Abstract
Prader-Willi syndrome (PWS) is a complex genetic syndrome characterized by hyperphagia, intellectual disability, hypotonia and hypothalamic dysfunction. Adults with PWS often have hormone deficiencies, hypogonadism being the most common. Untreated male hypogonadism can aggravate PWS-related health issues including muscle weakness, obesity, osteoporosis, and fatigue. Therefore, timely diagnosis and treatment of male hypogonadism is important. In this article, we share our experience with hypogonadism and its treatment in adult males with PWS and present a review of the literature. In order to report the prevalence and type of hypogonadism, treatment regimen and behavioral issues, we retrospectively collected data on medical interviews, physical examinations, biochemical measurements and testosterone replacement therapy (TRT) in 57 Dutch men with PWS. Fifty-six (98%) of the patients had either primary, central or combined hypogonadism. Untreated hypogonadism was associated with higher body mass index and lower hemoglobin concentrations. TRT was complicated by behavioral challenges in one third of the patients. Undertreatment was common and normal serum testosterone levels were achieved in only 30% of the patients. Based on the Dutch cohort data, review of the literature and an international expert panel discussion, we provide a practical algorithm for TRT in adult males with PWS in order to prevent undertreatment and related adverse health outcomes.
1. Introduction
Prader-Willi syndrome (PWS) is a rare genetic syndrome caused by the absence of expression of a cluster of paternally expressed genes on chromosome 15q11.2-q13, also called the ‘PWS region’. PWS can be caused by paternal deletion of (part of) the PWS region (60–75%), maternal uniparental disomy 15 (mUPD, 20–35%), imprinting center defect (ICD, 1–4%) or paternal chromosomal translocation (0.1%) [1,2]. Due to hypothalamic dysfunction, patients with PWS often have hormone deficiencies, hyperphagia, sleep disorders, abnormal temperature regulation and high pain threshold. PWS also has a characteristic neurobehavioral phenotype, including mild to moderate intellectual disability, autism-like features, obsessive compulsions, skin picking, and temper tantrums [3,4,5,6,7].
The most common hormone deficiency in PWS is hypogonadism. The reported prevalence of hypogonadism in adult males with PWS ranges from 57 to 100% [8,9,10,11,12,13,14,15,16,17,18,19,20]. Although hypogonadism in PWS can be the result of hypothalamic dysfunction, recent studies show that hypogonadism in PWS can also be the result of primary gonadal failure [16,17,21], or a combination of hypothalamic and gonadal dysfunction [16,22,23].
Hypogonadism can affect males with PWS at all ages. At birth and during infancy, boys with PWS can display cryptorchidism, scrotal hypoplasia and short penile length [24]. Later in life, small penile length in combination with large suprapubic fat may lead to voiding difficulties in young, obese adults with PWS [24]. Puberty is usually incomplete and delayed, although precocious adrenarche and, rarely, precocious puberty can also occur [25,26,27]. Primary testicular dysfunction is a major contributor to abnormal pubertal development in males with PWS [23]. In adulthood, individuals with PWS often have low levels of sex steroids [8,15,18,19,28,29,30]. Males with PWS are believed to be infertile and no cases of paternity have been reported in the literature [21,24].
Male hypogonadism is associated with fatigue, depression, decreased muscle strength and mass, increased fat mass, decreased sexual quality of life, and an increased risk of osteoporosis [31,32,33] and cardiovascular disease [32,34]. As many of these factors are already prevalent in PWS [7], it is important to detect hypogonadism and start testosterone replacement therapy (TRT) at an early stage. However, TRT is a delicate matter as it may be complicated by challenging behavior [26,35].
In the current article, we share our experience with hypogonadism and its treatment in a Dutch cohort of adult males with PWS. We report the prevalence and type of hypogonadism, treatment regimen and behavioral issues encountered in adult males with PWS. Based on our findings, a thorough review of the literature and the clinical expertise of an international expert panel discussion, we provide a practical algorithm for the treatment of hypogonadism in adult males with PWS.
2. Materials and Methods
Ethical review and approval were waived for this study by the Medical Ethics Committee of the Erasmus University Medical Center.
In this retrospective study, we included adult males who visited the multidisciplinary outpatient clinic of our PWS reference center in the Erasmus University Medical Center, Rotterdam, the Netherlands, between January 2015 and December 2020 and underwent our routine systematic health screening. As described previously (see [36]), this screening consists of a structured interview, a complete physical examination, a medical questionnaire, a review of the medical file, biochemical measurements and, if indicated and feasible, additional tests.
As part of regular patient care, primary caregivers were asked to fill out a medical questionnaire. In this questionnaire, subjective complaints (daytime sleepiness, fatigue, sexual complaints and temper tantrums) were scored on a 5-point Likert scale (1 = rarely or never, 2 = not often and/or not severe, 3 = quite often and/or quite severe, 4 = often and/or severe, 5 = very often and/or very severe). A score of 3 or higher was considered clinically relevant.
During the visit, blood samples were taken for general medical screening, including evaluation of gonadal function (luteinizing hormone (LH), follicle-stimulating hormone (FSH), total testosterone and sex hormone binding globulin (SHBG)) and the hematopoietic system (hemoglobin and hematocrit).
Before 1 February 2018, testosterone concentrations were measured using the PerkinElmer CHS™ MSMS Steroids Kit and an ultra-performance liquid chromatography–tandem mass spectrometer (UPLC-MS/MS) (reference range 10.0–30.0 nmol/L). After that date, testosterone concentrations were measured using an in-house assay and a UPLC-MS/MS (reference range 10.0–30.0 nmol/L). Before 1 February 2019, LH and FSH concentrations were measured using the Siemens Immulite 2000XPi (reference range 1.5–8.0 IU/L for LH and 2.0–7.0 IU/L for FSH). After that date, LH and FSH concentrations were measured using the Fujirebio Lumipulse G1200 (reference range 1.0–5.5 IU/L for LH and 0.8–5.1 IU/L for FSH). Before 15 June 2020 SHBG, concentrations were measured using the Siemens Immulite 2000XPi (reference range 10–70 nmol/L). After that date, SHBG concentrations were measured using the IDS-ISYS (reference range 10–70 nmol/L). Hemoglobin and hematocrit were measured using the Sysmex XN1000 analyzer (reference ranges 8.6–10.5 mmol/L and 0.4–0.5 L/L, respectively). LH and FSH measurements changed methods during the study with a different calibration, testosterone and SHBG measurements also changed methods, but they were calibrated similarly, as checked by external quality assessment schemes.
The visits to our outpatient clinic are always in the afternoon. In one visit, the patients are seen by the multidisciplinary team, after which blood is collected for general health screening. Although testosterone is preferably measured in the morning [37], in our clinic this was not feasible. Hypogonadism was defined as an afternoon total testosterone value below 10.0 nmol/L (2.88 ng/mL) with normal SHBG and sparse facial hair. Only if hypogonadism was not clearly present from clinical features (prepubertal status, underdeveloped genitals and/or absent virilization), a separate morning testosterone analysis was done to confirm hypogonadism. Pubic hair Tanner stage is often relatively advanced in PWS men due to normal or increased production of adrenal androgens, however, this does not represent testicular development or gonadal hormone secretion, and therefore, pubic hair was not considered in the diagnosis of hypogonadism [27]. Due to hyperphagia, it was not feasible to obtain fasting testosterone measurements. If patients already used TRT before the first visit to our outpatient clinic, this was also considered as indicating presence of hypogonadism. Only LH, FSH and testosterone values from before the start of TRT were included.
When TRT was initiated at our outpatient clinic, a daily dose of 10 mg transdermal testosterone gel was administered, which was increased by 10 mg every 4 weeks until serum testosterone concentrations within the normal range were reached. When adverse effects occurred, the TRT dose was not further increased or was decreased, depending on the severity of the adverse effects. After TRT was started, SHBG measurements were not routinely repeated.
We defined short-acting injections as injections that have to be administered every 1–6 weeks, and long-acting injections as injections that have to be administered every 12 weeks.
Hypothalamic dysfunction of the LH/testosterone axis was defined as a low or normal LH concentration with a low testosterone concentration, while testicular dysfunction was defined as a high LH concentration with a low testosterone concentration. Hypothalamic dysfunction of the FSH/inhibin B axis was defined as a low or normal FSH concentration, while testicular dysfunction was defined as a high FSH concentration. Inhibin B was not measured, but based on previous research, we would expect that inhibin B levels would be low in most males [16,18].
Patients that were treated by the pediatric endocrinologist at our reference center during childhood, received transitional care during transition to the multidisciplinary outpatient clinic for adults with PWS. Transitional care included a shared visit with both the pediatric and the adult endocrinologist, followed by alternating visits at the pediatric and adult department until the final transfer to adult endocrinology.
2.1. Literature Search
In collaboration with the Erasmus MC Medical Library, we performed a literature search on 24 September 2020 and last updated the search on 3 June 2021. We searched the following databases: Embase, Medline (Ovid), Web of Science Core Collection and Cochrane Central Register of Controlled Trials. We reviewed the literature for articles reporting the prevalence of hypogonadism and laboratory measurements (e.g., testosterone, LH, FSH, SHBG, inhibin B) in males with PWS. Search terms included ‘Prader-Willi Syndrome’, ‘gonadal disease’, ‘hypogonadism’, ‘puberty’, and relevant laboratory measurements. For the full search strategy, see Table S1. We excluded conference abstracts, non-original research articles, articles that were not available in English, and articles that included less than ten adults (males and females) with PWS. When articles reported on adults and children and the prevalence of hypogonadism or laboratory values were not available for adults only, we contacted the authors to retrieve information for the adults separately. When articles reported on overlapping populations, the article with the most patients or, when the number of patients was similar, the most recent article was included. Although this search strategy resulted in articles on hypogonadism in both males and females with PWS, only the articles that provide information on hypogonadism in males are reported here.
2.2. Expert Opinion
An international panel of PWS experts was asked to fill out a survey on their experience with the treatment of hypogonadism in adult males with PWS. Clinical recommendations have been made based on this survey, the results of the cohort study and the literature review. None of the experts had a financial interest in any of the modalities of TRT.
2.3. Data Analysis
Statistical analysis was performed using R version 3.6.3 (https://cran.r-project.org/, accessed on 16 September 2021). Descriptive statistics for continuous variables are reported as median (interquartile range (IQR)). For dichotomous variables we display the number and the percentage of people, n (%). For the comparison of untreated male hypogonadism compared to no hypogonadism or treated hypogonadism, we used the Wilcoxon rank sum test for continuous variables and a Chi-squared test for dichotomous variables. To correct for age, we used linear and logistic regression models, respectively, with a likelihood ratio test. To compare testosterone or SHBG concentrations between genotypes and between patients who used and did not use growth hormone (GH) treatment, we used a Wilcoxon rank sum test. To correct for age, we used a linear regression model with a likelihood ratio test. To investigate the relationship between testosterone or SHBG concentrations, and body mass index (BMI) and age, the Kendall rank correlation test was used. To explore the relationship between testosterone or SHBG concentrations, and BMI corrected to age, a linear regression model and a likelihood ratio test were used. For all analysis involving FSH and LH measurements, a linear regression model with a likelihood ratio test was used and a variable indicating whether the measurement was performed before or after 01-02-2019 (when the method was changed with a different calibration) was included in the model. As this was an exploratory analysis, no correction for multiple testing was performed. p-values < 0.05 were considered statistically significant.
3. Results
3.1. Baseline Characteristics
Baseline characteristics are shown in Table 1. We included 57 adult males with a median age of 29 years (IQR 20–40) (range 18–72 years). Only one patient was more than 60 years old and 21 patients were younger than 25 years old. One patient was excluded from the analysis. We did not screen for hypogonadism in this individual as TRT was unfeasible due to serious behavioral challenges that were already present before the first visit to our outpatient clinic.

Table 1.
Baseline characteristics of 57 adult males with Prader-Willi syndrome.
3.2. Hypogonadism
Hypogonadism was present in 56 of 57 males (98%). In 28 males (49%), hypogonadism had been previously diagnosed and 24 males were already receiving TRT. Our screening revealed hypogonadism in another 28 patients (49%). Most frequent modes of testosterone administration at the first visit to our center were transdermal gel (n = 12, 50%) and intramuscular injections (n = 10, 42%). Nine males used short-acting intramuscular injections (Sustanon®) and one used long-acting intramuscular injections (testosterone undecanoate, Nebido®). For practical and/or behavioral reasons (see also discussion section), 8 patients switched from oral TRT (n = 2) or intramuscular injections (n = 6) to transdermal testosterone gel after their first visit (Table 2). The current and highest dose of transdermal testosterone gel of each patient is shown in Figure 1a,b.

Table 2.
Hypogonadism in male adults with PWS.
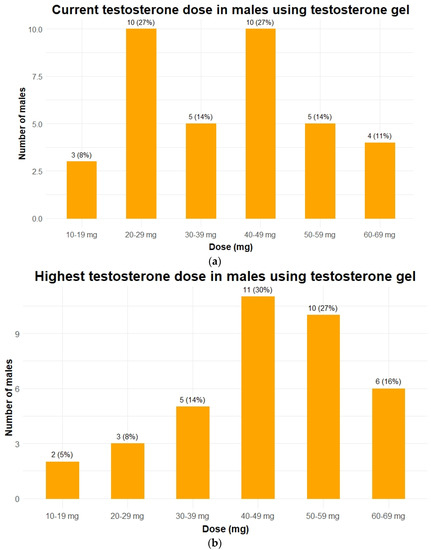
Figure 1.
Testosterone dose in 37 males with PWS using testosterone gel. Data is given as n (%). (a) The testosterone doses each patient received during the last visit to the outpatient clinic. When patients died or were transferred to another hospital, the last known dose was given; (b) the highest dose of testosterone gel ever received while visiting our outpatient clinic for each patient. To make both graphs comparable only patients who currently still use testosterone gel are depicted in panel b (in 5 patients testosterone replacement therapy was discontinued completely).
Figure 2 shows serum testosterone concentrations according to the current testosterone dose in 22 males. This figure shows that while higher testosterone doses lead to higher serum testosterone concentrations, low testosterone concentrations can also be seen in patients using higher doses of testosterone gel. Serum testosterone levels in the normal range were reached in 17 (30%) patients. Of the 70% not reaching normal range testosterone levels, 9 patients never started TRT at all (Figure 3a), due to fear of adverse events (n = 4), increased age (n = 2), or loss to follow-up (n = 3) (Figure 3b). In 27 males, TRT dose could not be increased, either due to challenging behavior (n = 18) or for unknown reasons (n = 9). Three (5%) patients had inadequate testosterone doses because they were still gradually increasing testosterone dose at the time of publication of this manuscript.
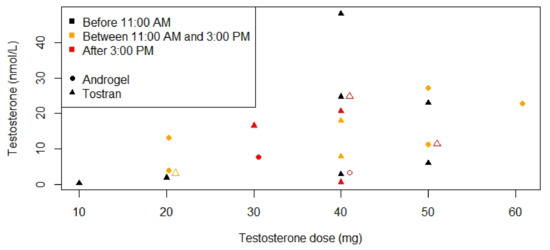
Figure 2.
Serum testosterone concentrations according to testosterone dose for patients using testosterone gel. Laboratory measurements were only available for 22 males. Testosterone gel was administered by the patient in the morning. Testosterone measurements before 11:00 A.M. are depicted in black, between 11:00 A.M. and 3:00 P.M. in orange and after 3:00 P.M. in red. Only two brands were used; Androgel® is depicted with circles and Tostran® with triangles. When two points overlapped, one of the points was moved 1 mg to the right and this point is depicted with an open circle or triangle instead of closed.
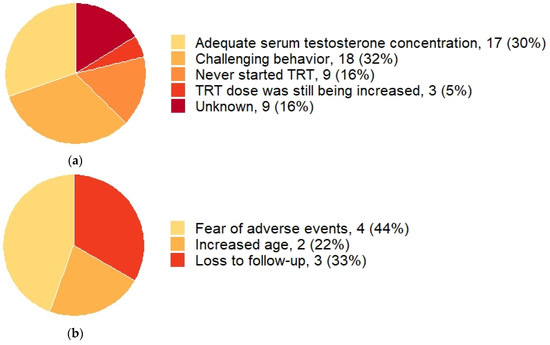
Figure 3.
Reasons for not increasing testosterone doses and reasons for not initiating testosterone replacement therapy. Abbreviations: testosterone replacement therapy (TRT). Data is given as n (%). (a) The reasons for not further increasing testosterone doses in adult males with PWS and hypogonadism (n = 56). (b) The reasons for never initiating TRT in adult males with PWS and hypogonadism (n = 9).
In 18 patients, testosterone dose had to be decreased due to challenging behavior, of whom the majority (83%) had serum testosterone concentrations below the reference range. Seventeen of them used transdermal gel (10 mg daily, n = 3; 20 mg daily, n = 2; 30 mg daily, n = 2; 40 mg daily, n = 2; 50 mg daily, n = 5; 60 mg daily, n = 2; and 69 mg daily, n = 1) and one used short-acting testosterone injections (200 mg every 4 weeks). In 11 (61%) behavior improved after testosterone dose reduction.
In 5 patients TRT was stopped completely, because even 10 mg transdermal testosterone gel was followed by unacceptable behavioral challenges (n = 4) or depressive symptoms (n = 1).
Problems with compliance were fairly common, with non-compliance confirmed in 6 patients (11%) and suspected in 5 (9%).
Eight males, of whom two used short-acting testosterone injections and six with untreated hypogonadism before screening, had enlarged breasts, either due to gynaecomastia or lipomastia. In the two patients using TRT it was unknown whether breast enlargement was related to TRT. Six patients with enlarged breasts had obesity and two were overweight. In the other 49 males, the medical records did not mention gynaecomastia or lipomastia. Estradiol levels were not available.
3.3. Effect of Untreated Hypogonadism
We compared males with and without untreated hypogonadism at the first visit to our outpatient clinic. After correction for age, we found a significant difference in BMI (median (IQR): 29 kg/m2 (27–38) in males with untreated hypogonadism and 26 kg/m2 [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37] in males with treated or no hypogonadism, p = 0.001). Three patients (12%) with treated or no hypogonadism had obesity, compared to 14 (44%) patients with untreated hypogonadism. Hemoglobin was significantly lower in males with untreated hypogonadism (median 8.2 nmol/L (IQR 8.0–9.0)) than in males with treated or no hypogonadism (median 9.3 nmol/L (IQR 8.6–9.7), p = 0.03). Although not significant, anemia was less prevalent in patients with treated or no hypogonadism (n = 4, 17%), compared to patients with untreated hypogonadism (n = 11, 34%). After correction for age, subjective complaints did not differ between the males with untreated hypogonadism and the males with treated or no hypogonadism (Table 3).

Table 3.
Effect of untreated hypogonadism in adult males with PWS.
We investigated the relationship between testosterone, BMI and age (Figure 4 and Figure 5) because in the normal population these parameters affect serum testosterone concentrations. Testosterone concentrations measured before 11:00 A.M. seemed to be negatively associated with BMI and age, but this association was not significant (p = 0.4 and p = 0.3, respectively). For the relationships between laboratory values (testosterone, LH, FSH and SHBG) and genotype, GH treatment, BMI, and age, see Tables S2 and S3.
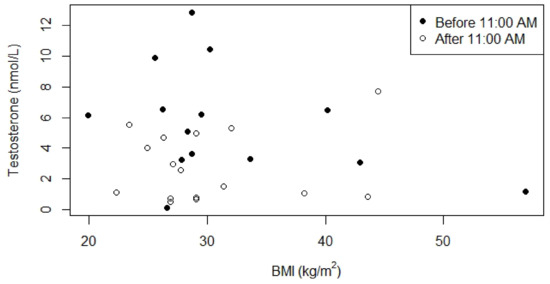
Figure 4.
Relationship between serum testosterone concentrations and BMI for males who were not receiving testosterone replacement therapy. p-value for the relationship between BMI and serum testosterone concentration measured before 11:00 A.M. was 0.4 (0.1 after correction for age), Kendall’s Tau was -0.19. p-value for the relationship between BMI and serum testosterone concentration measured after 11:00 A.M. was 0.9 (1.0 after correction for age), Kendall’s Tau was 0.03.
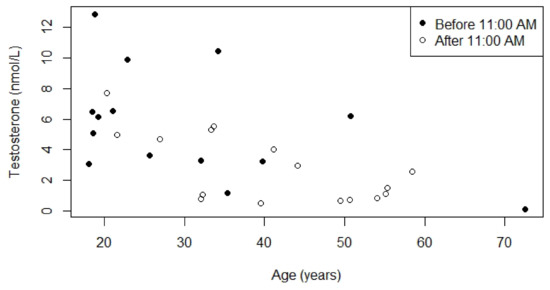
Figure 5.
Relationship between serum testosterone concentrations and age for males who were not receiving testosterone replacement therapy. p-value for the relationship between age and serum testosterone concentration measured before 11:00 A.M. was 0.3, Kendall’s Tau was −0.23. p-value for the relationship between age and serum testosterone concentration measured after 11:00 A.M. was 0.2, Kendall’s Tau was −0.27.
3.4. Types of Hypogonadism
Pre-TRT LH and FSH levels were available in 33 males. Seven patients had central hypogonadism (21%), seven had primary hypogonadism (21%), but the majority had a combination of hypothalamic and testicular dysfunction (n = 18, 55%), Table 4.

Table 4.
LH and FSH values in males with PWS.
3.5. Literature Review
We found 13 articles that described hypogonadism in adult males with PWS and fulfilled the inclusion criteria (Table 5 and Table 6). Most articles defined hypogonadism as a low serum testosterone concentration. The prevalence of hypogonadism ranged from 57% to 100%, with 6 of 10 articles reporting a prevalence of ≥90%. Central hypogonadism was the most common form of hypogonadism, while primary and mixed forms of hypogonadism were also reported. Multiple articles reported laboratory measurements and showed that testosterone and inhibin B values were below the reference range in most patients.

Table 5.
Literature review hypogonadism in male adults with PWS (Part 1).

Table 6.
Literature review hypogonadism in male adults with PWS (Part 2).
3.6. Expert Panel and Clinical Recommendations
Eleven experts (C.P., M.C., A.P.G., C.H., T.P.M., G.G., An.C., As.C., H.J.H., J.L.M. and L.C.G.d.G.) shared their experience with the treatment of hypogonadism in adult males with PWS (Table 7, Table 8 and Table 9). The most frequently used types of TRT were transdermal gel and short-acting injections. Starting dose and dose increase for each modality varied between experts. Additionally, one expert stated that he routinely measured estradiol concentrations in adult males with PWS, while three experts stated that they measured estradiol only in males with gynaecomastia. The other seven experts never measured estradiol in males. The advantages and disadvantages of injections and transdermal gel reported by the experts, supplemented with advantages and disadvantages mentioned in the Endocrine Society Clinical Practice Guideline for testosterone therapy in men with hypogonadism [38], are summarized in Table 10. Based on this cohort study, a review of the literature and the expert panel discussion, we have made recommendations for the screening and treatment of hypogonadism in adult males with PWS (Table 10 and Figure 6).

Table 7.
Expert panel discussion (Part 1).

Table 8.
Expert panel discussion (Part 2).

Table 9.
Expert panel discussion (Part 3).

Table 10.
Recommendations for different testosterone formulations.
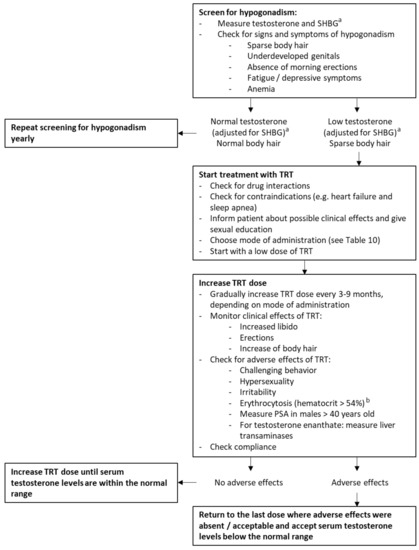
Figure 6.
Recommendations for hypogonadism in adult males with PWS. Abbreviations: sex hormone binding globulin (SHBG), testosterone replacement therapy (TRT). a Instead of total testosterone and SHBG, free testosterone can also be measured to diagnose hypogonadism in males with PWS. b Based on the Endocrine Society Clinical Practice Guideline for testosterone therapy in men with hypogonadism [38].
4. Discussion
Hypogonadism is present in nearly all adult males with PWS (98%). Although untreated hypogonadism was associated with obesity and decreased serum hemoglobin concentrations, adequate treatment leading to normal serum testosterone levels was only achieved in about one third of the patients.
4.1. Type of Hypogonadism
Although PWS is characterized by its hypothalamic dysfunction, hypogonadism in PWS can also be of testicular origin. MKRN3, NDN, and SNORD116, genes that are located in the PWS critical region, have been associated with GnRH secretion and hypothalamic dysfunction leading to hypogonadism [39]. Testicular dysfunction in men with PWS could be related to abnormal histology of the tubules and absence of spermatogonia [20,40,41]. C15orf2, another gene in the PWS critical region, is expressed in the testes and might play a role in spermatogenesis and therefore in the disturbance of the FSH/inhibin B axis [35,42].
The maturation of Leydig and Sertoli cells in PWS occurs independently [16]. Therefore, the LH/testosterone and FSH/inhibin B axes can be affected separately, either at the central or primary level. This leads to three forms of hypogonadism in PWS: central (low/normal LH with low testosterone and low/normal FSH with low inhibin B), primary (elevated LH with low testosterone and elevated FSH with low inhibin B), and a combination of hypothalamic and testicular dysfunction. We found a high prevalence of this mixed form of hypogonadism (55%). Central (21%) and primary hypogonadism (21%) were equally common. As previous reports show that most adult males with PWS have low inhibin B values [16,18], we assume that this is also true for our population. In that case, FSH levels in our patients might be in the normal range due to hypothalamic dysfunction of the FSH/inhibin B axis, with normal FSH levels being inadequately low for low inhibin B levels. However, as inhibin B measurements are not routinely measured as part of our standard patient care, we cannot draw any firm conclusions about the presence of hypothalamic dysfunction of the FSH/inhibin B axis.
4.2. Undertreatment
In one third of the patients, normal serum testosterone levels could not be achieved due to challenging behavior. Although this challenging behavior seemed related to the start of TRT or an increase in testosterone dose, it was not possible to exclude placebo effect or other factors that might aggravate challenging behavior. Eighteen patients required testosterone dose reduction due to the development of challenging behavior. In only 11 (61%) of these patients did a reduction in TRT dose reduce the challenging behaviors, suggesting that TRT may not necessarily be the cause of the increase in challenging behaviors.
Remarkably, low testosterone concentrations were still seen in patients with higher prescribed testosterone doses. This might be due to non-compliance or variability in biochemical measurements. Additionally, although SHBG concentrations were normal initially, they may have decreased over time.
4.3. Importance of Treatment of Hypogonadism
Untreated hypogonadism can aggravate PWS-related health issues including osteoporosis, decreased muscle mass and increased fat mass, fatigue, and impaired cardiovascular health.
4.3.1. Osteoporosis
TRT increases bone mineral density in hypogonadal males without [43] and with PWS [17,44]. Therefore, it is important to treat hypogonadism to avoid osteoporosis and subsequent fractures.
4.3.2. Muscle and Fat
Higher testosterone concentrations are associated with decreased fat mass and increased fat-free mass, muscle volume, and muscle strength [43,45]. A significant decrease in body fat percentage and increase in lean body mass in males with PWS has been demonstrated after two years of TRT [17]. Patients with PWS already have a decreased muscle mass and an increased fat mass, related to impaired exercise tolerance, hyperphagia and impaired GH secretion [36,46]. This abnormal body composition leads to a vicious cycle of low muscle mass, poor exercise tolerance and little physical activity, which further decreases muscle mass. Treatment of hypogonadism in males with PWS is important to increase exercise tolerance and improve muscle mass and strength to help break the vicious cycle.
After correction for age, we found that males with untreated hypogonadism had a significantly higher BMI compared to males who did not have hypogonadism or received TRT. However, patients receiving TRT are probably more likely to receive other interventions that may influence body weight, such as GH replacement, physiotherapy or dietary treatment.
4.3.3. Fatigue
Treatment of non-PWS male hypogonadism can have beneficial effects on vitality and quality of life, and reduce fatigue and depressive symptoms [43,47,48,49], although studies have reported mixed results [50].
The relation between hypogonadism and fatigue may be partly explained by anemia. In the general population, treatment of male hypogonadism increases hemoglobin levels and reduces anemia [43,45]. In our population, males with untreated hypogonadism had significantly lower hemoglobin levels than those without untreated hypogonadism.
At baseline, we did not find a significant difference in subjective complaints of fatigue and daytime sleepiness between males with and without untreated hypogonadism. We did not systematically assess the psychological effects after the start of TRT. However, in our clinical experience, we did see improvements in mood and vitality in many males after the start of TRT. Further research is needed to longitudinally assess the effect of TRT on fatigue and quality of life in adult males with PWS.
4.3.4. Cardiovascular Health
Cardiovascular (CV) risk factors, including obesity, hypertension, Type 2 diabetes mellitus, sleep apnea and hypercholesterolemia are prevalent in adults with PWS [36], leading to a high risk of CV disease and CV mortality at a young age [51,52]. Hypogonadism has been associated with poor CV outcomes [53,54] and TRT may improve CV health, although contradictory data have been reported and more research is needed [53,54].
4.4. TRT Warnings and Precautions
TRT can cause behavioral challenges, irritability, and aggressive behavior [35]. However, Kido et al. [17] found no difference in the Modifier Overt Aggression Scale (MOAS) after two years of TRT in males with PWS and did not observe challenging behaviors caused by TRT. In our population, we did see behavioral challenges during TRT. In 18 (32%) males, the testosterone dose was decreased because of behavioral challenges, leading to inadequate serum testosterone concentrations. These differences between our study and Kido et al. [17] can partly be explained by the fact that, as opposed to Kido et al., we did not exclude patients based on behavioral problems at baseline, and that Kido et al. used a different form of TRT, namely monthly intramuscular injections of 125 mg testosterone enanthate, which is half of the conventional dose. However, short-acting testosterone injection regimes (2–4 weekly) might be expected to increase the risk of behavioral problems as a result of supra-physiological testosterone concentrations shortly after injection compared to the most frequently used modality in our population, testosterone gel [38].
As TRT can induce libido and sexual activity in patients who are used to lifelong hypogonadism, it is important to inform the patients and their caregivers about these possibly confusing new feelings. A clear ‘code of conduct’ should be discussed with regard to sexual activity before starting TRT, in order to prevent inappropriate sexual behavior. It is important to ask about sexuality, sexual function, libido and erections to identify problems and to evaluate the effect of TRT. When discussing sexuality, it is important to use direct and very simple language.
A majority of the physicians of the expert panel discussion reported that normal testosterone values could be reached without causing behavioral problems in their population of adults with PWS. This could be related to the use of testosterone injections instead of transdermal gel or a slower increase in testosterone dose. Additionally, in the Dutch cohort a neuropsychologist was involved in the multidisciplinary care for adults with PWS, which could have led to greater identification of behavioral issues resulting from TRT. Further research is needed to accurately assess the differences in behavioral challenges between all treatment regimens and centers.
4.5. Recommendations
Based on the combined clinical experience of all co-authors, we propose clinical recommendations for the treatment of hypogonadism in adult males with PWS for TRT, see Table 10 and Figure 6. We wish to highlight issues that are especially relevant when treating hypogonadism in males with PWS. For the non-PWS specific aspects of TRT, we recommend referring to the general guidelines for the treatment of hypogonadism in men for topics not discussed here [38]. As clinical practice differed greatly among experts, we provide ranges for the possible starting dose and dose increase of TRT.
4.5.1. Interpretation of Hormone Levels
Whenever possible, testosterone concentrations should be measured in the morning. When a low total testosterone concentration is found, we recommend measurement of SHBG levels before starting TRT. SHBG levels can be low due to obesity, which is often present in patients with PWS [55]. During follow-up, SHBG measurement may need to be repeated if obesity or insulin resistance develop or worsen [38]. Alternatively, free testosterone levels can be measured instead of total testosterone and SHBG.
4.5.2. Sleep Apnea
Sleep apnea is common in PWS [8,56,57] and TRT can worsen symptoms of obstructive sleep apnea [57]. Therefore, we recommend screening for obstructive sleep apnea before starting TRT, and if present to treat this condition. After the start of TRT, polysomnography should be performed if clinical signs of sleep apnea develop.
4.5.3. Drug Interactions
As several drugs interact with TRT, we recommend checking for possible drug interactions before starting TRT. As use of psychotropic and anti-epileptic drugs is common in adults with PWS, it is especially important to check for interactions with drugs like selective serotonin reuptake inhibitors, anti-epileptic medication and psychostimulants like modafinil [58,59,60,61,62,63]. As these drugs may influence serum testosterone concentrations, adjustment of the dose of TRT might be needed. TRT can also interact with growth hormone (GH) treatment. As TRT can increase insulin-like growth factor 1 (IGF-1) concentrations [45,64,65], it is important to evaluate and, if necessary, adjust the GH dose after initiation of TRT.
4.5.4. Cardiac Failure
As the use of androgens might induce fluid retention [38,66] and cardiac problems are common in patients with PWS [57], we recommend excluding or appropriately managing heart failure before starting TRT. As patients with PWS are often unable to accurately express their cardiac symptoms due to intellectual disability and a high pain threshold [7], and leg edema is not a reliable marker of heart failure in patients with PWS [67], heart disease in adults with PWS can easily remain undiagnosed. Therefore, we recommend arranging an echocardiogram, checking serum N-terminal pro b-type natriuretic peptide (NT-proBNP) concentrations and/or consulting a cardiologist prior to the commencement of TRT in case of pitting edema or exercise-related shortness of breath. It should be noted that NT-proBNP can be false-negative in patients with obesity [68].
4.5.5. Challenging Behavior
To avoid the development or worsening of aggression, hypersexuality and temper tantrums, we recommend starting with a low dose of TRT and gradually increasing the dose every 3–6 months for testosterone gel and short-acting injections and every 3–9 months for long-acting injections. If increasing the dose is impossible due to altered (sexual) behavior, we recommend returning to the last dose where behavior was still acceptable.
4.5.6. Mode of Administration
Among the clinicians participating in the international expert panel, many different treatment regimens were used. Due to the need for gradual increase and the possibility for rapid dose reduction in case of behavioral challenges, our general recommendation is to use transdermal gel instead of injections when initiating TRT [69,70]. However, once established on a final transdermal TRT dose with satisfactory behavioral profile, it may be possible to switch to intramuscular injections. We advise against using oral testosterone preparations because of the risk of liver damage and increased intestinal conversion to dihydrotestosterone, preventing aromatisation to estrogen and thus hindering bone protection [38,71,72,73].
4.5.7. Erythrocytosis
As long-term treatment with testosterone might generate erythrocytosis, we recommend to measure hemoglobin and hematocrit regularly during TRT, similar to the recommendations for TRT in non-PWS males [38]. When erythrocytosis occurs, TRT should be withheld until hematocrit has returned to the normal range. Then, TRT can be resumed at a lower dose [38].
4.5.8. Prostate and Liver
We recommend measurement of prostate specific antigen (PSA) in men who are over 40 years old, as the long-term effects of TRT on the prostate in PWS are unknown. A urology consult should be obtained if PSA levels increase above baseline during TRT. TRT in non-PWS men does not seem to be associated with benign prostatic hyperplasia or lower urinary tract symptoms [74]. In addition, increased levels of liver transaminases may occur during treatment with testosterone enanthate and should be monitored [75,76].
4.5.9. Non-Compliance
Non-compliance is frequent in adults with PWS [77], even compared to non-PWS adults with intellectual disability [78]. Although many patients are grateful to receive TRT and have no problems with adhering to their TRT regime, we found that non-compliance to TRT was often seen (certain non-compliance in 11% and a high suspicion of non-compliance in 9%), especially when the patient administered his own medication. However, as figures about non-compliance are, by definition, unreliable, actual non-compliance may be more frequent. Therefore, we recommend asking about barriers that may reduce compliance such as practical barriers (e.g., inability to administer testosterone gel, lack of caregivers who can administer the gel) and other concerns (e.g., fear of adverse events). As indicated by multiple experts during our survey, compliance might be better in patients receiving monthly or three-monthly testosterone injections, compared to testosterone gel that requires daily administration.
4.6. Role of PWS Reference Centers
The TRT-related challenges may cause physicians to refrain from prescribing TRT in males with PWS. However, we want to stress the importance of adequate treatment as undertreatment can have serious health consequences. PWS reference centers can be contacted for consultation or, if geographically possible, referral. If there is no PWS reference center available, we recommend the use our algorithm for treatment of hypogonadism in men with PWS (Table 10 and Figure 6).
4.7. Strengths and Limitations
To our knowledge, we are the first to provide a practical flowchart for the screening and treatment of hypogonadism in males with PWS [39]. Another strength of our study is the relatively large cohort, given the fact that PWS is a rare syndrome. In addition, we have provided a comprehensive literature review of male hypogonadism in adults with PWS. However, our study also has some limitations. First, there was limited overlap in age between the group of males with untreated hypogonadism and the group of males with treated or no hypogonadism, possibly leading to residual confounding. Second, due to the circadian rhythm of testosterone, we analyzed the testosterone levels drawn before 11:00 A.M. and the testosterone levels drawn after 11:00 A.M. separately [37]. As few males had morning testosterone measurements and none had fasting testosterone measurements, we had limited power to investigate which factors influenced endogenous testosterone values. Third, physical examination reports did not always include details about lipomastia or gynaecomastia. Therefore, we cannot rule out that some men had breast enlargement that was not specifically described in their medical records. In addition, we did not measure estradiol concentrations, thus we were not able to investigate the relationship between breast enlargement and estradiol. Finally, we had too few DEXA-scans available to evaluate the effect of TRT on bone mineral density, lean body mass, and fat percentage. Further research should determine the effect of TRT on these clinical effects of TRT, as they may be more important parameters to measure the effectiveness of TRT than serum testosterone measurements, although these parameters may also be influenced by GH treatment.
5. Conclusions
In conclusion, hypogonadism was present in nearly all males with PWS (98%) and was often a combination of hypothalamic and testicular dysfunction. Although untreated hypogonadism was associated with obesity and decreased serum hemoglobin concentrations, treatment leading to serum testosterone levels within the normal range was only achieved in one third of the patients attending our center. In order to prevent undertreatment due to behavioral challenges or other PWS-related challenges, we provide a practical algorithm for TRT in adult males with PWS.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcm10194361/s1, Table S1: full search strategy (Embase), Table S2: laboratory values in adult males with PWS (Part 1), Table S3: laboratory values in adult males with PWS (Part 2).
Author Contributions
Conceptualization, K.P. and L.C.G.d.G.; methodology, K.P. and L.C.G.d.G.; formal analysis, K.P.; investigation, K.P., Y.B.B., and L.C.G.d.G.; resources, L.C.G.d.G.; data curation, K.P., Y.B.B., and L.C.G.d.G.; writing—original draft preparation, K.P.; writing—review and editing, K.P., Y.B.B., A.G.W.R., K.D., C.P., M.C., A.P.G., C.H., T.P.M., G.G., A.C. (Antonino Crinò), A.C. (Assumpta Caixàs), T.E.-G., H.J.H., V.G.-T., M.G.B., J.L.M., S.A.A.v.d.B., A.J.v.d.L., L.C.G.d.G.; visualization, K.P.; supervision, L.C.G.d.G. and A.J.v.d.L.; project administration, L.C.G.d.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. Ethical review and approval were waived for this study by the Institutional Review Board (or Ethics Committee) of the Erasmus University Medical Center (Protocol Code MEC-2018-1389, 24 September 2018), as this is not applicable for retrospective data collection from patient records.
Informed Consent Statement
Informed consent was obtained from subjects involved in the study or anonymized patient data was collected.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Acknowledgments
We wish to thank Sabrina Meertens-Gunput from the Erasmus MC Medical Library for developing and Maarten F.M. Engel and Wichor M. Bramer from the Erasmus MC Medical Library for updating the search strategies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cheon, C.K. Genetics of Prader-Willi syndrome and Prader-Will-Like syndrome. Ann. Pediatr. Endocrinol. Metab. 2016, 21, 126–135. [Google Scholar] [CrossRef]
- Butler, M.G.; Hartin, S.N.; Hossain, W.A.; Manzardo, A.M.; Kimonis, V.; Dykens, E.; Gold, J.A.; Kim, S.J.; Weisensel, N.; Tamura, R.; et al. Molecular genetic classification in Prader-Willi syndrome: A multisite cohort study. J. Med. Genet. 2019, 56, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Angulo, M.A.; Butler, M.G.; Cataletto, M.E. Prader-Willi syndrome: A review of clinical, genetic, and endocrine findings. J. Endocrinol. Investig. 2015, 38, 1249–1263. [Google Scholar] [CrossRef]
- Goldstone, A.P.; Holland, A.J.; Hauffa, B.P.; Hokken-Koelega, A.C.; Tauber, M.; Speakers Contributors at the Second Expert Meeting of the Comprehensive Care of Patients with PWS. Recommendations for the diagnosis and management of Prader-Willi syndrome. J. Clin. Endocrinol. Metab. 2008, 93, 4183–4197. [Google Scholar] [CrossRef]
- Cassidy, S.B. Prader-Willi syndrome. J. Med. Genet. 1997, 34, 917–923. [Google Scholar] [CrossRef]
- Holm, V.A.; Cassidy, S.B.; Butler, M.G.; Hanchett, J.M.; Greenswag, L.R.; Whitman, B.Y.; Greenberg, F. Prader-Willi syndrome: Consensus diagnostic criteria. Pediatrics 1993, 91, 398–402. [Google Scholar]
- Cassidy, S.B.; Schwartz, S.; Miller, J.L.; Driscoll, D.J. Prader-Willi syndrome. Gen. Med. 2012, 14, 10–26. [Google Scholar] [CrossRef]
- Partsch, C.A.; Lämmer, C.; Gillessen-Kaesbach, G.; Pankau, R. Adult patients with Prader-Willi syndrome: Clinical characteristics, life circumstances and growth hormone secretion. Growth Horm. IGF Res. 2000, 10, S81–S85. [Google Scholar] [CrossRef]
- Whittington, J.; Holland, A.; Webb, T.; Butler, J.; Clarke, D.; Boer, H. Relationship between clinical and genetic diagnosis of Prader-Willi syndrome. J. Med. Genet. 2002, 39, 926–932. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grugni, G.; Morabito, F.; Crinò, A. Gonadal function and its disorders in simple obesity and in Prader-Willi syndrome. In Prader-Willi Syndrome as a Model for Obesity; Eiholzer, U., l’Allemad, D., Zipf, W., Eds.; Karger: Basel, Switzerland, 2003; pp. 140–155. [Google Scholar] [CrossRef]
- Höybye, C.; Thorén, M.; Böhm, B. Cognitive, emotional, physical and social effects of growth hormone treatment in adults with Prader-Willi syndrome. J. Intellect. Disabil. Res. 2005, 49, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.L.; Goldstone, A.P.; Couch, J.A.; Shuster, J.; He, G.; Driscoll, D.J.; Liu, Y.; Schmalfuss, I.M. Pituitary abnormalities in Prader-Willi syndrome and early onset morbid obesity. Am. J. Med. Genet. A 2008, 146A, 570–577. [Google Scholar] [CrossRef]
- Brandau, D.T.; Theodoro, M.; Garg, U.; Butler, M.G. Follicle stimulating and leutinizing hormones, estradiol and testosterone in Prader-Willi syndrome. Am. J. Med. Genet. A 2008, 146A, 665–669. [Google Scholar] [CrossRef]
- Sode-Carlsen, R.; Farholt, S.; Rabben, K.F.; Bollerslev, J.; Schreiner, T.; Jurik, A.G.; Christiansen, J.S.; Höybye, C. Body composition, endocrine and metabolic profiles in adults with Prader-Willi syndrome. Growth Horm. IGF Res. 2010, 20, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Van Nieuwpoort, I.C.; Sinnema, M.; Castelijns, J.A.; Twisk, J.W.; Curfs, L.M.; Drent, M.L. The GH/IGF-I axis and pituitary function and size in adults with Prader-Willi syndrome. Horm. Res. Paediatr. 2011, 75, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Radicioni, A.F.; Di Giorgio, G.; Grugni, G.; Cuttini, M.; Losacco, V.; Anzuini, A.; Spera, S.; Marzano, C.; Lenzi, A.; Cappa, M.; et al. Multiple forms of hypogonadism of central, peripheral or combined origin in males with Prader-Willi syndrome. Clin. Endocrinol. 2012, 76, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Kido, Y.; Sakazume, S.; Abe, Y.; Oto, Y.; Itabashi, H.; Shiraishi, M.; Yoshino, A.; Tanaka, Y.; Obata, K.; Murakami, N.; et al. Testosterone replacement therapy to improve secondary sexual characteristics and body composition without adverse behavioral problems in adult male patients with Prader-Willi syndrome: An observational study. Am. J. Med. Genet. A 2013, 161A, 2167–2173. [Google Scholar] [CrossRef]
- Hirsch, H.J.; Eldar-Geva, T.; Bennaroch, F.; Pollak, Y.; Gross-Tsur, V. Sexual dichotomy of gonadal function in Prader-Willi syndrome from early infancy through the fourth decade. Hum. Reprod. 2015, 30, 2587–2596. [Google Scholar] [CrossRef]
- Coupaye, M.; Tauber, M.; Cuisset, L.; Laurier, V.; Bieth, E.; Lacorte, J.M.; Oppert, J.M.; Clement, K.; Poitou, C. Effect of Genotype and Previous GH Treatment on Adiposity in Adults With Prader-Willi Syndrome. J. Clin. Endocrinol. Metab. 2016, 101, 4895–4903. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, S.; Matsui, F.; Matsuoka, K.; Iijima, M.; Takeuchi, M.; Ida, S.; Matsumoto, F.; Mizokami, A. Gonadal function and testicular histology in males with Prader-Willi syndrome. Endocrinol. Diabetes Metab. 2019, 2, e00049. [Google Scholar] [CrossRef]
- Heksch, R.; Kamboj, M.; Anglin, K.; Obrynba, K. Review of Prader-Willi syndrome: The endocrine approach. Transl. Pediatr. 2017, 6, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Eiholzer, U.; l’Allemand, D.; Rousson, V.; Schlumpf, M.; Gasser, T.; Girard, J.; Grüters, A.; Simoni, M. Hypothalamic and gonadal components of hypogonadism in boys with Prader-Labhart- Willi syndrome. J. Clin. Endocrinol. Metab. 2006, 91, 892–898. [Google Scholar] [CrossRef]
- Hirsch, H.J.; Eldar-Geva, T.; Benarroch, F.; Rubinstein, O.; Gross-Tsur, V. Primary testicular dysfunction is a major contributor to abnormal pubertal development in males with Prader-Willi syndrome. J. Clin. Endocrinol. Metab. 2009, 94, 2262–2268. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Formoso, G.; Pugliese, G.; Ruggeri, R.M.; Scarano, E.; Colao, A.; Restare. Prader- Willi syndrome: An uptodate on endocrine and metabolic complications. Rev. Endocr. Metab. Disord. 2019, 20, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Linnemann, K.; Schroder, C.; Mix, M.; Kruger, G.; Fusch, C. Prader-Labhart-Willi syndrome with central precocious puberty and empty sella syndrome. Acta Paediatr. 1999, 88, 1295–1297. [Google Scholar] [CrossRef]
- Crinò, A.; Schiaffini, R.; Ciampalini, P.; Spera, S.; Beccaria, L.; Benzi, F.; Bosio, L.; Corrias, A.; Gargantini, L.; Salvatoni, A.; et al. Hypogonadism and pubertal development in Prader-Willi syndrome. Eur. J. Pediatr. 2003, 162, 327–333. [Google Scholar] [CrossRef]
- Siemensma, E.P.; de Lind van Wijngaarden, R.F.; Otten, B.J.; de Jong, F.H.; Hokken-Koelega, A.C. Pubarche and serum dehydroepiandrosterone sulphate levels in children with Prader-Willi syndrome. Clin. Endocrinol. 2011, 75, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Laurance, B.M.; Brito, A.; Wilkinson, J. Prader-Willi Syndrome after age 15 years. Arch. Dis. Child. 1981, 56, 181–186. [Google Scholar] [CrossRef]
- Marzullo, P.; Marcassa, C.; Campini, R.; Eleuteri, E.; Minocci, A.; Priano, L.; Temporelli, P.; Sartorio, A.; Vettor, R.; Liuzzi, A.; et al. The impact of growth hormone/insulin-like growth factor-I axis and nocturnal breathing disorders on cardiovascular features of adult patients with Prader-Willi syndrome. J. Clin. Endocrinol. Metab. 2005, 90, 5639–5646. [Google Scholar] [CrossRef] [PubMed]
- Hoybye, C.; Hilding, A.; Jacobsson, H.; Thoren, M. Metabolic profile and body composition in adults with Prader-Willi syndrome and severe obesity. J. Clin. Endocrinol. Metab. 2002, 87, 3590–3597. [Google Scholar] [CrossRef] [PubMed]
- Richard-Eaglin, A. Male and Female Hypogonadism. Nurs. Clin. North. Am. 2018, 53, 395–405. [Google Scholar] [CrossRef]
- Kloner, R.A.; Carson, C., 3rd; Dobs, A.; Kopecky, S.; Mohler, E.R., 3rd. Testosterone and Cardiovascular Disease. J. Am. Coll. Cardiol. 2016, 67, 545–557. [Google Scholar] [CrossRef]
- Molina-Vega, M.; Muñoz-Garach, A.; Damas-Fuentes, M.; Fernández-García, J.C.; Tinahones, F.J. Secondary male hypogonadism: A prevalent but overlooked comorbidity of obesity. Asian J. Androl. 2018, 20, 531–538. [Google Scholar] [CrossRef]
- Boese, A.C.; Kim, S.C.; Yin, K.J.; Lee, J.P.; Hamblin, M.H. Sex differences in vascular physiology and pathophysiology: Estrogen and androgen signaling in health and disease. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H524–H545. [Google Scholar] [CrossRef] [PubMed]
- Noordam, C.; Höybye, C.; Eiholzer, U. Prader-Willi Syndrome and Hypogonadism: A Review Article. Int. J. Mol. Sci. 2021, 22, 2705. [Google Scholar] [CrossRef] [PubMed]
- Pellikaan, K.; Rosenberg, A.G.W.; Kattentidt-Mouravieva, A.A.; Kersseboom, R.; Bos-Roubos, A.G.; Veen-Roelofs, J.M.C.; van Wieringen, N.; Hoekstra, F.M.E.; van den Berg, S.A.A.; van der Lely, A.J.; et al. Missed Diagnoses and Health Problems in Adults With Prader-Willi Syndrome: Recommendations for Screening and Treatment. J. Clin. Endocrinol. Metab. 2020, 105, e4671–e4687. [Google Scholar] [CrossRef]
- Crawford, E.D.; Poage, W.; Nyhuis, A.; Price, D.A.; Dowsett, S.A.; Gelwicks, S.; Muram, D. Measurement of testosterone: How important is a morning blood draw? Curr. Med. Res. Opin. 2015, 31, 1911–1914. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Brito, J.P.; Cunningham, G.R.; Hayes, F.J.; Hodis, H.N.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Wu, F.C.; Yialamas, M.A. Testosterone Therapy in Men With Hypogonadism: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018, 103, 1715–1744. [Google Scholar] [CrossRef]
- Napolitano, L.; Barone, B.; Morra, S.; Celentano, G.; La Rocca, R.; Capece, M.; Morgera, V.; Turco, C.; Caputo, V.F.; Spena, G.; et al. Hypogonadism in Patients with Prader Willi Syndrome: A Narrative Review. Int. J. Mol. Sci. 2021, 22, 1993. [Google Scholar] [CrossRef] [PubMed]
- Vogels, A.; Moerman, P.; Frijns, J.P.; Bogaert, G.A. Testicular histology in boys with Prader-Willi syndrome: Fertile or infertile? J. Urol. 2008, 180, 1800–1804. [Google Scholar] [CrossRef]
- Katcher, M.L.; Bargman, G.J.; Gilbert, E.F.; Opitz, J.M. Absence of spermatogonia in the Prader-Willi syndrome. Eur. J. Pediatr. 1977, 124, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Färber, C.; Gross, S.; Neesen, J.; Buiting, K.; Horsthemke, B. Identification of a testis-specific gene (C15orf2) in the Prader-Willi syndrome region on chromosome 15. Genomics 2000, 65, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Snyder, P.J.; Peachey, H.; Berlin, J.A.; Hannoush, P.; Haddad, G.; Dlewati, A.; Santanna, J.; Loh, L.; Lenrow, D.A.; Holmes, J.H.; et al. Effects of testosterone replacement in hypogonadal men. J. Clin. Endocrinol. Metab. 2000, 85, 2670–2677. [Google Scholar] [CrossRef] [PubMed]
- Donze, S.H.; Kuppens, R.J.; Bakker, N.E.; van Alfen-van der Velden, J.; Hokken-Koelega, A.C.S. Bone mineral density in young adults with Prader-Willi syndrome: A randomized, placebo-controlled, crossover GH trial. Clin. Endocrinol. 2018, 88, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Woodhouse, L.; Casaburi, R.; Singh, A.B.; Bhasin, D.; Berman, N.; Chen, X.; Yarasheski, K.E.; Magliano, L.; Dzekov, C.; et al. Testosterone dose-response relationships in healthy young men. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E1172–E1181. [Google Scholar] [CrossRef]
- Grugni, G.; Marzullo, P. Diagnosis and treatment of GH deficiency in Prader-Willi syndrome. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cunningham, G.; Dobs, A.; Iranmanesh, A.; Matsumoto, A.M.; Snyder, P.J.; Weber, T.; Berman, N.; Hull, L.; Swerdloff, R.S. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J. Clin. Endocrinol. Metab. 2004, 89, 2085–2098. [Google Scholar] [CrossRef]
- Wagner, G.J.; Rabkin, J.G.; Rabkin, R. Testosterone as a treatment for fatigue in HIV+ men. Gen. Hosp. Psychiatry 1998, 20, 209–213. [Google Scholar] [CrossRef]
- Pexman-Fieth, C.; Behre, H.M.; Morales, A.; Kan-Dobrosky, N.; Miller, M.G. A 6-month observational study of energy, sexual desire, and body proportions in hypogonadal men treated with a testosterone 1% gel. Aging Male 2014, 17. [Google Scholar] [CrossRef]
- Petering, R.C.; Brooks, N.A. Testosterone Therapy: Review of Clinical Applications. Am. Fam. Physician 2017, 96, 441–449. [Google Scholar]
- Butler, M.G.; Manzardo, A.M.; Heinemann, J.; Loker, C.; Loker, J. Causes of death in Prader-Willi syndrome: Prader-Willi Syndrome Association (USA) 40-year mortality survey. Genet. Med. 2017, 19, 635–642. [Google Scholar] [CrossRef]
- Pacoricona Alfaro, D.L.; Lemoine, P.; Ehlinger, V.; Molinas, C.; Diene, G.; Valette, M.; Pinto, G.; Coupaye, M.; Poitou-Bernert, C.; Thuilleaux, D.; et al. Causes of death in Prader-Willi syndrome: Lessons from 11 years’ experience of a national reference center. Orphanet J. Rare Dis. 2019, 14, 238. [Google Scholar] [CrossRef]
- Diaconu, R.; Donoiu, I.; Mirea, O.; Bălşeanu, T.A. Testosterone, cardiomyopathies, and heart failure: A narrative review. Asian J. Androl. 2021, 23, 348–356. [Google Scholar] [CrossRef]
- Cittadini, A.; Isidori, A.M.; Salzano, A. Testosterone therapy and cardiovascular diseases. Cardiovasc. Res. 2021. [Google Scholar] [CrossRef]
- Cooper, L.A.; Page, S.T.; Amory, J.K.; Anawalt, B.D.; Matsumoto, A.M. The association of obesity with sex hormone-binding globulin is stronger than the association with ageing—Implications for the interpretation of total testosterone measurements. Clin. Endocrinol. 2015, 83, 828–833. [Google Scholar] [CrossRef]
- Richards, A.; Quaghebeur, G.; Clift, S.; Holland, A.; Dahlitz, M.; Parkes, D. The upper airway and sleep apnoea in the Prader-Willi syndrome. Clin. Otolaryngol. Allied Sci. 1994, 19, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Proffitt, J.; Osann, K.; McManus, B.; Kimonis, V.E.; Heinemann, J.; Butler, M.G.; Stevenson, D.A.; Gold, J.A. Contributing factors of mortality in Prader-Willi syndrome. Am. J. Med. Genet. A 2019, 179, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Paragliola, R.M.; Prete, A.; Kaplan, P.W.; Corsello, S.M.; Salvatori, R. Treatment of hypopituitarism in patients receiving antiepileptic drugs. Lancet Diabetes Endocrinol. 2015, 3, 132–140. [Google Scholar] [CrossRef]
- Hansen, C.H.; Larsen, L.W.; Sørensen, A.M.; Halling-Sørensen, B.; Styrishave, B. The six most widely used selective serotonin reuptake inhibitors decrease androgens and increase estrogens in the H295R cell line. Toxicol. In Vitro 2017, 41, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Svalheim, S.; Sveberg, L.; Mochol, M.; Taubøll, E. Interactions between antiepileptic drugs and hormones. Seizure 2015, 28, 12–17. [Google Scholar] [CrossRef]
- Daniel, W.A. The influence of long-term treatment with psychotropic drugs on cytochrome P450: The involvement of different mechanisms. Expert Opin. Drug Metab. Toxicol. 2005, 1, 203–217. [Google Scholar] [CrossRef]
- Drobnis, E.Z.; Nangia, A.K. Psychotropics and Male Reproduction. Adv. Exp. Med. Biol. 2017, 1034, 63–101. [Google Scholar] [CrossRef] [PubMed]
- Pavlidi, P.; Kokras, N.; Dalla, C. Antidepressants’ effects on testosterone and estrogens: What do we know? Eur. J. Pharmacol. 2021, 899, 173998. [Google Scholar] [CrossRef] [PubMed]
- Veldhuis, J.D.; Frystyk, J.; Iranmanesh, A.; Orskov, H. Testosterone and estradiol regulate free insulin-like growth factor I (IGF-I), IGF binding protein 1 (IGFBP-1), and dimeric IGF-I/IGFBP-1 concentrations. J. Clin. Endocrinol. Metab. 2005, 90, 2941–2947. [Google Scholar] [CrossRef]
- Gibney, J.; Wolthers, T.; Johannsson, G.; Umpleby, A.M.; Ho, K.K. Growth hormone and testosterone interact positively to enhance protein and energy metabolism in hypopituitary men. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E266–E271. [Google Scholar] [CrossRef]
- Kenyon, A.; Knowlton, K.; Sandiford, I.; Koch, F.C.; Lotwin, G. A comparative study of the metabolic effects of testosterone propionate in normal men and women and in eunuchoidism1,2. Endocrinology 1940, 26, 26–45. [Google Scholar] [CrossRef]
- Sinnema, M.; Maaskant, M.A.; van Schrojenstein Lantman-de Valk, H.M.; van Nieuwpoort, I.C.; Drent, M.L.; Curfs, L.M.; Schrander-Stumpel, C.T. Physical health problems in adults with Prader-Willi syndrome. Am. J. Med. Genet. A 2011, 155A, 2112–2124. [Google Scholar] [CrossRef]
- Krauser, D.G.; Lloyd-Jones, D.M.; Chae, C.U.; Cameron, R.; Anwaruddin, S.; Baggish, A.L.; Chen, A.; Tung, R.; Januzzi, J.L., Jr. Effect of body mass index on natriuretic peptide levels in patients with acute congestive heart failure: A ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) substudy. Am. Heart J. 2005, 149, 744–750. [Google Scholar] [CrossRef]
- Abadilla, K.A.; Dobs, A.S. Topical testosterone supplementation for the treatment of male hypogonadism. Drugs 2012, 72, 1591–1603. [Google Scholar] [CrossRef]
- Swerdloff, R.S.; Wang, C.; Cunningham, G.; Dobs, A.; Iranmanesh, A.; Matsumoto, A.M.; Snyder, P.J.; Weber, T.; Longstreth, J.; Berman, N. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J. Clin. Endocrinol. Metab. 2000, 85, 4500–4510. [Google Scholar] [CrossRef]
- Margo, K.; Winn, R. Testosterone treatments: Why, when, and how? Am. Fam. Physician 2006, 73, 1591–1598. [Google Scholar] [PubMed]
- Bond, P.; Llewellyn, W.; Van Mol, P. Anabolic androgenic steroid-induced hepatotoxicity. Med. Hypotheses 2016, 93, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Falahati-Nini, A.; Riggs, B.L.; Atkinson, E.J.; O’Fallon, W.M.; Eastell, R.; Khosla, S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J. Clin. Investig. 2000, 106, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Baas, W.; Kohler, T.S. Testosterone replacement therapy and voiding dysfunction. Transl. Androl. Urol. 2016, 5, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Rajalakshmi, M.; Jeyaraj, D.A.; Sharma, R.S.; Bajaj, J.S. Effects of long-term use of testosterone enanthate. II. Effects on lipids, high and low density lipoprotein cholesterol and liver function parameters. Int. J. Androl. 1999, 22, 347–355. [Google Scholar] [CrossRef]
- Sadowska-Krępa, E.; Kłapcińska, B.; Nowara, A.; Jagsz, S.; Szołtysek-Bołdys, I.; Chalimoniuk, M.; Langfort, J.; Chrapusta, S.J. High-dose testosterone supplementation disturbs liver pro-oxidant/antioxidant balance and function in adolescent male Wistar rats undergoing moderate-intensity endurance training. PeerJ 2020, 8, e10228. [Google Scholar] [CrossRef] [PubMed]
- Feighan, S.M.; Hughes, M.; Maunder, K.; Roche, E.; Gallagher, L. A profile of mental health and behaviour in Prader-Willi syndrome. J. Intellect. Disabil. Res. 2020, 64, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.J.; Boer, H.; Chung, M.C.; Sturmey, P.; Webb, T. Maladaptive behaviour in Prader-Willi syndrome in adult life. J. Intellect. Disabil. Res. 1996, 40 Pt 2, 159–165. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).



