A Multi-Center Cohort Study on Characteristics of Pain, Its Impact and Pharmacotherapeutic Management in Patients with ALS
Abstract
:1. Introduction
2. Methods
2.1. Patient Recruitment
2.2. Patient Characteristics
2.3. Telephone Interviews and Patient-Reported Assessment Instruments
2.4. Evaluation of Pain Patients without Pain Medication
2.5. Statistics
3. Results
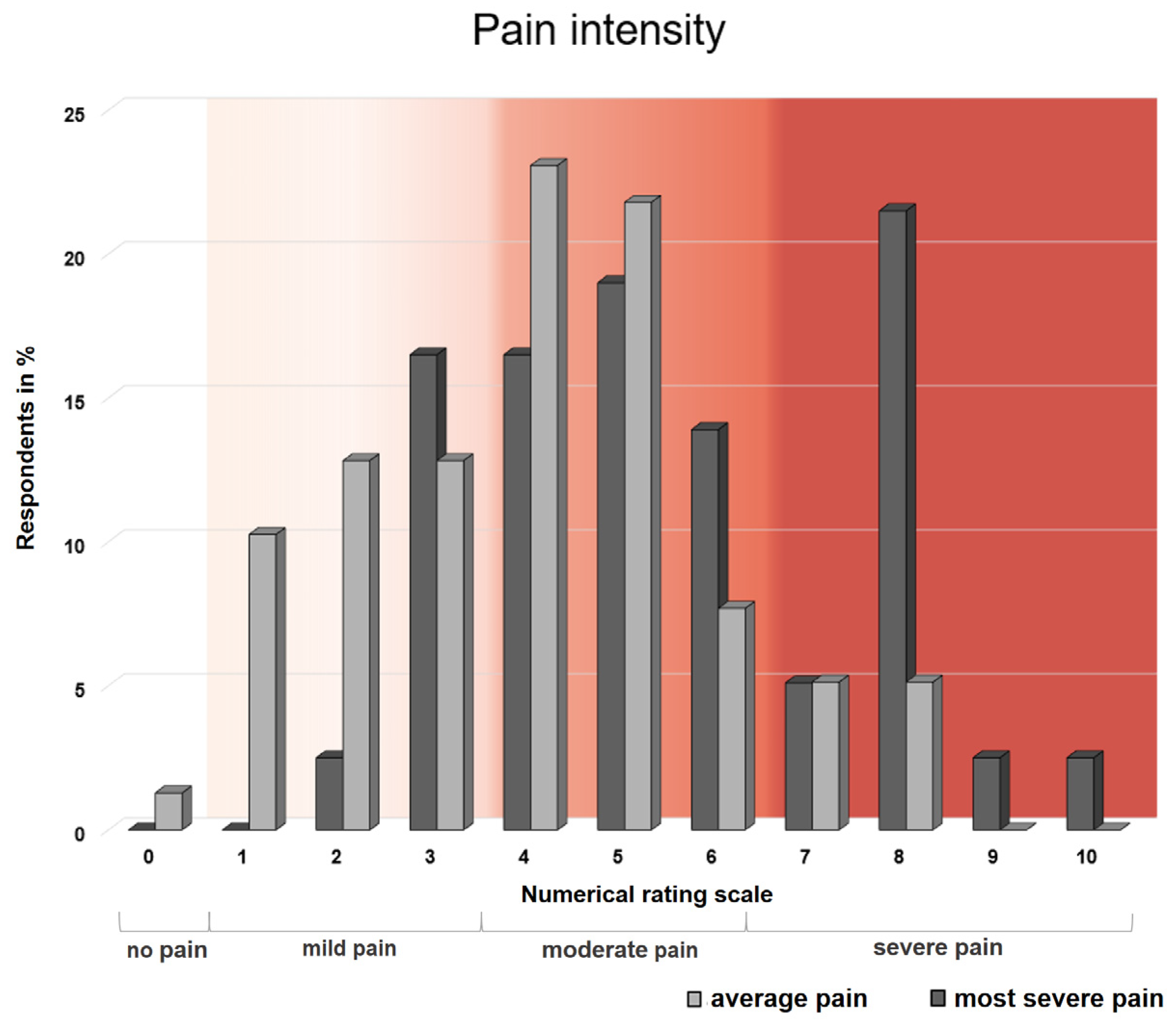
3.1. Pain Intensity
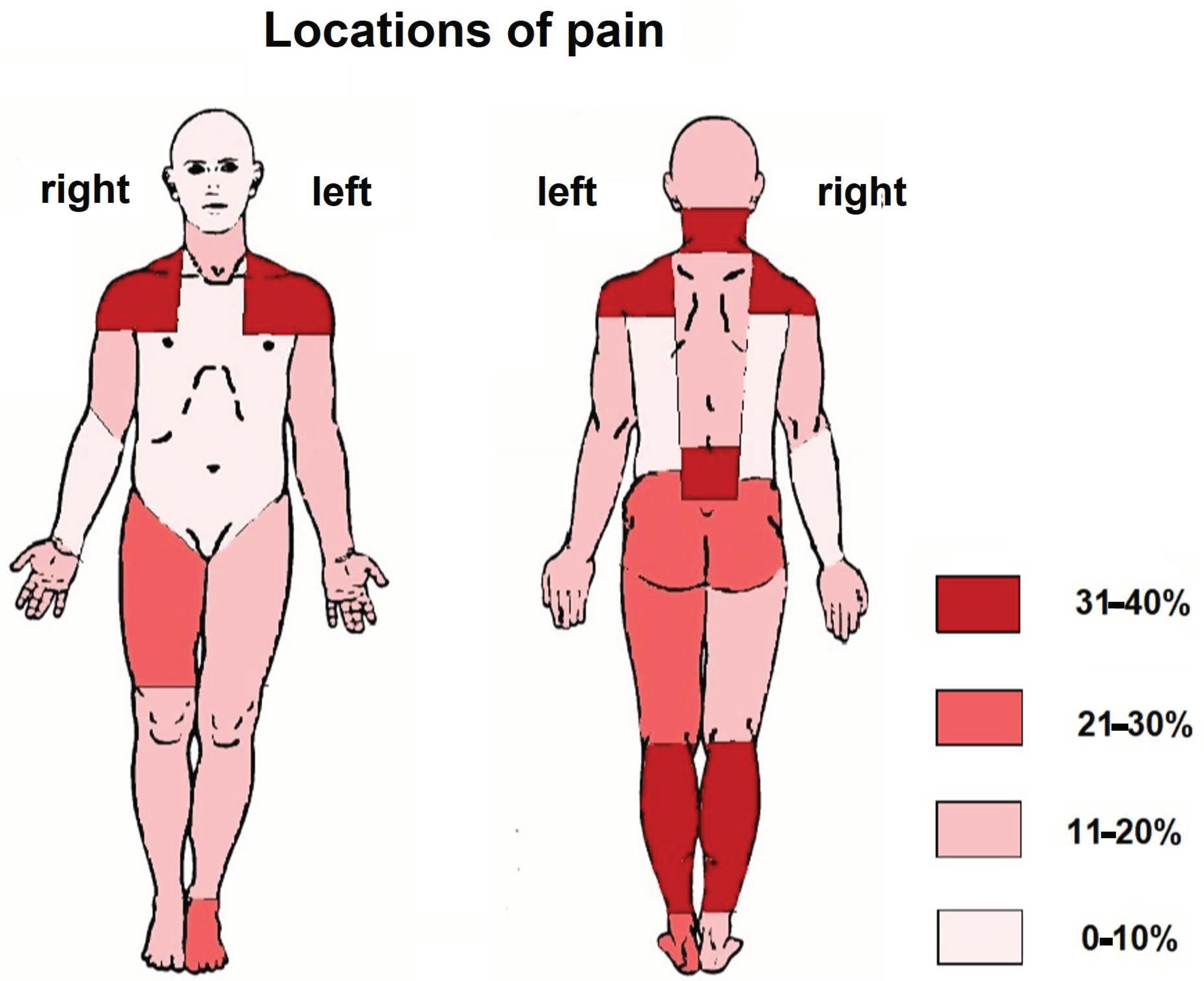
3.2. Locations of Pain
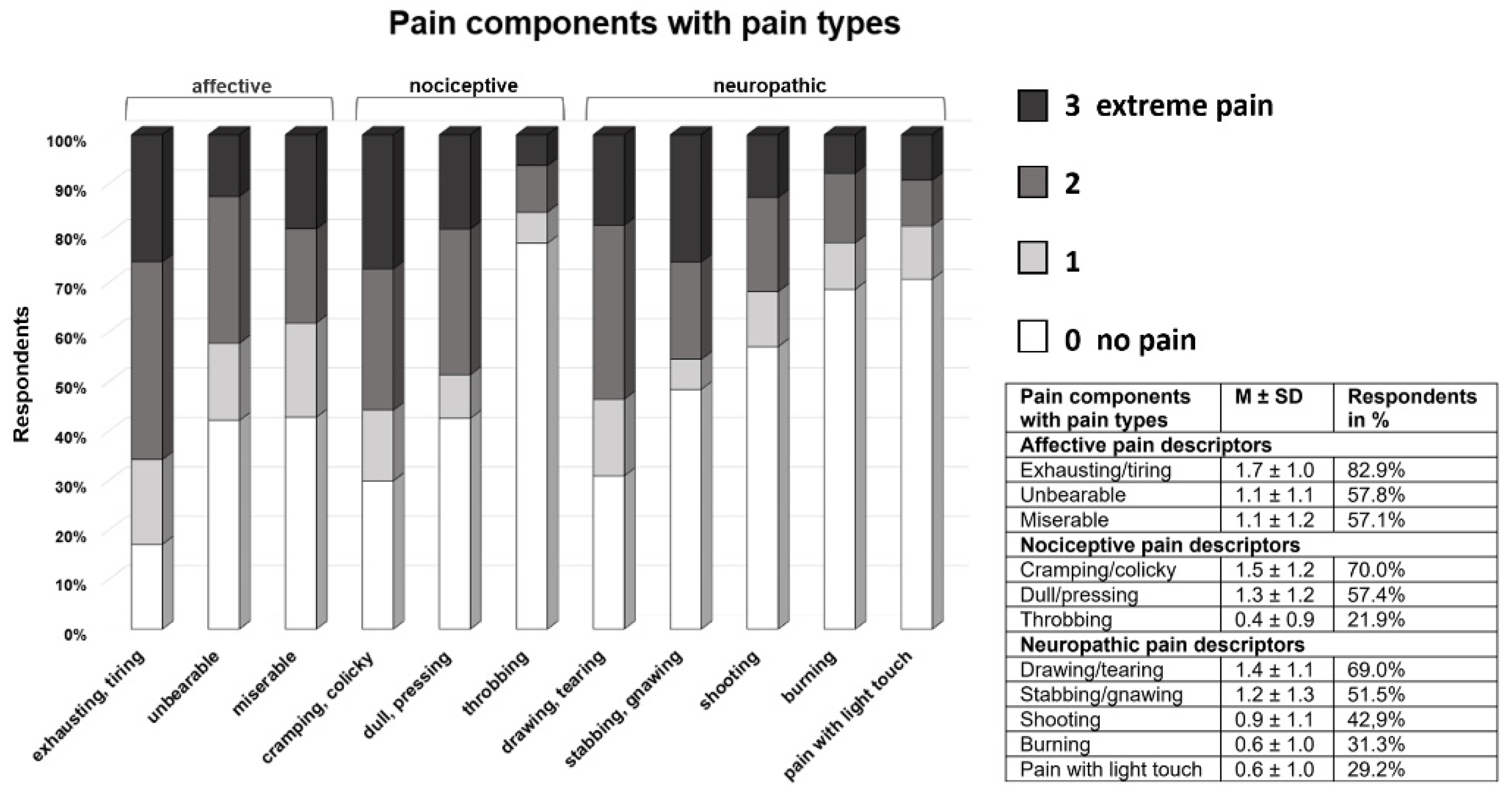
3.3. Pain Perception
3.4. Pain Interference with Daily Functions
3.5. Evaluation of Pharmacological Pain Therapy and the Patients’ Perceptions of Pain Relief
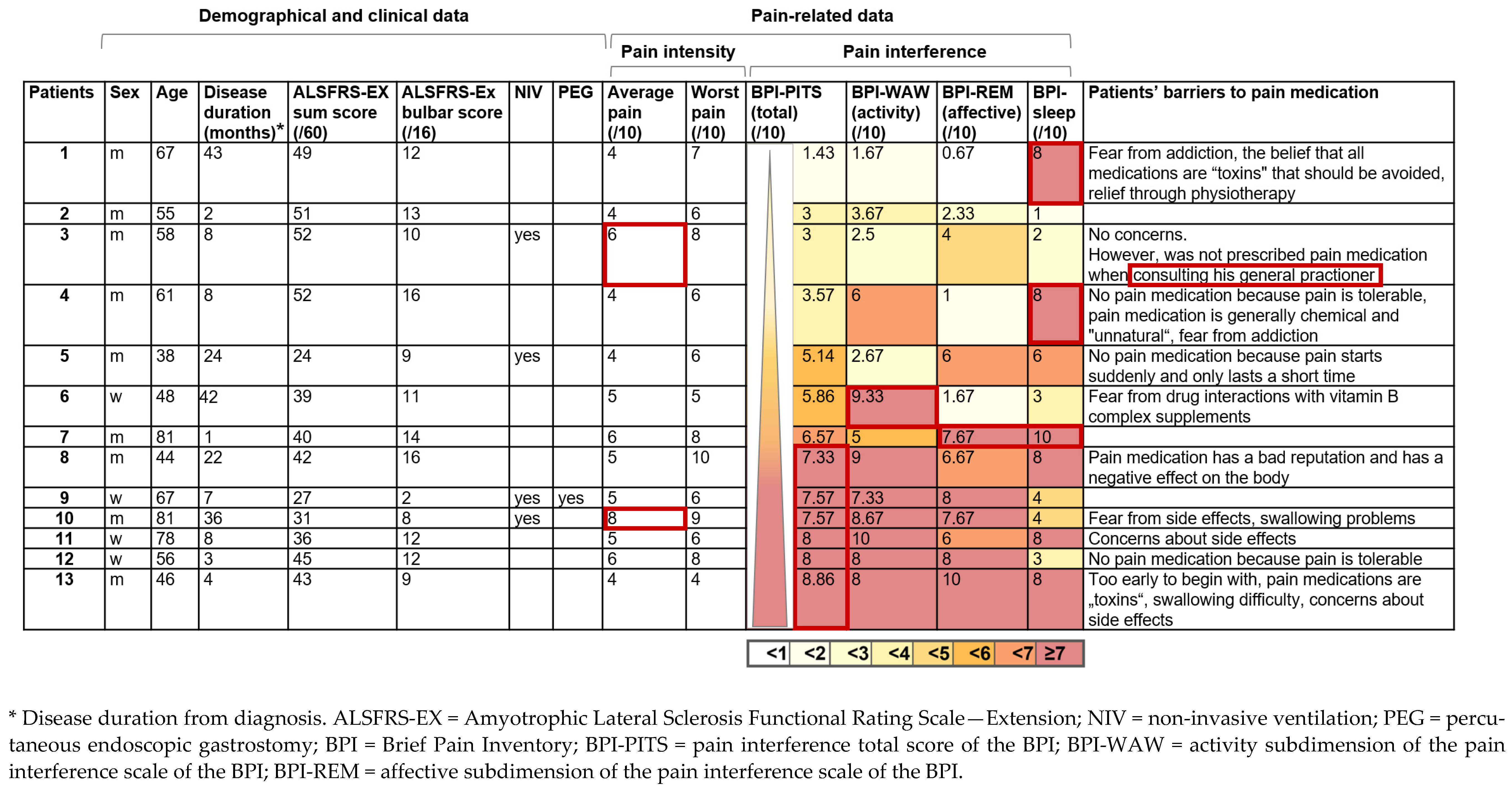
3.6. Characterization of the Pain Patients without Pain Medication
4. Discussion
4.1. Pain Characteristics and Impact
4.2. Pharmacotherapeutic Pain Management
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ringel, S.P.; Murphy, J.R.; Alderson, M.K.; Bryan, W.; England, J.D.; Miller, R.G.; Petajan, J.H.; Smith, S.A.; Roelofs, R.I.; Ziter, F.; et al. The natural history of amyotrophic lateral sclerosis. Neurology 1993, 43, 1316–1322. [Google Scholar] [CrossRef]
- Jenkins, T.M.; Hollinger, H.; McDermott, C.J. The evidence for symptomatic treatments in amyotrophic lateral sclerosis. Curr. Opin. Neurol. 2014, 27, 524–531. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, K.; Murphy, A.; McDonnell, E.; Shapiro, J.; Simpson, E.; Glass, J.; Mitsumoto, H.; Forshew, D.; Miller, R.; Atassi, N. Improving symptom management for people with amyotrophic lateral sclerosis. Muscle Nerve 2018, 57, 20–24. [Google Scholar] [CrossRef]
- Ganzini, L.; Johnston, W.S.; Hoffman, W.F. Correlates of suffering in amyotrophic lateral sclerosis. Neurology 1999, 52, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Wallace, V.C.J.; Ellis, C.M.; Burman, R.; Knights, C.; Shaw, C.E.; Al-Chalabi, A. The evaluation of pain in amyotrophic lateral sclerosis: A case controlled observational study. Amyotroph. Lateral Scler. Frontotemporal. Degener 2014, 15, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, F.; Skudlarek, A.; Berndt, J.; Kornhuber, M.E. Characteristics of pain in amyotrophic lateral sclerosis. Brain Behav. 2015, 5, e00296. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.C.G.; Galhardoni, R.; Silva, V.; Jorge, F.M.H.; Yeng, L.T.; Callegaro, D.; Chadi, G.; Teixeira, M.J.; Ciampi de Andrade, D. Beyond weakness: Characterization of pain, sensory profile and conditioned pain modulation in patients with motor neuron disease: A controlled study. Eur. J. Pain 2018, 22, 72–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wicks, P. Reassessing received wisdom in ALS–pain is common when studied systematically. Eur. J. Neurol. 2012, 19, 531–532. [Google Scholar] [CrossRef]
- Edge, R.; Mills, R.; Tennant, A.; Diggle, P.J.; Young, C.A.; TONiC Study Group. Do pain, anxiety and depression influence quality of life for people with amyotrophic lateral sclerosis/motor neuron disease? A national study reconciling previous conflicting literature. J. Neurol. 2020, 267, 607–615. [Google Scholar] [CrossRef] [Green Version]
- Stephens, H.E.; Lehman, E.; Raheja, D.; Yang, C.; Walsh, S.; Mcarthur, D.B.; Simmons, Z. Pain in amyotrophic lateral sclerosis: Patient and physician perspectives and practices. Amyotroph. Lateral Scler. Frontotemporal Degener. 2016, 17, 21–29. [Google Scholar] [CrossRef]
- Pizzimenti, A.; Aragona, M.; Onesti, E.; Inghilleri, M. Depression, pain and quality of life in patients with amyotrophic lateral sclerosis: A cross-sectional study. Funct. Neurol. 2013, 28, 115–119. [Google Scholar] [CrossRef]
- Chiò, A.; Canosa, A.; Gallo, S.; Moglia, C.; Ilardi, A.; Cammarosano, S.; Papurello, D.; Calvo, A. Pain in amyotrophic lateral sclerosis: A population-based controlled study. Eur. J. Neurol. 2012, 19, 551–555. [Google Scholar] [CrossRef]
- Ng, L.; Khan, F.; Young, C.A.; Galea, M. Cochrane Neuromuscular Group. Symptomatic treatments for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst. Rev. 2017, 8, 1–22. [Google Scholar]
- Brettschneider, J.; Kurent, J.; Ludolph, A. Cochrane Neuromuscular Group. Drug therapy for pain in amyotrophic lateral sclerosis or motor neuron disease. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moisset, X.; Cornut-Chauvinc, C.; Clavelou, P.; Pereira, B.; Dallel, R.; Guy, N. Is there pain with neuropathic characteristics in patients with amyotrophic lateral sclerosis? A cross-sectional study. Pall. Med. 2016, 30, 486–494. [Google Scholar] [CrossRef]
- Ishida, N.; Hongo, S.; Kumano, A.; Hatta, H.; Zakoji, N.; Hirutani, M.; Yamamoto, Y.; Aono, H.; Tuigi, M.; Suzuki, R.; et al. Relationship between pain and functional status in patients with amyotrophic lateral sclerosis: A multicenter cross-sectional study. J. Palliat. Med. 2018, 21, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L. World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2000, 1, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.R.; Wicks, P.; Brownstein, C.A.; Massagli, M.P.; Toronjo, M.; Talbot, K.; Al-Chalabi, A. Concordance between site of onset and limb dominance in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2011, 82, 853–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roche, J.C.; Rojas-Garcia, R.; Scott, K.M.; Scotton, W.; Ellis, C.E.; Burman, R.; Wijesekera, L.; Turner, M.R.; Leigh, P.N.; Shaw, C.E.; et al. A proposed staging system for amyotrophic lateral sclerosis. Brain 2012, 135, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Balendra, R.; Jones, A.; Jivraj, N.; Knights, C.; Ellis, C.M.; Burman, R.; Turner, M.R.; Leigh, P.N.; Shaw, C.E.; Al-Chalabi, A. Estimating clinical stage of amyotrophic lateral sclerosis from the ALS Functional Rating Scale. Amyotroph. Lateral Scler. Front. Degener. 2014, 15, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Wicks, P.; Massagli, M.P.; Wolf, C.; Heywood, J. Measuring function in advanced ALS validation of ALSFRS-EX extension items. Eur. J. Neurol. 2009, 16, 353–359. [Google Scholar] [CrossRef]
- Abdulla, S.; Vielhaber, S.; Körner, S.; Machts, J.; Heinze, H.J.; Dengler, R.; Petri, S. Validation of the German version of the extended ALS functional rating scale as a patient-reported outcome measure. J. Neurol. 2013, 260, 2242–2255. [Google Scholar] [CrossRef] [PubMed]
- Radbruch, L.; Loick, G.; Kiencke, P.; Lindena, G.; Sabatowski, R.; Grond, S.; Lehmann, K.A.; Cleeland, C.S. Validation of the German version of the Brief Pain Inventory. J. Pain Symptom Manag. 1999, 18, 180–187. [Google Scholar] [CrossRef]
- Brown, K.E.; Swift, I.; Spark, M.J. Pain severity cut-points and analgesic use by community-dwelling people for chronic pain. J. Pharm. Pract. Res. 2012, 42, 196–199. [Google Scholar] [CrossRef]
- Dworkin, R.H.; Turk, D.C.; Wyrwich, K.W.; Beaton, D.; Cleeland, C.S.; Farrar, J.T.; Haythornthwaite, J.A.; Jensen, M.P.; Kerns, R.D.; Ader, D.N.; et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J. Pain 2008, 9, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Cleeland, C.S. The Brief Pain Inventory User Guide. 2009. Available online: https://www.mdanderson.org/documents/Departments-and-Divisions/Symptom-Research/BPI_UserGuide.pdf (accessed on 26 June 2021).
- Miettinen, T.; Kautiainen, H.; Mäntyselkä, P.; Linton, S.J.; Kalso, E. Pain interference type and level guide the assessment process in chronic pain: Categorizing pain patients entering tertiary pain treatment with the Brief Pain Inventory. PLoS ONE 2019, 14, e0221437. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioural Sciences; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Ellis, C.M.; Simmons, A.; Jones, D.K.; Bland, J.; Dawson, J.M.; Horsfield, M.A.; Williams SCLeigh, P.N. Diffusion tensor MRI assesses corticospinal tract damage in ALS. Neurology 1999, 53, 1051–1058. [Google Scholar] [CrossRef]
- Fillingim, R.B.; Loeser, J.D.; Baron, R.; Edwards, R.R. Assessment of chronic pain: Domains, methods, and mechanisms. J. Pain 2016, 17 (Suppl. 9), T10–T20. [Google Scholar] [CrossRef] [Green Version]
- Rivera, I.; Ajroud-Driss, S.; Casey, P.; Heller, S.; Allen, J.; Siddique, T.; Sufit, R. Prevalence and characteristics of pain in early and late stages of ALS. Amyotroph. Lateral Scler. Front. Degener. 2013, 14, 369–372. [Google Scholar] [CrossRef]
- Handy, C.A.; Krudy, C.; Boulis, N.; Federici, T. Pain in amyotrophic lateral sclerosis: A neglected aspect of disease. Neurol. Res. Int. 2011, 2011, 403808. [Google Scholar] [CrossRef] [Green Version]
- Chiò, A.; Mora, G.; Lauria, G. Pain in amyotrophic lateral sclerosis. Lancet Neurol. 2017, 16, 144–157. [Google Scholar] [CrossRef]
- Bourke, S.C.; Tomlinson, M.; Williams, T.L.; Bullock, R.E.; Shaw, P.J.; Gibson, G.J. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: A randomized controlled trial. Lancet Neurol. 2006, 5, 140–147. [Google Scholar] [CrossRef]
- Dorst, J.; Ludolph, A.C. Non-invasive ventilation in amyotrophic lateral sclerosis. Ther. Adv. Neurol. Disord. 2019, 12, 1756286419857040. [Google Scholar] [CrossRef] [PubMed]
- Pagnini, F.; Lunetta, C.; Banfi, P.; Rossi, G.; Fossati, F.; Marconi, A.; Castelnuovo, G.; Corbo, M.; Molinari, E. Pain in amyotrophic lateral sclerosis: A psychological perspective. Neurol. Sci. 2012, 33, 1193–1196. [Google Scholar] [CrossRef]
- Gilron, I.; Tu, D.; Holden, R.R. Sensory and affective pain descriptors respond differentially to pharmacological interventions in neuropathic conditions. Clin. J. Pain 2013, 29, 124–131. [Google Scholar] [CrossRef]
- Vardeh, D.; Mannion, R.J.; Woolf, C.J. Towards a mechanism-based approach to pain diagnosis. J. Pain 2016, 17 (Suppl. 9), T50–T69. [Google Scholar] [CrossRef] [Green Version]
- Rudnicki, S.; McVey, A.L.; Jackson, C.E.; Dimachkie, M.M.; Barohn, R.J. Symptom management and end of life care. Neurol. Clin. 2015, 33, 889–908. [Google Scholar]
- Woo, A.; Lechner, B.; Fu, T.; Wong, C.S.; Chiu, N.; Lam, H.; Pulenzas, N.; Soliman, H.; DeAngelis, C.; Chow, E. Cut points for mild, moderate, and severe pain among cancer and non-cancer patients: A literature review. Ann. Palliat. Med. 2015, 4, 176–183. [Google Scholar]
- Sandstedt, P.; Littorin, S.; Johansson, S.; Gottberg, K.; Ytterberg, C.; Kierkegaard, M. Disability and contextual factors in patients with amyotrophic lateral sclerosis-A three-year observational study. J. Neuromuscul. Dis. 2018, 5, 439–449. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, M.D.; Ballantyne, J.C. Must we reduce pain intensity to treat chronic pain? Pain 2016, 157, 65–69. [Google Scholar] [CrossRef]
- El-Tallawy, S.N.; Nalamasu, R.; Salem, G.I.; LeQuang, J.A.K.; Pergolizzi, J.V.; Christo, P.J. Management of musculoskeletal pain: An update with emphasis on chronic musculoskeletal pain. Pain Ther. 2021, 10, 181–209. [Google Scholar] [CrossRef] [PubMed]

| Patients with Pain, n = 84 (56%) | Patients without Pain, n = 66 (44%) | p-Value | |
|---|---|---|---|
| Gender (female/male) | 24/60 (28.6/71.4%) | 20/46 (30.3/69.7%) | 0.817 |
| Patient age | 61.2 ± 11.8 | 66.6 ± 8.0 | 0.037 |
| Disease duration from diagnosis in months | 33.5 ± 38.5 | 37.7 ± 51.3 | 0.569 |
| Symptom onset | 0.135 | ||
| Bulbar | 14 (16.7%) | 19 (28.8%) | |
| Upper limb | 37 (44%) | 21 (31.8%) | |
| Lower limb | 31 (36.9%) | 22 (33.3%) | |
| King‘s clinical staging | 0.065 | ||
| Stage 1: Symptom onset/functional involvement of first region | 0 | 0 | |
| Stage 2A: Diagnosis | 7 (8.3%) | 5 (7.6%) | |
| Stage 2B: Functional involvement of a second region | 8 (9.5%) | 16 (24.2%) | |
| Stage 3: Functional involvement of a third region | 39 (46.4%) | 23 (34.8%) | |
| Stage 4A: Need for gastrostomy | 4 (4.8%) | 7 (10.6%) | |
| Stage 4B: Need for respiratory support (NIV) | 26 (31%) | 15 (22.7%) | |
| ALSFRS-EX | |||
| Sum score | 36.6 ± 12.9 | 42.6 ± 10.8 | 0.003 |
| Bulbar subscore | 11.6 ± 4.8 | 11.23 ± 3.4 | 0.344 |
| Fine motor subscore | 7.7 ± 4.3 | 10.0 ± 4.9 | 0.003 |
| Gross motor subscore | 8.2 ± 5.0 | 10.2 ± 4.6 | 0.012 |
| Respiratory subscore | 9.1 ± 3.2 | 10.2 ± 2.8 | 0.038 |
| Disease progression rate * | 0.9 ± 1.9 | 0.75 ± 0.9 | 0.639 |
| Pharmacotherapy * | Patients with Pain |
|---|---|
| Treatment for pain (n = 54 (64.3%)) | |
| Non-opioid analgesics (n = 45 (53.6%)) | |
| Non-steroidal anti-inflammatory drugs (NSAID) Metamizole Paracetamol COX-2 inhibitor drugs | 21 22 8 5 |
| Opioid analgesics (n = 16 (19%)) Low-potency opioids Tramadol Tilidine High-potency opioids Morphine Hydromorphone Fentanyl transdermal patch Buprenorphine sublingual tablet | 4 6 1 2 2 1 |
| Tricyclic antidepressants (n = 5 (6.0%)) ** | 5 |
| Anticonvulsants (n = 10 (12.0%)) Gabapentin Pregabalin | 2 8 |
| Treatment for cramping (n = 17 (20.2%)) | |
| Magnesium Quinine sulfate | 8 11 |
| Treatment for spasticity (n = 7 (8.3%)) | |
| Baclofen Tolperisone Intramuscular injections of botulinum toxin | 5 1 2 |
| Pain-Related Pharmacotherapy | Patients (n = 54) | Average Pain Relief in % | Degree of Pain Relief | ||||
|---|---|---|---|---|---|---|---|
| M ± SD | No | Minimal | Moderate | Substantial | No Information | ||
| Treatment with coanalgesics | 6 (11.1%) | 55 ± 39.4 | 1 (16.7%) | 1 (16.7%) | 0 | 4 (66.7%) | |
| Antidepressant alone | 3 | ||||||
| Anticonvulsant alone | 3 | ||||||
| Treatment with non-opioid analgesics | 32 (59.3%) | 43.8 ± 28 | 6 (18.8%) | 0 | 6 (18.8%) | 17 (53.1%) | 3 (9.4%) |
| Non-opioid analgesic alone | 23 | ||||||
| Combination of non-opioid analgesics | 3 | ||||||
| Non-opioid analgesic + antidepressant | 2 | ||||||
| Non-opioid analgesic + anticonvulsant | 4 | ||||||
| Opioid treatment with low-potency opioids | 10 (18.5%) | 36.7 ± 17.3 | 0 | 2 (20%) | 5 (50%) | 2 (20%) | 1 (10%) |
| Low-potency opioid alone | 1 | ||||||
| Low-potency opioid + anticonvulsant | 1 | ||||||
| Low-potency opioid + non-opioid analgesic | 7 | ||||||
| Low-potency opioid + non-opioid analgesic + anticonvulsant | 1 | ||||||
| Opioid treatment with high-potency opioids | 6 (11.1%) | 56.7 ± 23.4 | 0 | 1 (16.7%) | 1 (16.7%) | 4 (66.7%) | |
| High-potency opioid alone | 1 | ||||||
| High-potency opioid + non-opioid analgesic | 4 | ||||||
| High-potency opioid + non-opioid analgesic + anticonvulsant | 1 | ||||||
| Pain Patients without Pain Medication and NRS ≥4 (n = 13) | Pain Patients with Pain Medication (n = 54) | p-Value | |
|---|---|---|---|
| Gender (female/male) | 4/9 (30.8%/69.2%) | 18/36 (33.3%/66.7%) | 0.569 |
| Patient age | 60.0 ± 14.2 | 61.9 ± 12.5 | 0.625 |
| Disease duration from diagnosis in months | 16.0 ± 15.6 | 41.5 ± 43.9 | 0.001 |
| ALSFRS-EX | |||
| Sum score | 40.9 ± 9.3 | 33.0 ± 13.1 | 0.048 |
| Bulbar subscore | 11.1 ± 3.7 | 11.2 ± 4.8 | 0.906 |
| Fine motor subscore | 9.4 ± 4.2 | 6.6 ± 4.5 | 0.047 |
| Gross motor subscore | 10.3 ± 3.7 | 6.6 ± 4.7 | 0.006 |
| Respiratory subscore | 10.1 ± 1.4 | 8.8 ± 3.2 | 0.034 |
| PEG | 1 (7.7%) | 12 (23.1%) | 0.215 |
| NIV | 3 (23.1%) | 17 (32.7%) | 0.502 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogt, S.; Schlichte, I.; Schreiber, S.; Wigand, B.; Debska-Vielhaber, G.; Heitmann, J.; Meyer, T.; Dengler, R.; Petri, S.; Haghikia, A.; et al. A Multi-Center Cohort Study on Characteristics of Pain, Its Impact and Pharmacotherapeutic Management in Patients with ALS. J. Clin. Med. 2021, 10, 4552. https://doi.org/10.3390/jcm10194552
Vogt S, Schlichte I, Schreiber S, Wigand B, Debska-Vielhaber G, Heitmann J, Meyer T, Dengler R, Petri S, Haghikia A, et al. A Multi-Center Cohort Study on Characteristics of Pain, Its Impact and Pharmacotherapeutic Management in Patients with ALS. Journal of Clinical Medicine. 2021; 10(19):4552. https://doi.org/10.3390/jcm10194552
Chicago/Turabian StyleVogt, Susanne, Ina Schlichte, Stefanie Schreiber, Bernadette Wigand, Grazyna Debska-Vielhaber, Johanna Heitmann, Thomas Meyer, Reinhard Dengler, Susanne Petri, Aiden Haghikia, and et al. 2021. "A Multi-Center Cohort Study on Characteristics of Pain, Its Impact and Pharmacotherapeutic Management in Patients with ALS" Journal of Clinical Medicine 10, no. 19: 4552. https://doi.org/10.3390/jcm10194552
APA StyleVogt, S., Schlichte, I., Schreiber, S., Wigand, B., Debska-Vielhaber, G., Heitmann, J., Meyer, T., Dengler, R., Petri, S., Haghikia, A., & Vielhaber, S. (2021). A Multi-Center Cohort Study on Characteristics of Pain, Its Impact and Pharmacotherapeutic Management in Patients with ALS. Journal of Clinical Medicine, 10(19), 4552. https://doi.org/10.3390/jcm10194552







