A Prospective Study of Association of Micronutrients Deficiencies during Pregnancy and Neonatal Outcome among Women after Bariatric Surgery
Abstract
1. Introduction
2. Methods
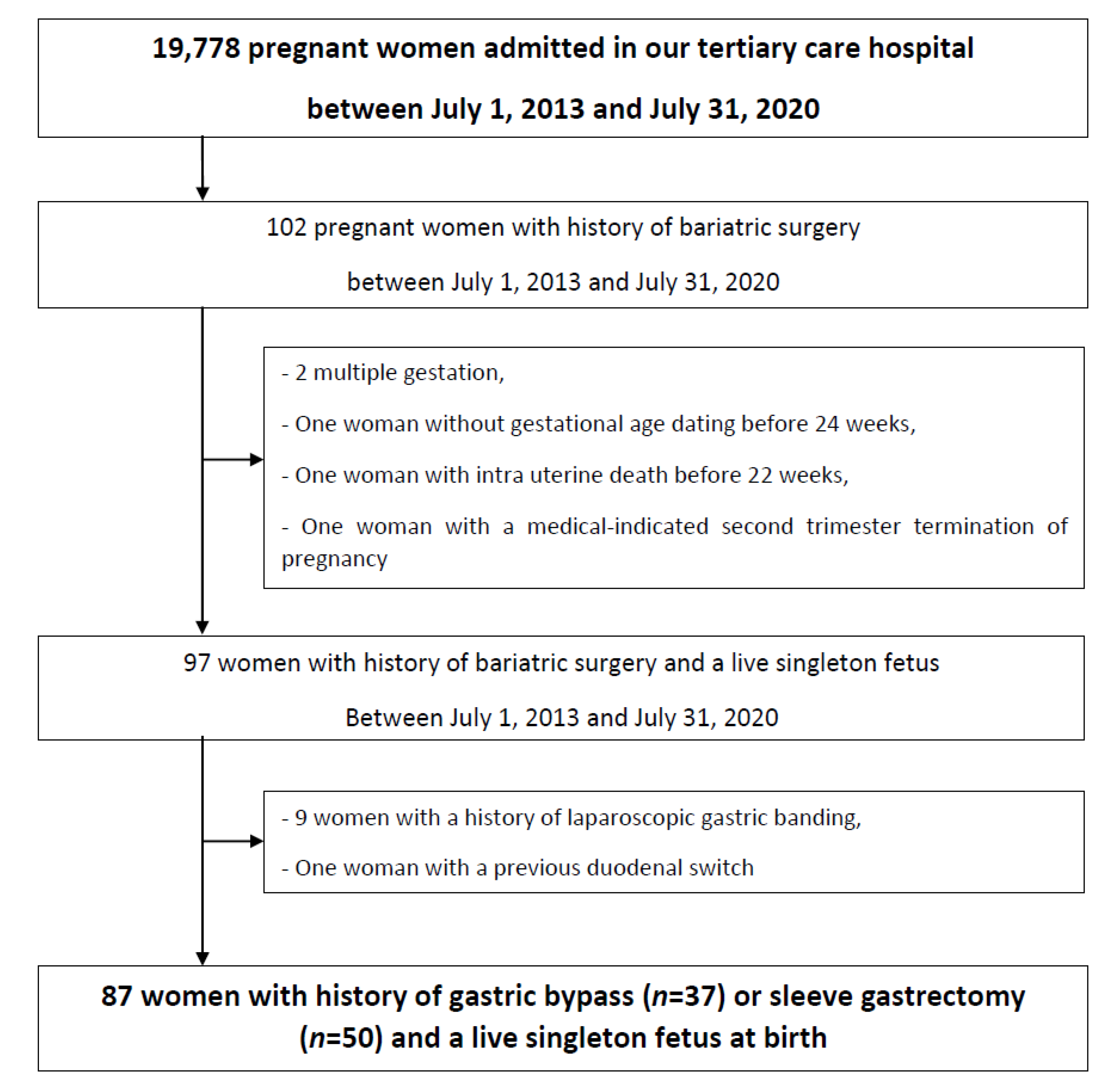
2.1. Patient Selection
2.2. Management of Pregnant Women with Previous Bariatric Surgery
2.3. Maternal and Perinatal Outcome
2.4. Biological Tests
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- INSERM/KANTAR HEALTH/ROCHE. ObEpi-Roche, Enquête Epidémiologique de Référence sur L’évolution de L’obésité et du Surpoids en France. 2012. Available online: https://www.roche.fr/content/dam/rochexx/roche-fr/roche_france/fr_FR/doc/obepi_2012.pdf (accessed on 1 November 2020).
- Robinson, H.E.; O’Connell, C.M.; Joseph, K.S.; McLeod, N.L. Maternal outcomes in pregnancies complicated by obesity. Obstet. Gynecol. 2005, 106, 1357–1364. [Google Scholar] [CrossRef]
- Yu, C.K.; Teoh, T.G.; Robinson, S. Obesity in pregnancy. BJOG 2006, 113, 1117–1125. [Google Scholar] [CrossRef]
- Arterburn, D.E.; Telem, D.A.; Kushner, F.; Courcoulas, A.P. Benefits and risks of bariatric surgery in adults: A review. JAMA 2020, 324, 879–887. [Google Scholar] [CrossRef]
- Roos, N.; Neovius, M.; Cnattingius, S.; Lagerros, Y.T.; Sääf, M.; Granath, F.; Stephansson, O. Perinatal outcomes after bariatric surgery: Nationwide population based matched cohort study. BMJ Open Diabetes Res. Care 2013, 347, f6460. [Google Scholar] [CrossRef]
- Johansson, K.; Cnattingius, S.; Näslund, I.; Roos, N.; Trolle Lagerros, Y.; Granath, F. Outcomes of pregnancy after bariatric surgery. N. Engl. J. Med. 2015, 372, 814–824. [Google Scholar] [CrossRef]
- Kwong, W.; Tomlinson, G.; Feig, D.S. Maternal and neonatal outcomes after bariatric surgery; a systematic review and meta-analysis: Do the benefits outweigh the risks? Am. J. Obstet. Gynecol. 2018, 218, 573–580. [Google Scholar] [CrossRef]
- Damti, P.; Friger, M.; Landau, D.; Sergienko, R.; Sheiner, E. Offspring of women following bariatric surgery and those of patients with obesity are at an increased risk for long-term pediatric endocrine morbidity. Arch. Gynecol. Obstet. 2019, 300, 1253–1259. [Google Scholar] [CrossRef]
- Zarshenas, N.; Tapsell, L.C.; Neale, E.P.; Batterham, M.; Talbot, M.L. The relationship between bariatric surgery and diet quality: A systematic review. Obes. Surg. 2020, 30, 1768–1792. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Apovian, C.; Brethauer, S.; Garvey, T.W.; Joffe, A.M.; Kim, J.; Kushner, R.F.; Lindquist, R.; Pessah-Pollack, R.; Seger, J.; et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures-2019 update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, the Obesity Society, American Society for Metabolic and Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Obesity 2020, 28, o1–o58. [Google Scholar]
- Ciangura, C.; Coupaye, M.; Deruelle, P.; Gascoin, G.; Calabrese, D.; Cosson, E.; Ducarme, G.; Gaborit, B.; Lelièvre, B.; Mandelbrot, L.; et al. Clinical practice guidelines for childbearing female candidates for bariatric surgery, pregnancy, and post-partum management after bariatric surgery. Obes. Surg. 2019, 29, 3722–3734. [Google Scholar] [CrossRef]
- Jans, G.; Matthys, C.; Bogaerts, A.; Lannoo, M.; Verhaeghe, J.; Van Der Schueren, B.; Devlieger, R. Maternal micronutrient deficiencies and related adverse neonatal outcomes after bariatric surgery: A systematic review. Adv. Nutr. 2015, 6, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Hazart, J.; Le Guennec, D.; Accoceberry, M.; Lemery, D.; Mulliez, A.; Farigon, N.; Lahaye, C.; Miolanne-Debouit, M.; Boirie, Y. Maternal nutritional deficiencies and small-for-gestational-age neonates at birth of women who have undergone bariatric surgery. J. Pregnancy 2017, 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mead, N.C.; Sakkatos, P.; Sakellaropoulos, G.C.; Adonakis, G.L.; Alexandrides, T.; Kalfarentzos, F. Pregnancy outcomes and nutritional indices after 3 types of bariatric surgery performed at a single institution. Surg. Obes. Relat. Dis. 2014, 10, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Coupaye, M.; Legardeur, H.; Sami, O.; Calabrese, D.; Mandelbrot, L.; LeDoux, S. Impact of Roux-en-Y gastric bypass and sleeve gastrectomy on fetal growth and relationship with maternal nutritional status. Surg. Obes. Relat. Dis. 2018, 14, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Ledoux, S.; Calabrese, D.; Bogard, C.; Dupré, T.; Castel, B.; Msika, S.; Larger, E.; Coupaye, M. Long-term evolution of nutritional deficiencies after gastric bypass: An assessment according to compliance to medical care. Ann. Surg. 2014, 259, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 13. Management of Diabetes in Pregnancy. Diabetes Care. 2017, 40, s114–s119. [Google Scholar] [CrossRef]
- International Association of Diabetes Pregnancy Study Groups. Recommendations on the diagnosis and classification of hyperglycaemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef]
- Cosson, E.; Pigeyre, M.; Ritz, P. Diagnosis and management of patients with significantly abnormal glycaemic profiles during pregnancy after bariatric surgery: PRESAGE (Pregnancy with significantly abnormal glycaemic exposure—bariatric patients). Diabetes Metab. 2018, 44, 376–379. [Google Scholar] [CrossRef]
- Hadlock, F.P.; Harrist, R.; Sharman, R.S.; Deter, R.L.; Park, S.K. Estimation of fetal weight with the use of head, body, and femur measurements—A prospective study. Am. J. Obstet. Gynecol. 1985, 151, 333–337. [Google Scholar] [CrossRef]
- Vayssière, C.; Sentilhes, L.; Langer, B.; Malan, V.; Marcorelles, P.; Nizard, J.; Perrotin, F.; Salomon, L.J.; Senat, M.V.; Serry, A.; et al. Fetal growth restriction and intra-uterine growth restriction: Guidelines for clinical practice from the French College of Gynaecologists and Obstetricians. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 193, 10–18. [Google Scholar] [CrossRef]
- Institute of Medicine. Weight Gain During Pregnancy: Reexamining the Guidelines; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Pusl, T.; Beuers, U. Intrahepatic cholestasis of pregnancy. Orphanet J. Rare Dis. 2007, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. Task force on hypertension in pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ task force on hypertension in pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar]
- Vayssière, C.; Haumonté, J.-B.; Chantry, A.; Coatleven, F.; Debord, M.P.; Gomez, C.; Le Ray, C.; Lopez, E.; Salomon, L.J.; Senat, M.V.; et al. Prolonged and post-term pregnancies: Guidelines for clinical practice from the French College of Gynecologists and Obstetricians (CNGOF). Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 169, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Salomon, L.J.; Bernard, J.P.; de Stavola, B.; Kenward, M.; Ville, Y. Birth weight and size: Charts and equations. J. Gynecol. Obstet. Biol. Reprod. 2007, 36, 50–56. [Google Scholar] [CrossRef]
- Abbassi-Ghanavati, M.; Greer, L.G.; Cunningham, F.G. Pregnancy and laboratory studies: A reference table for clinicians. Obstet. Gynecol. 2010, 115, 868–869. [Google Scholar] [CrossRef]
- Kjaer, M.M.; Lauenborg, J.; Breum, B.M.; Nilas, L. The risk of adverse pregnancy outcome after bariatric surgery: A nationwide register-based matched cohort study. Am. J. Obstet. Gynecol. 2013, 208, 464. [Google Scholar] [CrossRef]
- Santulli, P.; Mandelbrot, L.; Facchiano, E.; Dussaux, C.; Ceccaldi, P.-F.; LeDoux, S.; Msika, S. Obstetrical and neonatal outcomes of pregnancies following gastric bypass surgery: A retrospective cohort study in a French referral centre. Obes. Surg. 2010, 20, 1501–1508. [Google Scholar] [CrossRef]
- Ducarme, G.; Parisio, L.; Santulli, P.; Carbillon, L.; Mandelbrot, L.; Luton, D. Neonatal outcomes in pregnancies after bariatric surgery: A retrospective multi-centric cohort study in three French referral centers. J. Matern. Neonatal Med. 2012, 26, 275–278. [Google Scholar] [CrossRef]
- Guelinckx, I.; Devlieger, R.; Donceel, P.; Bel, S.; Pauwels, S.; Bogaerts, A.; Thijs, I.; Schurmans, K.; Deschilder, P.; VanSant, G. Lifestyle after bariatric surgery: A multicenter, prospective cohort study in pregnant women. Obes. Surg. 2012, 22, 1456–1464. [Google Scholar] [CrossRef]
- Maggard, M.; Yermilov, I.; Livingston, E.; Shekelle, P.; Li, Z.; Maglione, M.; Newberry, S.; Suttorp, M.; Hilton, L.; Santry, H.; et al. Pregnancy and fertility following bariatric surgery: A systematic review. Obstet. Anesth. Dig. 2009, 29, 179. [Google Scholar] [CrossRef]
- Rottenstreich, A.; Elazary, R.; Goldenshluger, A.; Pikarsky, A.J.; Elchalal, U.; Ben-Porat, T. Maternal nutritional status and related pregnancy outcomes following bariatric surgery: A systematic review. Surg. Obes. Relat. Dis. 2019, 15, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Ruz, M.; Carrasco, F.; Rojas, P.; Codoceo, J.; Inostroza, J.; Basfi-Fer, K.; Csendes, A.; Papapietro, K.; Pizarro, F.; Olivares, M.; et al. Zinc absorption and zinc status are reduced after Roux-en-Y gastric bypass: A randomized study using 2 supplements. Am. J. Clin. Nutr. 2011, 94, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Durán, C.; Weisstaub, G. Zinc supplementation and growth of the fetus and low birth weight infant. J. Nutr. 2003, 133, 1494S–1497S. [Google Scholar] [CrossRef] [PubMed]
- Hovdenak, N.; Haram, K. Influence of mineral and vitamin supplements on pregnancy outcome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 164, 127–132. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Zadeh, M.H.; Farsani, G.M.; Zamaninour, N. Selenium status after roux-en-Y gastric bypass: Interventions and recommendations. Obes. Surg. 2019, 29, 3743–3748. [Google Scholar] [CrossRef]
- Toh, S.Y.; Zarshenas, N.; Jorgensen, J. Prevalence of nutrient deficiencies in bariatric patients. Nutrition 2009, 25, 1150–1156. [Google Scholar] [CrossRef]
- Sawaya, R.A.; Jaffe, J.; Friedenberg, L.; Friedenberg, F.K. Vitamin, mineral, and drug absorption following bariatric surgery. Curr. Drug Metab. 2012, 13, 1345–1355. [Google Scholar] [CrossRef]
- Smelt, H.J.M.; Pouwels, S.; Smulders, J.F.; Hazebroek, E.J. Patient adherence to multivitamin supplementation after bariatric surgery: A narrative review. J. Nutr. Sci. 2020, 9. [Google Scholar] [CrossRef]
| Roux-en-Y Gastric Bypass, n = 37 (42.5%) | Sleeve Gastrectomy, n = 50 (57.5%) | p-Value | |
|---|---|---|---|
| Age, years | 34.3 ± 4.9 | 31.1 ± 4.6 | 0.003 |
| Caucasian | 36 (97.3) | 47 (94.0) | 0.63 |
| Pre-pregnancy BMI, kg/m2 | 29.8 ± 5.6 | 32.9 ± 7.1 | 0.03 |
| Obesity (BMI ≥ 30 kg/m2) | 16 (43.2) | 32 (64.0) | 0.06 |
| Pre-existing type 1 or 2 diabetes | 2 (5.4) | 3 (6.0) | 0.93 |
| Chronic hypertension | 1 (2.7) | 1 (2.0) | 0.85 |
| Smoking during pregnancy | 7 (18.9) | 19 (38.0) | 0.06 |
| Time to conception from BS, months | 64.5 ± 47.0 | 38.1 ± 28.1 | 0.002 |
| Nulliparity | 10 (27.0) | 16 (32.0) | 0.62 |
| Gestational weight gain, kg | 10.2 ± 5.4 | 10.3 ± 4.7 | 0.87 |
| Adequate weight gain during pregnancy | 25 (67.6) | 30 (60.0) | 0.47 |
| GDM | 11 (29.7) | 13 (26.0) | 0.70 |
| Pregnancy-induced hypertension/Pre-eclampsia | 2 (5.4) | 5 (10.0) | 0.48 |
| Intrahepatic cholestasis of pregnancy | 0 | 1 (2.0) | 0.66 |
| Additional supplementations after blood tests | 12 (32.4) | 16 (32.0) | 0.96 |
| Gestational age at delivery, weeks | 39.2 ± 1.1 | 39.0 ± 1.8 | 0.51 |
| Spontaneous labor | 19 (51.4) | 23 (46.0) | 0.63 |
| Induced labor | 16 (43.2) | 16 (32.0) | 0.29 |
| Mode of delivery | |||
| Planned cesarean delivery before labor | 2 (5.4) | 11 (22.0) | 0.03 |
| Cesarean delivery during labor | 3 (8.1) | 5 (10.0) | 0.79 |
| Vaginal delivery | 32 (86.5) | 34 (68.0) | 0.05 |
| Postpartum hemorrhage | 3 (8.1) | 4 (8.0) | 1.00 |
| Pre-term delivery (<37 wk) | 0 | 5 (10.0) | 0.07 |
| Birth weight, g | 3227 ± 499 | 3151 ± 373 | 0.42 |
| Birth weight z-score | 0.2 ± 1.1 | 0.2 ± 0.9 | 0.83 |
| SGA | 4 (10.8) | 3 (6.0) | 0.45 |
| Birth weight < 2500 g | 3 (8.1) | 1 (2.0) | 0.23 |
| LGA | 3 (8.1) | 2 (4.0) | 0.65 |
| Birth weight > 4000 g | 1 (2.7) | 0 | 0.66 |
| Cephalic perimeter, cm | 33.0 ± 1.7 | 33.0 ± 1.5 | 0.83 |
| 5 min Apgar score < 7 | 3 (8.1) | 3 (6.0) | 0.70 |
| pH < 7.10 | 0 | 3 (6.0) | 0.26 |
| Need for resuscitation or intubation | 0 | 0 | - |
| NICU admission | 4 (10.8) | 10 (20.0) | 0.25 |
| Neonatal death | 0 | 0 | - |
| Neonatal morbidity | 7 (18.9) | 11 (22.0) | 0.74 |
| Roux-en-Y Gastric Bypass, n = 37 (42.5%) | Sleeve Gastrectomy, n = 50 (57.5%) | p-Value | |
|---|---|---|---|
| Gestational age at blood samples, weeks | 27.9 ± 3.2 | 28.7 ± 2.6 | 0.18 |
| Additional supplementations after blood tests | 12 (32.4) | 16 (32.0) | 0.96 |
| Se (µg/L) | 66.3 ± 13.5 | 67.4 ± 10.2 | 0.68 |
| Deficiency * | 8 (21.6) | 7 (14.0) | 0.36 |
| Zinc (mg/L) | 1.0 ± 2.5 | 1.0 ± 2.0 | 0.93 |
| Deficiency * | 7 (18.9) | 0 | 0.002 |
| Vitamin A1 (µg/L) | 496.4 ± 623.6 | 503.3 ± 171.0 | 0.94 |
| Deficiency * | 13 (35.1) | 12 (24.0) | 0.26 |
| Vitamin B1 (µg/L) | 54.6 ± 18.2 | 55.4 ± 15.2 | 0.83 |
| Deficiency * | 4 (10.8) | 2 (4.0) | 0.40 |
| Vitamin B6 (µg/L) | 5.1 ± 6.5 | 3.8 ± 3.5 | 0.24 |
| Deficiency * | 29 (78.4) | 44 (88.0) | 0.23 |
| Vitamin C (mg/L) | 4.1 ± 3.0 | 4.8 ± 3.4 | 0.32 |
| Deficiency * | 30 (81.1) | 33 (66.0) | 0.12 |
| Vitamin E (mg/L) | 12.4 ± 3.6 | 13.2 ± 4.0 | 0.11 |
| Deficiency * | 8 (21.6) | 4 (8.0) | 0.07 |
| Variables | Birth Weight | Birth Weight Z-Score | ||
|---|---|---|---|---|
| Spearman’s Correlation Coefficient | p-Value | Spearman’s Correlation Coefficient | p-Value | |
| Age, years | 0.010 | 0.93 | 0.012 | 0.91 |
| BMI, kg/m2 | 0.188 | 0.08 | 0.191 | 0.11 |
| Time to conception from BS, months | 0.126 | 0.25 | 0.047 | 0.67 |
| Gestational weight gain, kg | 0.100 | 0.36 | −0.087 | 0.42 |
| Selenium deficiency | −0.230 | 0.03 | −0.265 | 0.01 |
| Zinc deficiency | −0.189 | 0.08 | −0.165 | 0.13 |
| Vitamin A deficiency | −0.034 | 0.76 | −0.048 | 0.67 |
| Vitamin B1 deficiency | −0.246 | 0.06 | −0.238 | 0.06 |
| Vitamin B6 deficiency | −0.055 | 0.61 | −0.033 | 0.76 |
| Vitamin C deficiency | −0.088 | 0.42 | −0.224 | 0.06 |
| Vitamin E deficiency | −0.094 | 0.39 | −0.128 | 0.42 |
| Gestational age at birth, weeks | 0.424 | <0.001 | −0.281 | 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ducarme, G.; Planche, L.; Abet, E.; Desroys du Roure, V.; Ducet-Boiffard, A. A Prospective Study of Association of Micronutrients Deficiencies during Pregnancy and Neonatal Outcome among Women after Bariatric Surgery. J. Clin. Med. 2021, 10, 204. https://doi.org/10.3390/jcm10020204
Ducarme G, Planche L, Abet E, Desroys du Roure V, Ducet-Boiffard A. A Prospective Study of Association of Micronutrients Deficiencies during Pregnancy and Neonatal Outcome among Women after Bariatric Surgery. Journal of Clinical Medicine. 2021; 10(2):204. https://doi.org/10.3390/jcm10020204
Chicago/Turabian StyleDucarme, Guillaume, Lucie Planche, Emeric Abet, Valérie Desroys du Roure, and Amélie Ducet-Boiffard. 2021. "A Prospective Study of Association of Micronutrients Deficiencies during Pregnancy and Neonatal Outcome among Women after Bariatric Surgery" Journal of Clinical Medicine 10, no. 2: 204. https://doi.org/10.3390/jcm10020204
APA StyleDucarme, G., Planche, L., Abet, E., Desroys du Roure, V., & Ducet-Boiffard, A. (2021). A Prospective Study of Association of Micronutrients Deficiencies during Pregnancy and Neonatal Outcome among Women after Bariatric Surgery. Journal of Clinical Medicine, 10(2), 204. https://doi.org/10.3390/jcm10020204





