Association between Serum Phosphate Levels and the Development of Aortic Stenosis in Patients Undergoing Hemodialysis
Abstract
:1. Introduction
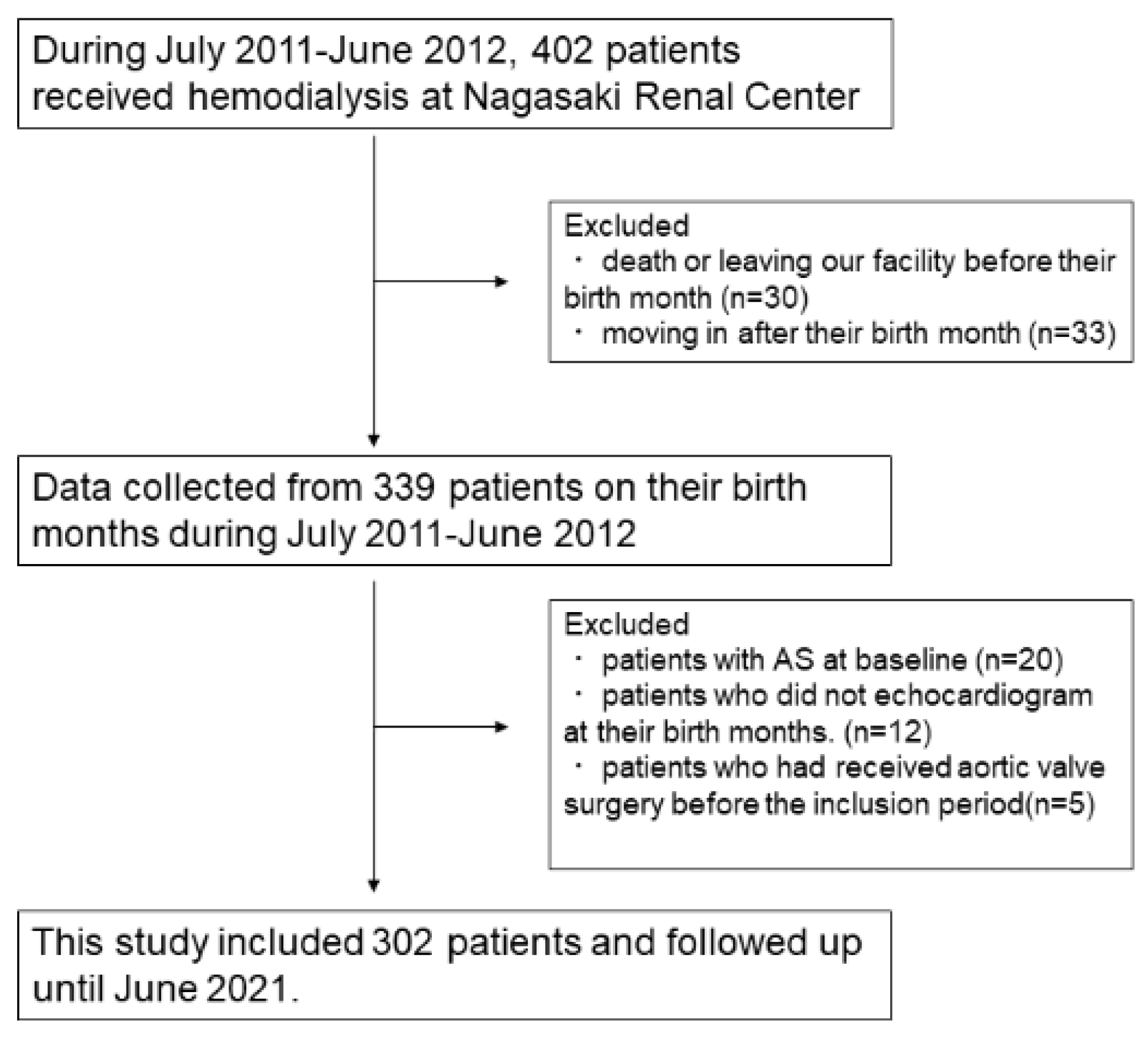
2. Materials and Methods
2.1. Patients
2.2. Data Collection
2.3. Statistical Analysis
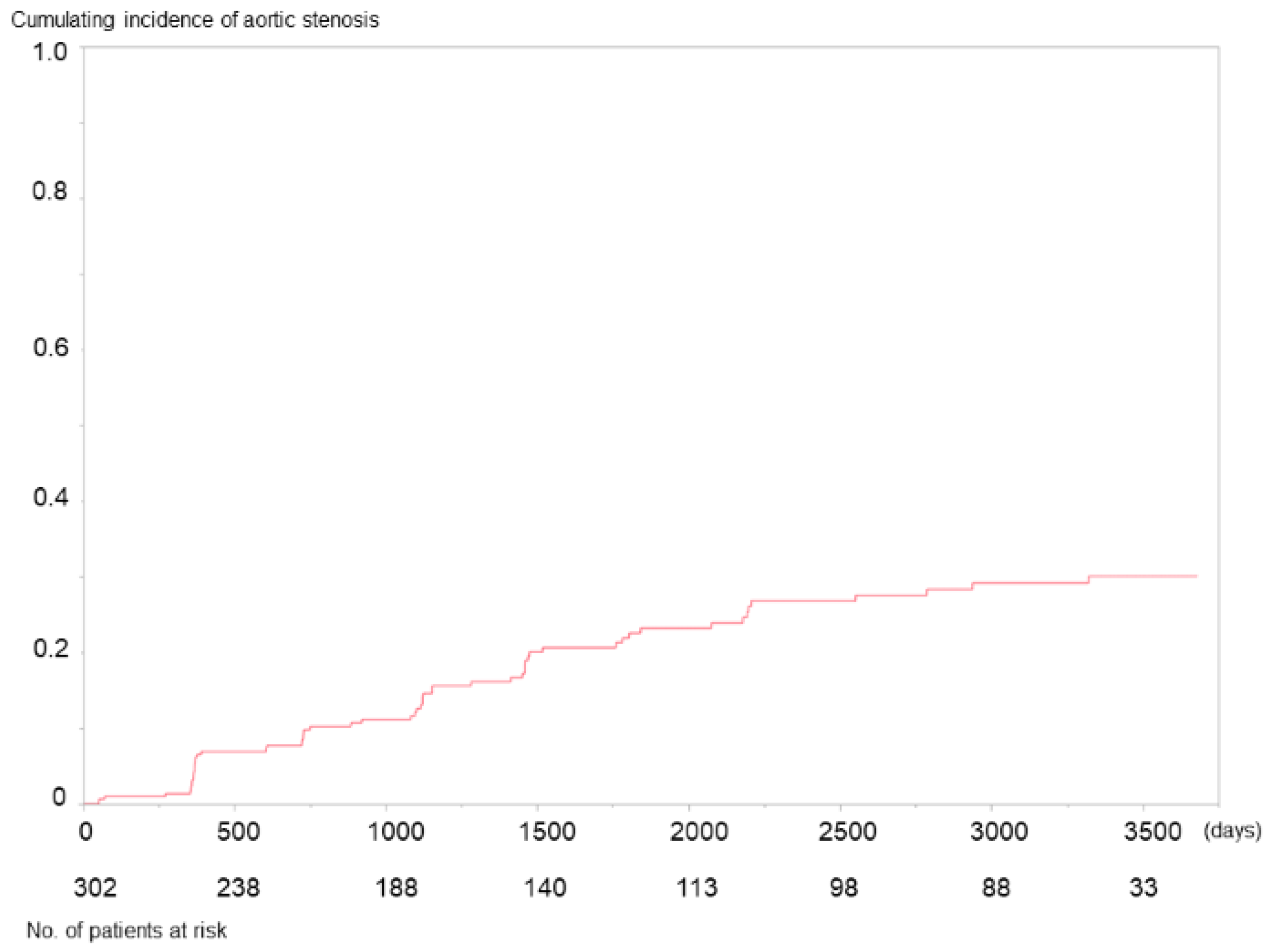
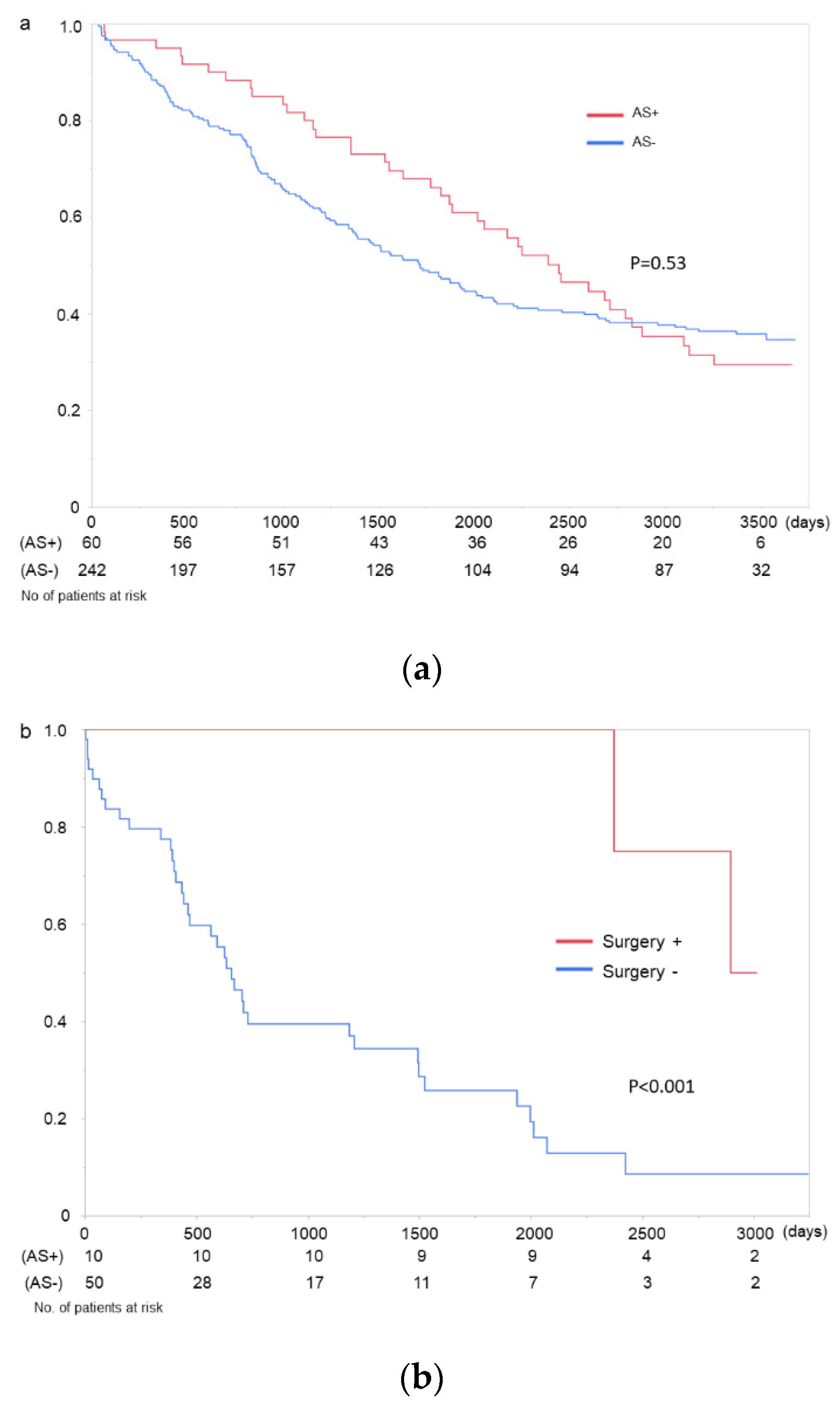
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samad, Z.; Sivak, J.A.; Phelan, M.; Schulte, P.J.; Patel, U.; Velazquez, E.J. Prevalence and outcomes of left-sided valvular heart disease associated with chronic kidney disease. J. Am. Heart Assoc. 2017, 6, e006044. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, Y.; Bellamy, M.F.; Baker, C.S.R. Aortic Stenosis in Dialysis Patients. Semin. Dial. 2017, 30, 224–231. [Google Scholar] [CrossRef]
- Lindroos, M.; Kupari, M.; Heikkilä, J.; Tilvis, R. Prevalence of aortic valve abnormalities in the elderly: An echocardiographic study of a random population sample. J. Am. Coll. Cardiol. 1993, 21, 1220–1225. [Google Scholar] [CrossRef] [Green Version]
- Danielsen, R.; Aspelund, T.; Harris, T.B.; Gudnason, V. The prevalence of aortic stenosis in the elderly in Iceland and predictions for the coming decades: The AGES-Reykjavík study. Int. J. Cardiol. 2016, 176, 916–922. [Google Scholar] [CrossRef] [Green Version]
- Nitta, K.; Goto, S.; Masakane, I.; Hanafusa, N.; Taniguchi, M.; Hasegawa, T.; Nakai, S.; Wada, A.; Hamano, T.; Hoshino, J.; et al. Annual dialysis data report for 2018, JSDT Renal Data Registry: Survey methods, facility data, incidence, prevalence, and mortality. Ren. Replace. Ther. 2020, 6, 41. [Google Scholar] [CrossRef]
- Ureña, P.; Malergue, M.C.; Goldfarb, B.; Prieur, P.; Guédon-Rapoud, C.; Pétrover, M. Evolutive aortic stenosis in hemodialysis patients: Analysis of risk factors. Nephrologie 1999, 20, 217–225. [Google Scholar]
- Ohara, T.; Hashimoto, Y.; Matsumura, A.; Suzuki, M.; Isobe, M. Accelerated progression and morbidity in patients with aortic stenosis on chronic dialysis. Circ. J. 2005, 69, 1535–1539. [Google Scholar] [CrossRef] [Green Version]
- Perkovic, V.; Hunt, D.; Griffin, S.V.; du Plessis, M.; Becker, G.J. Accelerated progression of calcific aortic stenosis in dialysis patients. Nephron. Clin. Pract. 2003, 94, c40–c45. [Google Scholar] [CrossRef]
- Bakri, K.; Goldsmith, D.J. Accelerated progression of calcific aortic stenosis in dialysis patients: What we still need to learn. Nephron. Clin. Pract. 2003, 94, c27–c28. [Google Scholar] [CrossRef]
- Ketteler, M.; Block, G.A.; Evenepoel, P.; Fukagawa, M.; Herzog, C.A.; McCann, L.; Moe, S.M.; Shroff, R.; Tonelli, M.A.; Toussaint, N.D.; et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease—Mineral and Bone Disorder (CKD-MBD) Guideline Update: What’s changed and why it matters. Kidney Int. 2017, 92, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Fujii, H.; Joki, N. Mineral metabolism and cardiovascular disease in CKD. Clin. Exp. Nephrol. 2017, 21, 53–63. [Google Scholar] [CrossRef]
- Yamada, S.; Tsuruya, K.; Taniguchi, M.; Tokumoto, M.; Fujisaki, K.; Hirakata, H.; Fujimi, S.; Kitazono, T. Association between serum phosphate levels and stroke risk in patients undergoing hemodialysis: The Q-Cohort Study. Stroke 2016, 47, 2189–2196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimamoto, S.; Yamada, S.; Hiyamuta, H.; Arase, H.; Taniguchi, M.; Tokumoto, M.; Nakano, T.; Tsuruya, K.; Kitazono, T. Association of serum phosphate concentration with the incidence of intervention for peripheral artery disease in patients undergoing hemodialysis: 10-year outcomes of the Q-Cohort Study. Atherosclerosis 2020, 304, 22–29. [Google Scholar] [CrossRef] [PubMed]
- London, G.M.; Pannier, B.; Marchais, S.J.; Guerin, A.P. Calcification of the aortic valve in the dialyzed patient. J. Am. Soc. Nephrol. 2000, 11, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, K.; Fujii, H.; Nakai, K.; Kono, K.; Goto, S.; Nishii, T.; Kono, A.; Nishi, S. Relationship between cardiac calcification and left ventricular hypertrophy in patients with chronic kidney disease at hemodialysis initiation. Heart Vessel. 2017, 32, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Adragao, T.; Pires, A.; Lucas, C.; Birne, R.; Magalhaes, L.; Goncalves, M.; Negrao, A.P. A simple vascular calcification score predicts cardiovascular risk in haemodialysis patients. Nephrol. Dial. Transplant. 2004, 19, 1480–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitamura, M.; Tateishi, Y.; Sato, S.; Kitamura, S.; Ota, Y.; Muta, K.; Yamashita, H.; Uramatsu, T.; Obata, Y.; Mochizuki, Y.; et al. Association between serum calcium levels and prognosis, hematoma volume, and onset of cerebral hemorrhage in patients undergoing hemodialysis. BMC Nephrol. 2019, 20, 210. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin III, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e1159–e1195. [Google Scholar] [CrossRef]
- Horstkotte, D.; Loogen, F. The natural history of aortic valve stenosis. Eur. Heart J. 1988, 9, 57–64. [Google Scholar] [CrossRef]
- Maher, E.R.; Young, G.; Smyth-Walsh, B.; Pugh, S.; Curtis, J.R. Aortic and mitral valve calcification in patients with end-stage renal disease. Lancet 1987, 330, 875–877. [Google Scholar] [CrossRef]
- Wang, A.Y.; Ho, S.S.; Wang, M.; Liu, E.K.H.; Ho, S.; Li, P.K.T.; Lui, S.F.; Sanderson, J.E. Cardiac valvular calcification as a marker of atherosclerosis and arterial calcification in end-stage renal disease. Arch. Intern. Med. 2005, 165, 327–332. [Google Scholar] [CrossRef] [Green Version]
- Floege, J. When man turns to stone: Extraosseous calcification in uremic patients. Kidney Int. 2004, 65, 2447–2462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukagawa, M.; Yokoyama, K.; Koiwa, F.; Taniguchi, M.; Shoji, T.; Kazama, J.J.; Komaba, H.; Ando, R.; Kakuta, T.; Fujii, H.; et al. Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther. Apher. Dial. 2013, 17, 247–288. [Google Scholar] [CrossRef] [PubMed]
- Isaka, Y.; Hamano, T.; Fujii, H.; Tsujimoto, Y.; Koiwa, F.; Sakaguchi, Y.; Tanaka, R.; Tomiyama, N.; Tatsugami, F.; Teramukai, S. Optimal phosphate control related to coronary artery calcification in dialysis patients. J. Am. Soc. Nephrol. 2021, 32, 723–735. [Google Scholar] [CrossRef]
- Block, G.A.; Raggi, P.; Bellasi, A.; Kooienga, L.; Spiegel, D.M. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007, 71, 438–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takei, T.; Otsubo, S.; Uchida, K.; Matsugami, K.; Mimuro, T.; Kabaya, T.; Akiba, T.; Nitta, K. Effects of sevelamer on the progression of vascular calcification in patients on chronic haemodialysis. Nephron. Clin. Pract. 2008, 108, c278–c283. [Google Scholar] [CrossRef]
- Fujii, H.; Kono, K.; Nakai, K.; Goto, S.; Nishii, T.; Kono, A.; Nishi, S. Effects of lanthanum carbonate on coronary artery calcification and cardiac abnormalities after initiating hemodialysis. Calcif. Tissue Int. 2018, 102, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Dweck, M.R.; Boon, N.A.; Newby, D.E. Calcific aortic stenosis: A disease of the valve and the myocardium. J. Am. Coll. Cardiol. 2012, 60, 1854–1863. [Google Scholar] [CrossRef] [Green Version]
- Côté, C.; Pibarot, P.; Després, J.P.; Mohty, D.; Cartier, A.; Arsenault, B.J.; Couture, C.; Mathieu, P. Association between circulating oxidised low-density lipoprotein and fibrocalcific remodelling of the aortic valve in aortic stenosis. Heart 2008, 94, 1175–1180. [Google Scholar] [CrossRef]
- Dweck, M.R.; Pawade, T.A.; Newby, D.E. Aortic stenosis begets aortic stenosis: Between a rock and a hard place? Heart 2015, 101, 919–920. [Google Scholar] [CrossRef]
- Jimbo, R.; Kawakami-Mori, F.; Mu, S.; Hirohama, D.; Majtan, B.; Shimizu, Y.; Yatomi, Y.; Fukumoto, S.; Fujita, T.; Shimosawa, T. Fibroblast growth factor 23 accelerates phosphate-induced vascular calcification in the absence of Klotho deficiency. Kidney Int. 2014, 85, 1103–1111. [Google Scholar] [CrossRef] [Green Version]
- K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am. J. Kidney Dis. 2005, 45, 16–153. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, T.; Yamamoto, H.; Miyata, H.; Kobayashi, J.; Masai, T.; Motomura, N. Surgical aortic valve replacement for aortic stenosis in dialysis patients—Analysis of Japan cardiovascular surgery database. Circ. J. 2020, 84, 1271–1276. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Kuratani, T.; Torikai, K.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Early outcomes in japanese dialysis patients treated with transcatheter aortic valve implantation. Circ. J. 2015, 79, 2713–2719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeji, Y.; Taniguchi, T.; Morimoto, T.; Saito, N.; Ando, K.; Shirai, S.; Sakaguchi, G.; Arai, Y.; Fuku, Y.; Kawase, Y.; et al. Transcatheter aortic valve implantation vs. Surgical aortic valve replacement for severe aortic stenosis in real-world clinical practice. Circ. J. 2020, 84, 806–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vindhyal, M.R.; Ndunda, P.; Khayyat, S.; Boppana, V.S.; Fanari, Z. Trans-catheter aortic valve replacement and surgical aortic valve replacement outcomes in patients with dialysis: Systematic review and meta-analysis. Cardiovasc. Revasc. Med. 2019, 20, 852–857. [Google Scholar] [CrossRef]
- Kang, D.H.; Park, S.J.; Lee, S.A.; Lee, S.; Kim, D.H.; Kim, H.K.; Yun, S.C.; Hong, G.R.; Song, J.M.; Chung, C.H.; et al. Early surgery or conservative care for asymptomatic aortic stenosis. N. Engl. J. Med. 2020, 382, 111–119. [Google Scholar] [CrossRef]
| AS (n = 60) | Without AS (n = 242) | p-Value | |
|---|---|---|---|
| Age (years) | 69.6 ± 11.0 | 65.7 ± 13.4 | 0.04 |
| Male (%) | 55.0 | 58.7 | 0.61 |
| Dialysis vintage a (years) | 5.3 (1.9–9.0) | 4.8 (2.0–10.5) | 0.58 |
| Dialysis time a (h) | 4 (3–4) | 4 (3–4) | 0.64 |
| Hypertension (%) | 91.7 | 84.2 | 0.12 |
| Diabetes mellitus (%) | 33.3 | 35.1 | 0.79 |
| Ischemic heart disease (%) | 40.0 | 33.5 | 0.35 |
| Cerebral hemorrhage (%) | 3.3 | 7.4 | 0.22 |
| Cerebral infarction (%) | 23.3 | 24.4 | 0.87 |
| Arteriosclerosis obliterans (%) | 16.7 | 17.4 | 0.90 |
| Cardiothoracic ratio (%) | 52.6 ± 5.4 | 51.7 ± 5.7 | 0.26 |
| Dry weight (kg) | 51.7 ± 8.9 | 52.7 ± 11.5 | 0.54 |
| Systolic blood pressure (mmHg) | 154 ± 23 | 149 ± 25 | 0.18 |
| Left ventricular ejection fraction (%) | 66 ± 9 | 65 ± 11 | 0.37 |
| Hemoglobin (g/dL) | 11.1 ± 1.2 | 10.8 ± 1.3 | 0.13 |
| Ferritina (ng/mL) | 57.5 (24.0–197.9) | 69.3 (25.7–178.9) | 0.73 |
| Transferrin saturation (%) | 26.4 ± 14.4 | 25.1 ± 14.1 | 0.53 |
| Albumin (g/dL) | 3.6 ± 0.3 | 3.6 ± 0.4 | 0.31 |
| Corrected calcium (mg/dL) | 9.3 ± 0.7 | 9.2 ± 0.7 | 0.53 |
| Phosphate (mg/dL) | 6.1 ± 1.7 | 5.5 ± 1.6 | 0.02 |
| Intact parathyroid hormone (pg/mL) | 91 (24–154) | 67 (27–157) | 0.68 |
| Alkaline phosphatase (IU/L) | 252 (179–342) | 250 (192–343) | 0.55 |
| Blood urea nitrogen (mg/dL) | 71.9 ± 16.7 | 67.7 ± 17.4 | 0.10 |
| Creatinine (mg/dL) | 11.0 ± 2.7 | 10.3 ± 3.4 | 0.19 |
| Total cholesterol (mg/dL) | 163 ± 31 | 162 ± 37 | 0.83 |
| Triglycerides (mg/dL) | 82 (68–135) | 92 (65–130) | 0.70 |
| C-reactive protein a (mg/dL) | 0.11 (0.07–0.43) | 0.18 (0.07–0.48) | 0.39 |
| ESA a (IU/week) | 4250 (1625–8000) | 4500 (2000–8000) | 0.85 |
| Calcium carbonate (%) | 41.7 | 50.0 | 0.25 |
| Lanthanum carbonate (%) | 50.0 | 28.5 | 0.002 |
| Sevelamer (%) | 1.7 | 3.7 | 0.39 |
| Cinacalcet (%) | 20.0 | 17.0 | 0.59 |
| Vitamin D (%) | 70.0 | 66.1 | 0.56 |
| Kt/V | 1.32 ± 0.38 | 1.35 ± 0.40 | 0.61 |
| Univariate | Model 1 | Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age/years | 1.05 | 1.03–1.08 | <0.001 | 1.07 | 1.04–1.10 | <0.001 | 1.06 | 1.03–1.09 | <0.001 |
| Male vs. female | 0.97 | 0.59–1.62 | 0.92 | 1.06 | 0.63–1.78 | 0.83 | |||
| HD vintage/year | 0.98 | 0.94–1.01 | 0.15 | 0.99 | 0.96–1.03 | 0.91 | |||
| HD time/h | 0.58 | 0.36–0.92 | 0.02 | 1.02 | 0.59–1.71 | 0.94 | |||
| DM | 1.18 | 0.69–2.03 | 0.54 | 0.97 | 0.53–1.76 | 0.91 | |||
| IHD history | 1.39 | 0.83–2.32 | 0.22 | ||||||
| Stroke history | 1.05 | 0.58–1.88 | 0.88 | ||||||
| CTR/% | 1.07 | 1.02–1.12 | 0.004 | 1.04 | 0.99–1.10 | 0.08 | |||
| DW/kg | 0.98 | 0.95–1.00 | 0.07 | 0.99 | 0.96–1.02 | 0.56 | |||
| SBP/10 mmHg | 1.07 | 0.97–1.19 | 0.19 | ||||||
| Hb/g/dL | 1.11 | 0.91–1.35 | 0.31 | ||||||
| Alb/g/dL | 0.57 | 0.29–1.15 | 0.12 | ||||||
| cCa/g/dL | 1.16 | 0.80–1.63 | 0.44 | 1.31 | 0.93–1.80 | 0.12 | |||
| P/mg/dL | 1.16 | 0.98–1.35 | 0.08 | 1.40 | 1.16–1.67 | <0.001 | 1.44 | 1.21–1.70 | <0.001 |
| iPTH/10 pg/mL | 1.00 | 0.98–1.02 | 0.77 | 1.00 | 0.98–1.02 | 0.94 | |||
| BUN/10 mg/dL | 1.07 | 0.91–1.26 | 0.40 | ||||||
| Cr/mg/dL | 0.97 | 0.90–1.05 | 0.47 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torigoe, M.; Kitamura, M.; Yamaguchi, K.; Uchino, T.; Torigoe, K.; Harada, T.; Funakoshi, S.; Yamamoto, K.; Maemura, K.; Eishi, K.; et al. Association between Serum Phosphate Levels and the Development of Aortic Stenosis in Patients Undergoing Hemodialysis. J. Clin. Med. 2021, 10, 4385. https://doi.org/10.3390/jcm10194385
Torigoe M, Kitamura M, Yamaguchi K, Uchino T, Torigoe K, Harada T, Funakoshi S, Yamamoto K, Maemura K, Eishi K, et al. Association between Serum Phosphate Levels and the Development of Aortic Stenosis in Patients Undergoing Hemodialysis. Journal of Clinical Medicine. 2021; 10(19):4385. https://doi.org/10.3390/jcm10194385
Chicago/Turabian StyleTorigoe, Miki, Mineaki Kitamura, Kosei Yamaguchi, Takumi Uchino, Kenta Torigoe, Takashi Harada, Satoshi Funakoshi, Kazuko Yamamoto, Koji Maemura, Kiyoyuki Eishi, and et al. 2021. "Association between Serum Phosphate Levels and the Development of Aortic Stenosis in Patients Undergoing Hemodialysis" Journal of Clinical Medicine 10, no. 19: 4385. https://doi.org/10.3390/jcm10194385
APA StyleTorigoe, M., Kitamura, M., Yamaguchi, K., Uchino, T., Torigoe, K., Harada, T., Funakoshi, S., Yamamoto, K., Maemura, K., Eishi, K., Mukae, H., & Nishino, T. (2021). Association between Serum Phosphate Levels and the Development of Aortic Stenosis in Patients Undergoing Hemodialysis. Journal of Clinical Medicine, 10(19), 4385. https://doi.org/10.3390/jcm10194385






