 95 (82–95) | 93 (75–93) | 0.50 |
| Outcome * | Uni-ENBD (n = 36) | Bi-ENBD (n = 39) | p Value |
|---|---|---|---|
| Technical success | 36 (100%) | 37 (95%) | 0.49 |
| Functional success | 21 (57%) | 22 (56%) | 0.99 |
| Additional drainage | 14 (39%) | 2 (5.3%) | <0.001 |
| ENBD | 12 (33%) | 2 (5.1%) | |
| PTBD | 1 (2.8%) | 0 | |
| Plastic stent | 1 (2.8%) | 0 | |
| Early adverse events | 7 (19%) | 12 (31%) | 0.30 |
| Cholangitis | 4 (11%) † | 4 (10%) | 0.99 |
| Cholecystitis | 0 | 1 (2.6%) | 0.99 |
| Pancreatitis | 4 (11%) † | 3 (7.7%) | 0.70 |
| Liver abscess | 0 | 1 (2.6%) | 0.99 |
| Gastrointestinal bleeding | 0 | 2 (5.1%) | 0.49 |
| Self-removal of ENBD | 0 | 1 (2.6%) | 0.99 |
| Subgroup | Total, n | FS | OR (95% CI) | |||
|---|---|---|---|---|---|---|
| Univariable | p Value | Multivariable * | p Value | |||
| Drainage | ||||||
| Uni-ENBD | 36 | 21 (58%) | 1 (referent) | 1 (referent) | ||
| Bi-ENBD | 39 | 22 (56%) | 0.92 (0.37–2.31) | 0.87 | 1.11 (0.42–2.95) | 0.83 |
| Outcomes * | Uni-SEMS (n = 19) | Bi-SEMS (n = 25) | p Value |
|---|---|---|---|
| Technical success | 19 (100%) | 25 (100%) | 0.99 |
| Early adverse events | 1 (5.3%) | 7 (28%) | 0.11 |
| Cholangitis | 1 (5.3%) † | 5 (20%) † | 0.21 |
| Pancreatitis | 0 | 1 (4.0%) | 0.99 |
| Acute obstruction | 1 (5.3%) † | 2 (8.0%) † | 0.99 |
| Late adverse events | 9 (47%) | 11 (44%) | 0.99 |
| Recurrent biliary obstruction | 8 (42%) | 11 (44%) | 0.99 |
| Tumor ingrowth | 6 (32%) | 6 (24%) | 0.74 |
| Sludge | 2 (11%) | 1 (4.0%) | 0.57 |
| Tumor bleeding | 0 | 2 (8.0%) | 0.50 |
| Cholangitis | 0 | 2 (8.0%) | 0.50 |
| Liver abscess | 1 (5.3%) | 1 (4.0%) ‡ | 0.99 |
| Intra-abdominal abscess | 0 | 1 (4.0%) § | 0.99 |
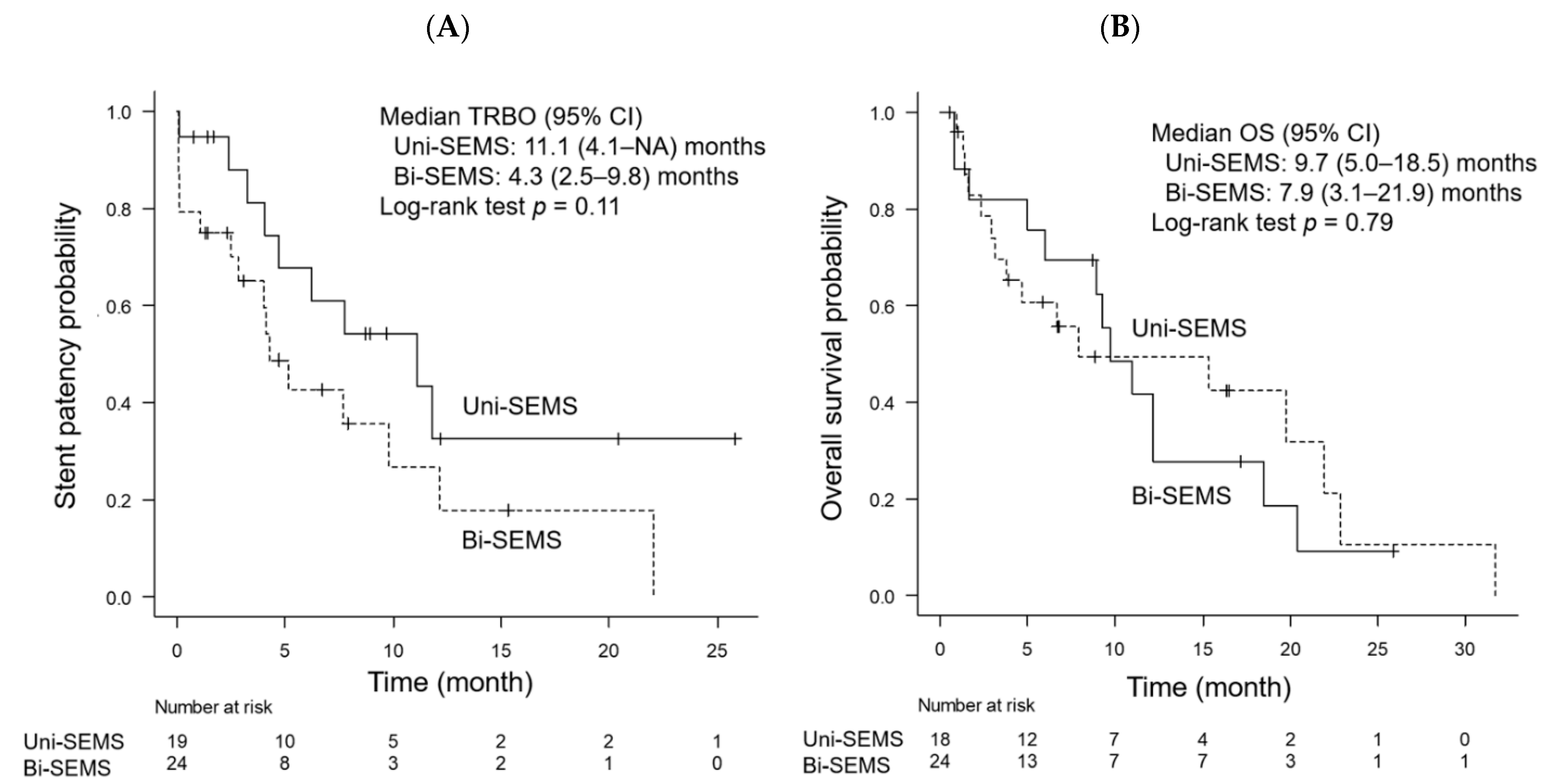
| Median TRBO, month | 11.1 | 4.3 | 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakuta, R.; Kogure, H.; Nakai, Y.; Kawakami, H.; Maguchi, H.; Mukai, T.; Iwashita, T.; Saito, T.; Togawa, O.; Matsubara, S.; et al. Unilateral versus Bilateral Endoscopic Nasobiliary Drainage and Subsequent Metal Stent Placement for Unresectable Malignant Hilar Obstruction: A Multicenter Randomized Controlled Trial. J. Clin. Med. 2021, 10, 206. https://doi.org/10.3390/jcm10020206
Hakuta R, Kogure H, Nakai Y, Kawakami H, Maguchi H, Mukai T, Iwashita T, Saito T, Togawa O, Matsubara S, et al. Unilateral versus Bilateral Endoscopic Nasobiliary Drainage and Subsequent Metal Stent Placement for Unresectable Malignant Hilar Obstruction: A Multicenter Randomized Controlled Trial. Journal of Clinical Medicine. 2021; 10(2):206. https://doi.org/10.3390/jcm10020206
Chicago/Turabian StyleHakuta, Ryunosuke, Hirofumi Kogure, Yousuke Nakai, Hiroshi Kawakami, Hiroyuki Maguchi, Tsuyoshi Mukai, Takuji Iwashita, Tomotaka Saito, Osamu Togawa, Saburo Matsubara, and et al. 2021. "Unilateral versus Bilateral Endoscopic Nasobiliary Drainage and Subsequent Metal Stent Placement for Unresectable Malignant Hilar Obstruction: A Multicenter Randomized Controlled Trial" Journal of Clinical Medicine 10, no. 2: 206. https://doi.org/10.3390/jcm10020206
APA StyleHakuta, R., Kogure, H., Nakai, Y., Kawakami, H., Maguchi, H., Mukai, T., Iwashita, T., Saito, T., Togawa, O., Matsubara, S., Hayashi, T., Maetani, I., Ito, Y., Hasebe, O., Itoi, T., Hanada, K., & Isayama, H. (2021). Unilateral versus Bilateral Endoscopic Nasobiliary Drainage and Subsequent Metal Stent Placement for Unresectable Malignant Hilar Obstruction: A Multicenter Randomized Controlled Trial. Journal of Clinical Medicine, 10(2), 206. https://doi.org/10.3390/jcm10020206









