Dependence of Heart Rate Variability Indices on the Mean Heart Rate in Women with Well-Controlled Type 2 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Protocol and Patients
2.2. Ethical Considerations
2.3. Materials
2.4. Blood Sample and Reagents
2.5. Electrocardiogram Recording and Beat Detection
2.6. HRV Analysis
2.7. Statistical Analysis
3. Results
3.1. Antropometric Measures
3.2. Metabolic Profile
3.3. HRV Indices between Groups and Maneuvers
3.4. Correlations between meanNN and HRV Indices
3.5. Multiple Regression Analysis: Supine and Active Standing
3.6. Multiple Regression Analysis: Supine and Rhythmic Breathing
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petersmann, A.; Müller-Wieland, D.; Müller, U.A.; Landgraf, R.; Nauck, M.; Freckmann, G.; Heinemann, L.; Schleicher, E. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2019, 127, S1–S7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global Prevalence of Diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gale, E.A.M.; Gillespie, K.M. Diabetes and gender. Diabetologia 2001, 44, 3–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Diabetes Federation, I. Mortality related to Diabetes. In IDF Diabetes Atlas; International Diabetes Federation: Brussels, Belgium, 2019; p. 54. [Google Scholar]
- Spallone, V. Update on the Impact, Diagnosis and Management of Cardiovascular Autonomic Neuropathy in Diabetes: What Is Defined, What Is New, and What Is Unmet. Diabetes Metab. J. 2019, 43, 3–30. [Google Scholar] [CrossRef]
- Pop-Busui, R.; Braffett, B.H.; Zinman, B.; Martin, C.; White, N.H.; Herman, W.H.; Genuth, S.; Gubitosi-Klug, R. Cardiovascular Autonomic Neuropathy and Cardiovascular Outcomes in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes Care 2017, 40, 94–100. [Google Scholar] [CrossRef] [Green Version]
- Freeman, R. Diabetic autonomic neuropathy. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 63–79. [Google Scholar]
- Cabezas-Cerrato, J.; Hermida, R.C.; Cabezas-Agrícola, J.M.; Ayala, D.E. Cardiac Autonomic Neuropathy, Estimated Cardiovascular Risk, and Circadian Blood Pressure Pattern in Diabetes Mellitus. Chronobiol. Int. 2009, 26, 942–957. [Google Scholar] [CrossRef]
- Balcıoğlu, A.S. Diabetes and cardiac autonomic neuropathy: Clinical manifestations, cardiovascular consequences, diagnosis and treatment. World J. Diabetes 2015, 6, 80. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Healh 2017, 5, 258. [Google Scholar] [CrossRef] [Green Version]
- Sacha, J. Why should one normalize heart rate variability with respect to average heart rate. Front. Physiol. 2013, 4, 306. [Google Scholar] [CrossRef] [Green Version]
- Sacha, J. Heart rate contribution to the clinical value of heart rate variability. Kardiol. Pol. 2014, 72, 919–924. [Google Scholar] [CrossRef]
- Lefrandt, J.D.; Smit, A.J.; Zeebregts, C.J.; Gans, R.O.B.; Hoogenberg, K.H. Autonomic Dysfunction in Diabetes: A Consequence of Cardiovascular Damage. Curr. Diabetes Rev. 2010, 6, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Macartney, M.J.; McLennan, P.L.; Peoples, G.E. Heart rate variability during cardiovascular reflex testing: The importance of underlying heart rate. J. Basic Clin. Physiol. Pharmacol. 2021, 32, 145–153. [Google Scholar] [CrossRef]
- Monfredi, O.; Lyashkov, A.E.; Johnsen, A.-B.; Inada, S.; Schneider, H.; Wang, R.; Nirmalan, M.; Wisloff, U.; Maltsev, V.A.; Lakatta, E.G.; et al. Biophysical Characterization of the Underappreciated and Important Relationship Between Heart Rate Variability and Heart Rate. Hypertension 2014, 64, 1334–1343. [Google Scholar] [CrossRef] [Green Version]
- Stauss, H.M. Heart Rate Variability: Just a Surrogate for Mean Heart Rate? Hypertension 2014, 64, 1184–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hoogenhuyze, D.; Weinstein, N.; Martin, G.J.; Weiss, J.S.; Schaad, J.W.; Sahyouni, X.N.; Fintel, D.; Remme, W.J.; Singer, D.H. Reproducibility and relation to mean heart rate of heart rate variability in normal subjects and in patients with congestive heart failure secondary to coronary artery disease. Am. J. Cardiol. 1991, 68, 1668–1676. [Google Scholar] [CrossRef]
- Sacha, J.; Barabach, S.; Statkiewicz-Barabach, G.; Sacha, K.; Müller, A.; Piskorski, J.; Barthel, P.; Schmidt, G. How to select patients who will not benefit from ICD therapy by using heart rate and its variability? Int. J. Cardiol. 2013, 168, 1655–1658. [Google Scholar] [CrossRef] [PubMed]
- Hayano, J.; Sakakibara, Y.; Yamada, A.; Yamada, M.; Mukai, S.; Fujinami, T.; Yokoyama, K.; Watanabe, Y.; Takata, K. Accuracy of assessment of cardiac vagal tone by heart rate variability in normal subjects. Am. J. Cardiol. 1991, 67, 199–204. [Google Scholar] [CrossRef]
- Tsuji, H.; Venditti, F.J.; Manders, E.S.; Evans, J.C.; Larson, M.G.; Feldman, C.L.; Levy, D. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation 1994, 90, 878–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michel-Chávez, A.; Estañol, B.; Gien-López, J.A.; Robles-Cabrera, A.; Huitrado-Duarte, M.E.; Moreno-Morales, R.; Becerra-Luna, B. Heart Rate and Systolic Blood Pressure Variability on Recently Diagnosed Diabetics. Arq. Bras. Cardiol. 2015, 105, 276–284. [Google Scholar] [CrossRef]
- Hoshi, R.A.; Santos, I.S.; Dantas, E.M.; Andreão, R.V.; Schmidt, M.I.; Duncan, B.B.; Mill, J.G.; Lotufo, P.A.; Bensenor, I. Decreased heart rate variability as a predictor for diabetes—A prospective study of the Brazilian longitudinal study of adult health. Diabetes Metab. Res. Rev. 2019, 35, e3175. [Google Scholar] [CrossRef]
- Coopmans, C.; Zhou, T.L.; Henry, R.M.A.; Heijman, J.; Schaper, N.C.; Koster, A.; Schram, M.T.; van der Kallen, C.J.H.; Wesselius, A.; den Engelsman, R.J.A.; et al. Both Prediabetes and Type 2 Diabetes Are Associated With Lower Heart Rate Variability: The Maastricht Study. Diabetes Care 2020, 43, 1126–1133. [Google Scholar] [CrossRef]
- Rivera, A.L.; Estañol, B.; Fossion, R.; Toledo-Roy, J.C.; Callejas-Rojas, J.A.; Gien-López, J.A.; Delgado-García, G.R.; Frank, A. Loss of Breathing Modulation of Heart Rate Variability in Patients with Recent and Long Standing Diabetes Mellitus Type II. PLoS ONE 2016, 11, e0165904. [Google Scholar] [CrossRef]
- Benichou, T.; Pereira, B.; Mermillod, M.; Tauveron, I.; Pfabigan, D.; Maqdasy, S.; Dutheil, F. Heart rate variability in type 2 diabetes mellitus: A systematic review and meta–analysis. PLoS ONE 2018, 13, e0195166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, A.S.; El ghormli, L.; Vajravelu, M.E.; Bacha, F.; Farrell, R.M.; Gidding, S.S.; Levitt Katz, L.E.; Tryggestad, J.B.; White, N.H.; Urbina, E.M. Heart Rate Variability and Cardiac Autonomic Dysfunction: Prevalence, Risk Factors, and Relationship to Arterial Stiffness in the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) Study. Diabetes Care 2019, 42, 2143–2150. [Google Scholar] [CrossRef] [Green Version]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research—Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Front. Psychol. 2017, 8, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilz, M.J.; Dütsch, M. Quantitative studies of autonomic function. Muscle Nerve 2006, 33, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, J.; TenVoorde, B.J.; Dekker, J.M.; Kingma, R.; Kostense, P.J.; Bouter, L.M.; Heethaar, R.M. Measures of cardiovascular autonomic nervous function: Agreement, reproducibility, and reference values in middle age and elderly subjects. Diabetologia 2003, 46, 330–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elstad, M.; O’Callaghan, E.L.; Smith, A.J.; Ben-Tal, A.; Ramchandra, R. Cardiorespiratory interactions in humans and animals: Rhythms for life. Am. J. Physiol. Circ. Physiol. 2018, 315, H6–H17. [Google Scholar] [CrossRef] [Green Version]
- Figuerola, S.; Quintanar, E.; Lerma, C. Development and validation of a graphical user interface for assessment of cardiorespiratory coupling. Proc. Congr. Int. Ing. Electrón. Mem. Electro 2019, 41, 148–153. [Google Scholar]
- Wessel, N.; Voss, A.; Malberg, H.; Ziehmann, C.; Voss, H.U.; Schirdewan, A.; Meyerfeldt, U.; Kurths, J. Nonlinear analysis of complex phenomena in cardiological data. Herzschrittmachertherapie Elektrophysiologie 2000, 11, 159–173. [Google Scholar] [CrossRef]
- Malik, M.; Bigger, J.T.; Camm, A.J.; Kleiger, R.E.; Malliani, A.; Moss, A.J.; Schwartz, P.J. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef] [Green Version]
- Zoungas, S.; Woodward, M.; Li, Q.; Cooper, M.E.; Hamet, P.; Harrap, S.; Heller, S.; Marre, M.; Patel, A.; Poulter, N.; et al. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 Diabetes. Diabetologia 2014, 57, 2465–2474. [Google Scholar] [CrossRef]
- Bays, H.E.; Chapman, R.H.; Grandy, S. The relationship of body mass index to diabetes mellitus, hypertension and dyslipidaemia: Comparison of data from two national surveys. Int. J. Clin. Pract. 2007, 61, 737–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Zhong, J.; Zhu, H.; Hu, R.; Fang, L.; Wang, M.; Zhang, J.; Guo, Y.; Bian, Z.; Chen, Z.; et al. Independent and interactive associations of heart rate and body mass index or blood pressure with type 2 diabetes mellitus incidence: A prospective cohort study. J. Diabetes Investig. 2019, 10, 1068–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayan, K.M.V.; Boyle, J.P.; Thompson, T.J.; Gregg, E.W.; Williamson, D.F. Effect of BMI on Lifetime Risk for Diabetes in the U.S. Diabetes Care 2007, 30, 1562–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aune, D.; Hartaigh, B.ó.; Vatten, L.J. Resting heart rate and the risk of type 2 diabetes: A systematic review and dose–response meta-analysis of cohort studies. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 526–534. [Google Scholar] [CrossRef] [Green Version]
- Casiglia, E.; Tikhonoff, V.; Virdis, A.; Masi, S.; Barbagallo, C.M.; Bombelli, M.; Bruno, B.; Cicero, A.F.G.; Cirillo, M.; Cirillo, P.; et al. Serum uric acid and fatal myocardial infarction: Detection of prognostic cut-off values: The URRAH (Uric Acid Right for Heart Health) study. J. Hypertens. 2020, 38, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Kohnert, K.-D. Utility of different glycemic control metrics for optimizing management of Diabetes. World J. Diabetes 2015, 6, 17. [Google Scholar] [CrossRef]
- Liu, J.; Tao, L.; Zhao, Z.; Mu, Y.; Zou, D.; Zhang, J.; Guo, X. Two-Year Changes in Hyperuricemia and Risk of Diabetes: A Five-Year Prospective Cohort Study. J. Diabetes Res. 2018, 2018, 6905720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Hsieh, M.-C.; Chang, S.-J. Metabolic syndrome, diabetes, and hyperuricemia. Curr. Opin. Rheumatol. 2013, 25, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Sassi, R.; Cerutti, S.; Lombardi, F.; Malik, M.; Huikuri, H.V.; Peng, C.-K.; Schmidt, G.; Yamamoto, Y.; Gorenek, B.; Lip, G.Y.H.; et al. Advances in heart rate variability signal analysis: Joint position statement by the e-Cardiology ESC Working Group and the European Heart Rhythm Association co-endorsed by the Asia Pacific Heart Rhythm Society. Europace 2015, 17, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Kurths, J.; Voss, A.; Saparin, P.; Witt, A.; Kleiner, H.J.; Wessel, N. Quantitative analysis of heart rate variability. Chaos Interdiscip. J. Nonlinear Sci. 1995, 5, 88–94. [Google Scholar] [CrossRef]
- Yamasaki, Y.; Ueda, N.; Kishimoto, M.; Tani, A.; Ishida, Y.; Kawamori, R.; Kamada, T. Assessment of early stage autonomic nerve dysfunction in diabetic subjects--application of power spectral analysis of heart rate variability. Diabetes Res. 1991, 17, 73–80. [Google Scholar] [PubMed]
- Howorka, K.; Pumprla, J.; Jirkovska, A.; Lacigova, S.; Nolan, J. Modified orthostatic load for spectral analysis of short-term heart rate variability improves the sensitivity of autonomic dysfunction assessment. J. Diabetes Complicat. 2010, 24, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Ernst, G. Heart-Rate Variability—More than Heart Beats? Front. Public Healh 2017, 5, 240. [Google Scholar] [CrossRef] [PubMed]
- Dick, T.E.; Hsieh, Y.-H.; Dhingra, R.R.; Baekey, D.M.; Galán, R.F.; Wehrwein, E.; Morris, K.F. Cardiorespiratory Coupling. Prog. Brain Res. 2014, 209, 191–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, M.A.; Santarelli, D.M.; O’Rourke, D. The physiological effects of slow breathing in the healthy human. Breathe 2017, 13, 298–309. [Google Scholar] [CrossRef]
- Matsutani, D.; Sakamoto, M.; Iuchi, H.; Minato, S.; Suzuki, H.; Kayama, Y.; Takeda, N.; Horiuchi, R.; Utsunomiya, K. Glycemic variability in continuous glucose monitoring is inversely associated with baroreflex sensitivity in type 2 diabetes: A preliminary report. Cardiovasc. Diabetol. 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewing, D.J.; Martyn, C.N.; Young, R.J.; Clarke, B.F. The Value of Cardiovascular Autonomic Function Tests: 10 Years Experience in Diabetes. Diabetes Care 1985, 8, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Vinik, A.I.; Maser, R.E.; Mitchell, B.D.; Freeman, R. Diabetic Autonomic Neuropathy. Diabetes Care 2003, 26, 1553–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eleftheriadou, A.; Williams, S.; Nevitt, S.; Brown, E.; Roylance, R.; Wilding, J.P.H.; Cuthbertson, D.J.; Alam, U. The prevalence of cardiac autonomic neuropathy in prediabetes: A systematic review. Diabetologia 2021, 64, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.K.; Barzilay, J.I.; Domitrovich, P.P.; Chaves, P.M.; Gottdiener, J.S.; Heckbert, S.R.; Kronmal, R.A. The relationship of heart rate and heart rate variability to non-diabetic fasting glucose levels and the metabolic syndrome: The Cardiovascular Health Study. Diabet. Med. 2007, 24, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Yamamoto, S.S.; Brosschot, J.F. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int. J. Cardiol. 2010, 141, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Solaro, N.; Malacarne, M.; Pagani, M.; Lucini, D. Cardiac Baroreflex, HRV, and Statistics: An Interdisciplinary Approach in Hypertension. Front. Physiol. 2019, 10, 478. [Google Scholar] [CrossRef]
- Nemeroff, C.B.; Goldschmidt-Clermont, P.J. Heartache and heartbreak—The link between depression and cardiovascular disease. Nat. Rev. Cardiol. 2012, 9, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lavin, M.; Hermosillo, A.G.; Rosas, M.; Soto, M.-E. Circadian studies of autonomic nervous balance in patients with fibromyalgia: A heart rate variability analysis. Arthritis Rheum. 1998, 41, 1966–1971. [Google Scholar] [CrossRef]
- Barantke, M.; Krauss, T.; Ortak, J.; Lieb, W.; Reppel, M.; Burgdorf, C.; Pramstaller, P.P.; Schunkert, H.; Bonnemeier, H. Effects of Gender and Aging on Differential Autonomic Responses to Orthostatic Maneuvers. J. Cardiovasc. Electrophysiol. 2008, 19, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Zhang, L.F.; Wang, X.B.; Cheng, J.H. Age dependency of heart rate variability, blood pressure variability and baroreflex sensitivity. Space Med. Med. Eng. 2000, 13, 318–322. [Google Scholar]
- Singh, D.; Vinod, K.; Saxena, S.C.; Deepak, K.K. Spectral evaluation of aging effects on blood pressure and heart rate variations in healthy subjects. J. Med. Eng. Technol. 2006, 30, 145–150. [Google Scholar] [CrossRef]
- Agashe, S.; Petak, S. Cardiac Autonomic Neuropathy in Diabetes Mellitus. Methodist Debakey Cardiovasc. J. 2018, 14, 251–256. [Google Scholar] [CrossRef]
- Echeverría, J.C.; Infante, O.; Pérez-Grovas, H.; González, H.; José, M.V.; Lerma, C. Effects of Orthostatism and Hemodialysis on Mean Heart Period and Fractal Heart Rate Properties of Chronic Renal Failure Patients. Artif. Organs 2017, 41, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Torres-Arellano, J.M.; Echeverría, J.C.; Ávila-Vanzzini, N.; Springall, R.; Toledo, A.; Infante, O.; Bojalil, R.; Cossío-Aranda, J.E.; Fajardo, E.; Lerma, C. Cardiac Autonomic Response to Active Standing in Calcific Aortic Valve Stenosis. J. Clin. Med. 2021, 10, 2004. [Google Scholar] [CrossRef] [PubMed]
- Lerma, C.; Echeverría, J.C.; Infante, O.; Pérez-Grovas, H.; González-Gómez, H. Sign and magnitude scaling properties of heart rate variability in patients with end-stage renal failure: Are these properties useful to identify pathophysiological adaptations? Chaos Interdiscip. J. Nonlinear Sci. 2017, 27, 093906. [Google Scholar] [CrossRef]
- Calderón-Juárez, M.; González-Gómez, G.H.; Echeverría, J.C.; Pérez-Grovas, H.; Lerma, C. Association between Mean Heart Rate and Recurrence Quantification Analysis of Heart Rate Variability in End-Stage Renal Disease. Entropy 2020, 22, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]

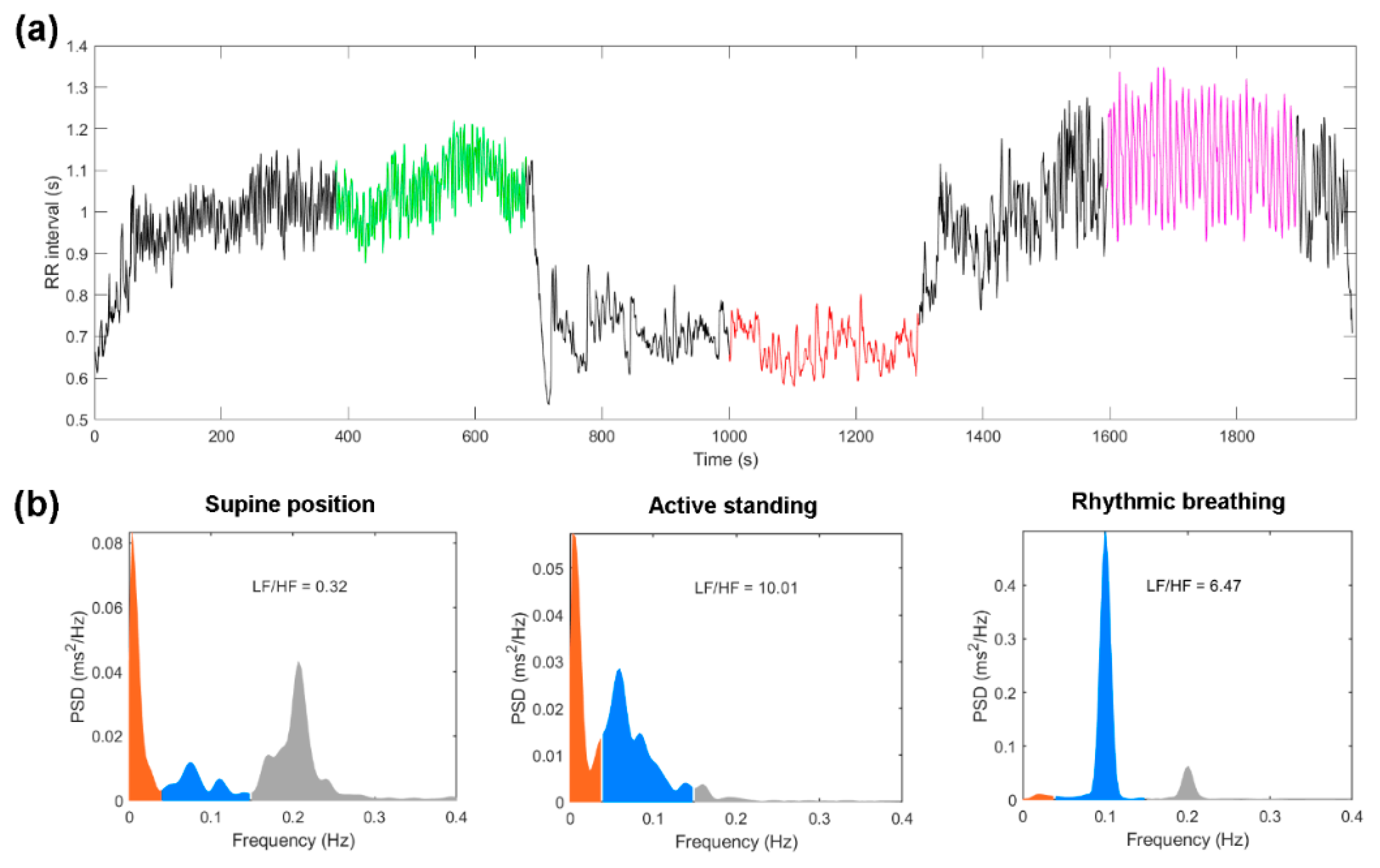
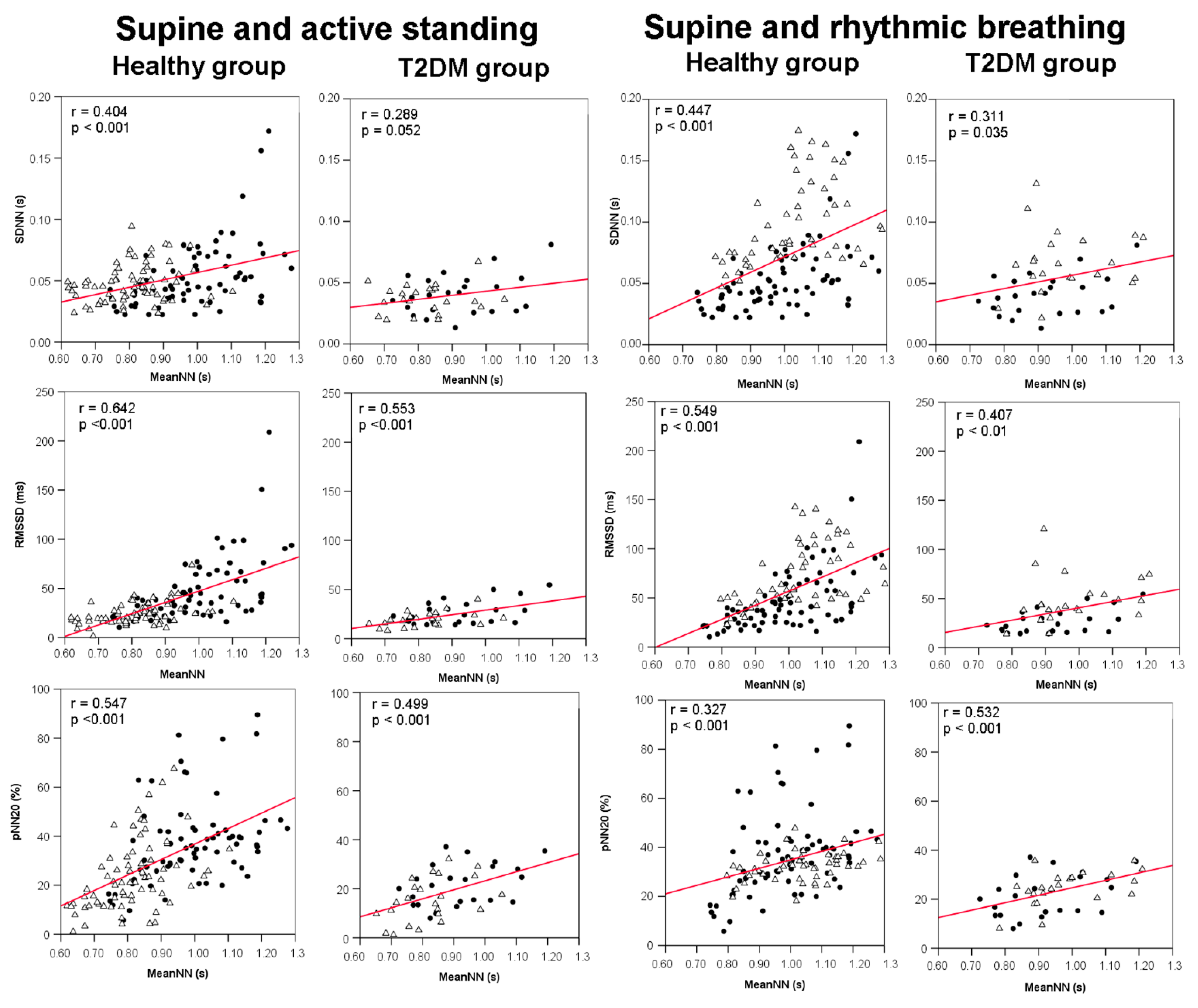
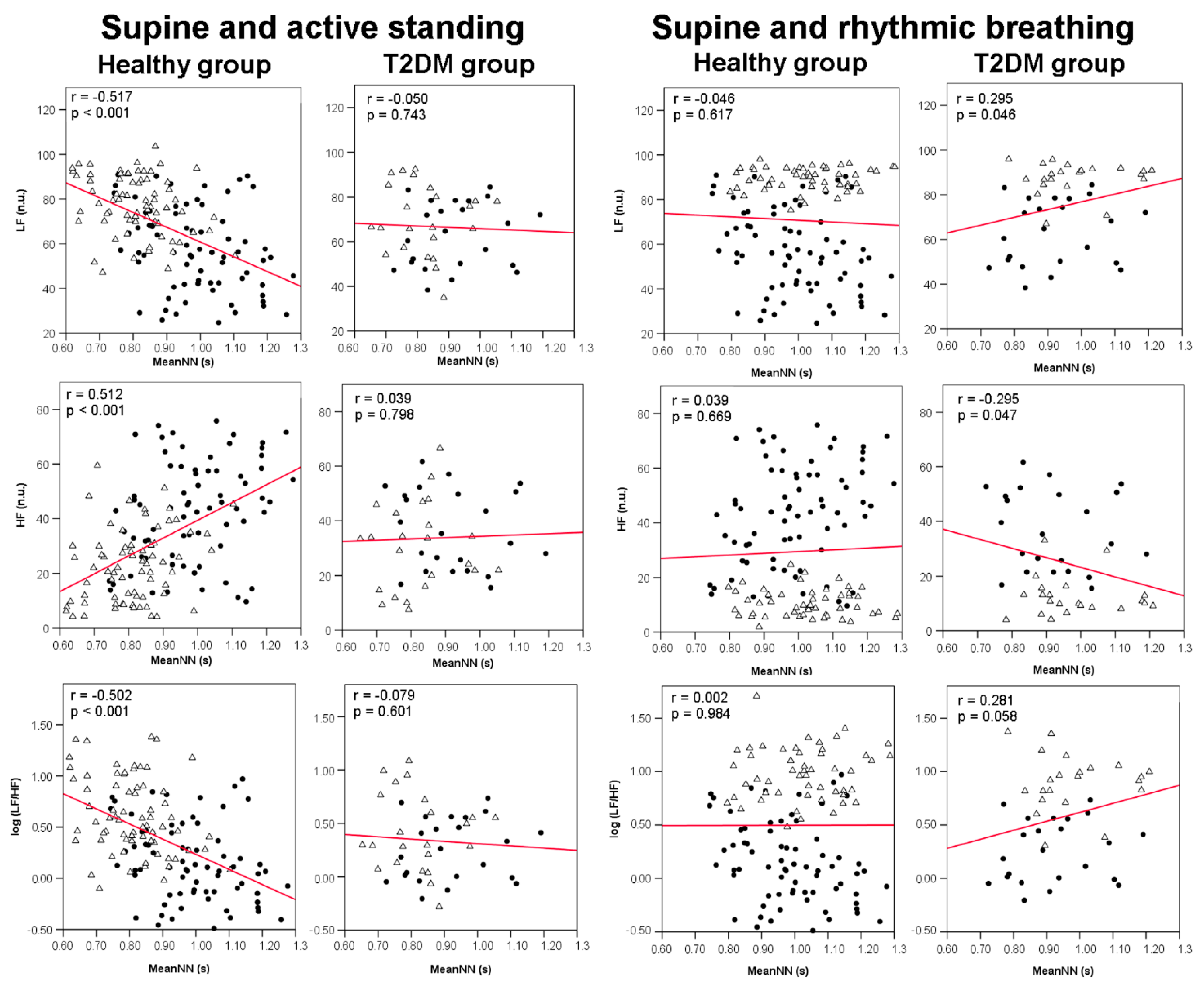
| Variable | Healthy Group (N = 44) | Diabetes Group (N = 19) | p-Value |
|---|---|---|---|
| Age (years) | 42 ± 7 | 52 ± 7 | <0.001 |
| Height (m) | 1.60 ± 0.07 | 1.55 ± 0.06 | 0.004 |
| Weight (kg) | 63 ± 7 | 63 ± 7 | 0.982 |
| BMI (kg/m2) | 25 ± 3 | 26 ± 2 | 0.018 |
| Waist (cm) | 86 ± 13 | 86 ± 7 | 0.817 |
| Systolic BP (mmHg) | 116 ± 13 | 119 ± 15 | 0.515 |
| Diastolic BP (mmHg) | 74 ± 9 | 73 ± 6 | 0.866 |
| Heart rate (bpm) | 61 ± 9 | 69 ± 7 | <0.001 |
| Variable | Healthy Group (N = 44) | Diabetes Group (N = 19) | p-Value |
|---|---|---|---|
| Glucose (mg/dL) | 79.86 ± 19.26 | 99.87 ± 20.09 | <0.001 |
| Total cholesterol (mg/dL) | 163.64 ± 40.40 | 166.96 ± 37.33 | 0.730 |
| HDL (mg/dL) | 45.74 ± 15.14 | 48.82 ± 11.94 | 0.380 |
| LDL (mg/dL) | 101.47 ± 33.47 | 105.75 ± 32.63 | 0.596 |
| Triglycerides (mg/dL) | 105.94 ± 54.39 | 113.30 ± 29.68 | 0.420 |
| Creatinine (mg/dL) | 0.62 ± 0.17 | 0.66 ± 0.15 | 0.327 |
| Uric acid (mg/dL) | 3.82 ± 0.96 | 4.66 ± 1.37 | 0.034 |
| Ureic nitrogen (mg/dL) | 11.51 ± 2.73 | 13.29 ± 4.66 | 0.116 |
| Variable | Healthy Group (N = 44) | Diabetes Group (N = 19) | p-Value |
|---|---|---|---|
| Supine position | |||
| MeanNN (s) | 0.990 ± 0.135 a | 0.920 ± 0.129 a,b | 0.018 |
| SDNN (s) | 0.054 ± 0.027 a,b | 0.041 ± 0.017 b | 0.022 |
| RMSSD (ms) | 47.289 ± 32.713 a,b | 26.722 ± 12.164 b | 0.001 |
| pNN20 (%) | 37.277 ± 17.440 a | 21.666 ± 8.605 a | <0.001 |
| LF (n.u.) | 57.225 ± 18.837 a,b | 63.060 ± 14.685 b | 0.231 |
| HF (n.u.) | 43.019 ± 18.641 a,b | 36.940 ± 14.685 b | 0.203 |
| log (LF/HF) | 0.151 ± 0.379 a,b | 0.257 ± 0.291 b | 0.310 |
| Active standing | |||
| MeanNN (s) | 0.811 ± 0.104 c | 0.822 ± 0.104 c | 0.714 |
| SDNN (s) | 0.047 ± 0.015 c | 0.037 ± 0.012 c | 0.008 |
| RMSSD (ms) | 24.354 ± 11.148 c | 19.371 ± 7.536 c | 0.052 |
| pNN20 (%) | 23.765 ± 14.395 c | 15.378 ± 9.040 c | 0.139 |
| LF (n.u.) | 77.512 ± 13.298 c | 70.153 ± 15.681 c | 0.034 |
| HF (n.u.) | 22.830 ± 13.256 c | 30.445 ± 15.858 c | 0.031 |
| log (LF/HF) | 0.612 ± 0.375 c | 0.418 ± 0.363 c | 0.037 |
| Rhythmic breathing | |||
| MeanNN (s) | 1.025 ± 0.126 | 1.000 ± 0.149 | 0.453 |
| SDNN (s) | 0.098 ± 0.034 | 0.068 ± 0.025 | <0.001 |
| RMSSD (ms) | 72.190 ± 32.088 | 49.767 ± 24.268 | 0.004 |
| pNN20 (%) | 32.075 ± 5.832 | 25.215 ± 7.544 | <0.001 |
| LF (n.u.) | 89.443 ± 5.160 | 87.828 ± 7.107 | 0.273 |
| HF (n.u.) | 10.591 ± 5.137 | 12.223 ± 7.112 | 0.267 |
| log (LF/HF) | 0.979 ± 0.250 | 0.913 ± 0.261 | 0.302 |
| Variables | Standardized β | β (C.I.95%) | p-Value | R2 |
|---|---|---|---|---|
| Predicted HRV index: SDNN (ms) | 0.286 | |||
| meanNN | 0.356 | 52.123 (34.20–70.06) | <0.001 | |
| Age | −0.374 | −0.948 (−1.26 to −0.64) | <0.001 | |
| Maneuver | Excluded variable | |||
| DM condition | Excluded variable | |||
| Predicted HRV index: RMSSD (ms) | 0.492 | |||
| meanNN | 0.585 | 99.558 (81.98–117.14) | <0.001 | |
| Age | −0.339 | −1.000 (−1.31 to −0.70) | <0.001 | |
| Maneuver | Excluded variable | |||
| DM condition | Excluded variable | |||
| Predicted HRV index: pNN20 (%) | 0.349 | |||
| meanNN | 0.510 | 58.317 (44.94–71.69) | <0.001 | |
| DM condition | −0.268 | −10.289 (−14.78 to −5.80) | <0.001 | |
| Maneuver | Excluded variable | |||
| Age | Excluded variable | |||
| Predicted HRV index: LF (n.u.) | 0.253 | |||
| meanNN | −0.248 | −31.338 (−50.27 to −12.41) | 0.001 | |
| Maneuver | 0.331 | 12.064 (6.60–17.52) | <0.001 | |
| DM condition | Excluded variable | |||
| Age | Excluded variable | |||
| Predicted HRV index: HF (n.u.) | 0.248 | |||
| meanNN | 0.241 | 30.320 (11.43–49.21) | 0.002 | |
| Maneuver | −0.331 | −12.006(−17.46 to −6.56) | <0.001 | |
| DM condition | Excluded variable | |||
| Age | Excluded variable | |||
| Predicted HRV index: log (LF/HF) | 0.249 | |||
| meanNN | −0.239 | -0.691 (−1.12 to −0.26) | 0.002 | |
| Maneuver | 0.334 | 0.278 (0.153–0.403) | <0.001 | |
| DM condition | Excluded variable | |||
| Age | Excluded variable | |||
| Variables | Standardized β | β (C.I.95%) | p-Value | R2 |
|---|---|---|---|---|
| Predicted HRV index: SDNN (ms) | 0.526 | |||
| meanNN | 0.241 | 62.414 (33.56–91.27) | <0.001 | |
| Age | −0.369 | −1.525 (−1.98 to −1.07) | <0.001 | |
| Maneuver | 0.492 | 17.451 (13.67–21.23) | <0.001 | |
| DM condition | Excluded variable | |||
| Predicted HRV index: RMSSD (ms) | 0.510 | |||
| meanNN | 0.351 | 84.576 (57.31–111.84) | <0.001 | |
| Age | −0.436 | −1.671 (−2.10 to −1.24) | <0.001 | |
| Maneuver | 0.282 | 9.282 (5.71–12.86) | <0.001 | |
| DM condition | Excluded variable | |||
| Predicted HRV index: pNN20 (%) | 0.265 | |||
| meanNN | 0.361 | 36.337 (22.82–49.86) | <0.001 | |
| DM condition | −0.316 | −9.675 (−13.75 to −5.60) | <0.001 | |
| Maneuver | −0.163 | −2.246 (−4.08 to −0.41) | 0.017 | |
| Age | Excluded variable | |||
| Predicted HRV index: LF (n.u.) | 0.551 | |||
| Age | 0.135 | 0.325 (0.08–0.57) | 0.010 | |
| Maneuver | 0.736 | 15.206 (13.09–17.32) | <0.001 | |
| meanNN | Excluded variable | |||
| DM condition | Excluded variable | |||
| Predicted HRV index: HF (n.u.) | 0.557 | |||
| Age | −0.135 | −0.324 (−0.57–−0.08) | 0.010 | |
| Maneuver | −0.740 | −15.278 (−17.38 to −13.18) | <0.001 | |
| meanNN | Excluded variable | |||
| DM condition | Excluded variable | |||
| Predicted HRV index: log (LF/HF) | 0.612 | |||
| Age | 0.124 | 0.007 (0.002–0.013) | 0.011 | |
| Maneuver | 0.778 | 0.392 (0.34–0.44) | <0.001 | |
| meanNN | Excluded variable | |||
| DM condition | Excluded variable | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robles-Cabrera, A.; Torres-Arellano, J.M.; Fossion, R.; Lerma, C. Dependence of Heart Rate Variability Indices on the Mean Heart Rate in Women with Well-Controlled Type 2 Diabetes. J. Clin. Med. 2021, 10, 4386. https://doi.org/10.3390/jcm10194386
Robles-Cabrera A, Torres-Arellano JM, Fossion R, Lerma C. Dependence of Heart Rate Variability Indices on the Mean Heart Rate in Women with Well-Controlled Type 2 Diabetes. Journal of Clinical Medicine. 2021; 10(19):4386. https://doi.org/10.3390/jcm10194386
Chicago/Turabian StyleRobles-Cabrera, Adriana, José M. Torres-Arellano, Ruben Fossion, and Claudia Lerma. 2021. "Dependence of Heart Rate Variability Indices on the Mean Heart Rate in Women with Well-Controlled Type 2 Diabetes" Journal of Clinical Medicine 10, no. 19: 4386. https://doi.org/10.3390/jcm10194386
APA StyleRobles-Cabrera, A., Torres-Arellano, J. M., Fossion, R., & Lerma, C. (2021). Dependence of Heart Rate Variability Indices on the Mean Heart Rate in Women with Well-Controlled Type 2 Diabetes. Journal of Clinical Medicine, 10(19), 4386. https://doi.org/10.3390/jcm10194386







