The Application of High-Dose Proton Pump Inhibitor Induction Treatment before Dual Therapy for Helicobacter pylori Eradication: An Open-Label Random Trial
Abstract
:1. Introduction
- 1.
- Three-day high-dose rabeprazole (20 mg, four times per day) induction treatment before H. pylori eradication would increase intragastric pH and induce active replication in H. pylori. The active replicative status enhances the bactericidal effects of amoxicillin.
- 2.
- After a 3-day high-dose rabeprazole induction treatment, rabeprazole and amoxicillin could be taken simultaneously. It is not necessary to separate rabeprazole (before meals) and amoxicillin (after meals). Simultaneous administration of rabeprazole and amoxicillin would improve drug compliance.
- 3.
- A total of 2 g instead of 3 g amoxicillin (500 mg, four times per day) might be adequate for dual therapy to achieve an acceptable eradication rate.
- 4.
- A modified schedule such as taking the medications during breakfast, lunch, dinner, and bedtime could adopt the patients’ daily activities. This schedule might remind patients to take drugs and achieve good drug compliance.
2. Materials and Methods
2.1. UBT
2.2. Treatment Regimens
2.3. Statistical Analysis
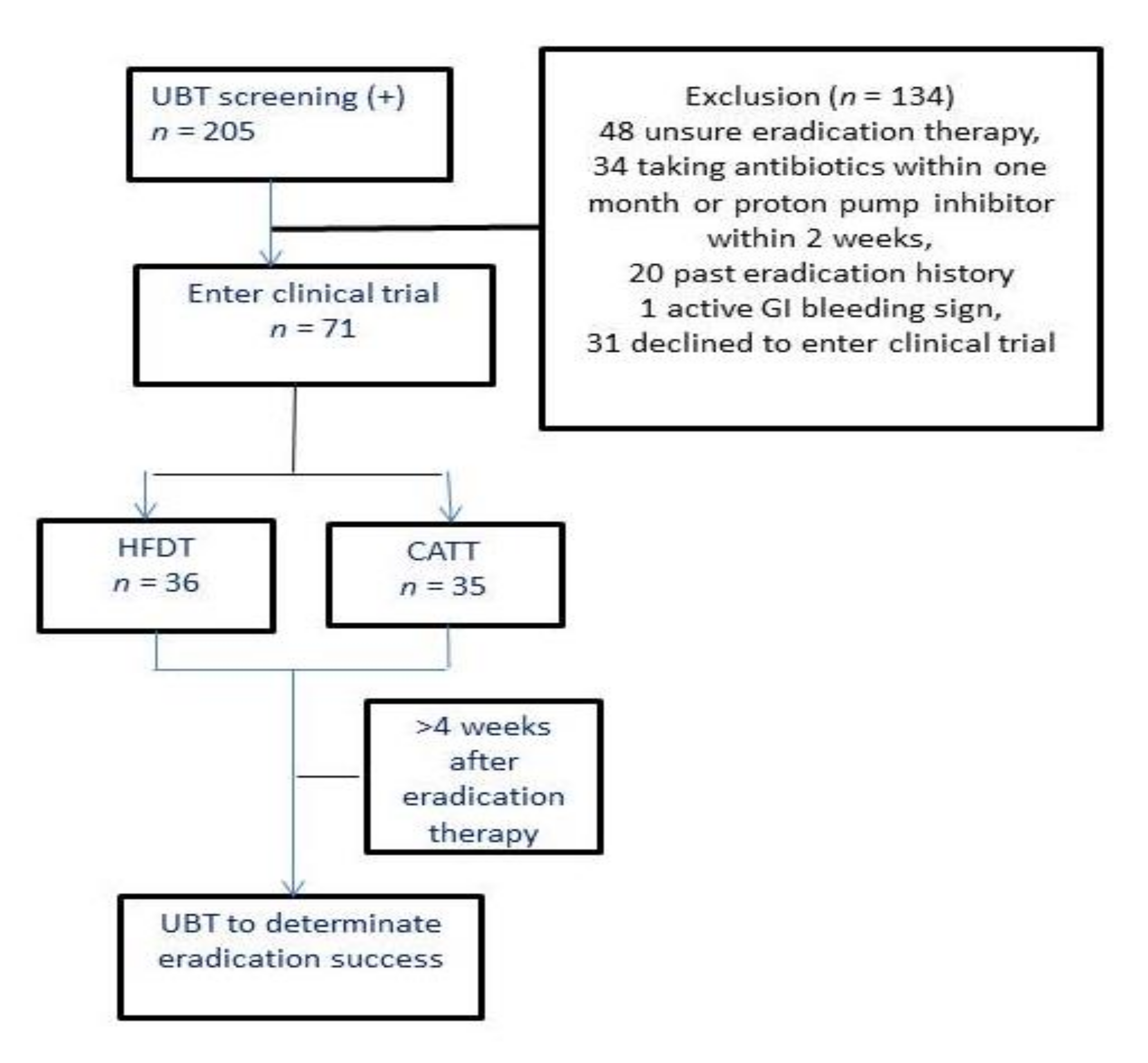
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McColl, K.E.L. Helicobacter pylori infection. N. Engl. J. Med. 2010, 362, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Atherton, J.; Axon, A.T.R.; Bazzoli, F.; Gensini, G.F.; Gisbert, J.P.; Graham, D.Y.; Rokkas, T. Management of Helicobacter pylori infection: The Maastricht IV/Florence Consensus Report. Gut 2012, 61, 646–664. [Google Scholar] [CrossRef] [Green Version]
- Graham, D.Y.; Fischbach, L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 2010, 59, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Vakil, N.; Vaira, D. Treatment for H. pylori infection: New challenges with antimicrobial resistance. J. Clin. Gastroenterol. 2013, 47, 383–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Megraud, F.; Lehours, P. Helicobacter pylori delection and antimicrobial susceptibility testing. Clin. Microbiol. Rev. 2007, 20, 280–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glupczynski, Y.; Megraud, F.; Lopez-Brea, M.; Andersen, L.P. European multicentre survey of in vitro antimicrobial resistance in Helicobacter pylori. Eur. J. Clin. Microbiol. Infect. Dis. 2001, 20, 820–823. [Google Scholar] [CrossRef]
- Meyer, J.M.; Silliman, N.P.; Wang, W.; Siepman, N.Y.; Sugg, J.E.; Morris, D.; Zhang, J.; Bhattacharyya, H.; King, E.C.; Hopkins, R.J. Risk factors for Helicobacter pylori resistance in the United States: The surveillance of H. pylori antimicrobial resistance partnership (SHARP) study, 1993–1999. Ann. Intern. Med. 2002, 136, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Berry, V.; Jennings, K.; Woodnutt, G. Bactericidal and morphological effects of amoxicillin on Helicobacter pylori. Antimicrob. Agents Chemother. 1995, 39, 1859–1861. [Google Scholar] [CrossRef] [Green Version]
- Craig, W.A. Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 1998, 26, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Lin, C.J. CYP2C19 genotypes in the pharmacokinetics/pharmacodynamics of proton pump inhibitor-based therapy of Helicobacter pylori infection. Expert Opin. Drug Metab. Toxicol. 2010, 6, 29–41. [Google Scholar] [CrossRef]
- Agrawal, A.; Tutuian, R.; Hila, A.; Freeman, J.; Castell, D.O. Ingestion of acidic foods mimics gastroesophageal reflux during pH monitoring. Dig. Dis. Sci. 2005, 50, 1916–1920. [Google Scholar] [CrossRef]
- Scott, D.; Weeks, D.; Melchers, K.; Sachs, G. The life and death of Helicobacter pylori. Gut 1998, 43 (Suppl. 1), S56–S60. [Google Scholar] [CrossRef]
- Scott, D.R.; Marcus, E.A.; Wen, Y.; Oh, J.; Sachs, G. Gene expression in vivo shows that Helicobacter pylori colonizes an acidic niche on the gastric surface. Proc. Natl. Acad. Sci. USA 2007, 104, 7235–7240. [Google Scholar] [CrossRef] [Green Version]
- Kawakami, Y.; Akahane, T.; Yamaguchi, M.; Oana, K.; Takahashi, Y.; Okimura, Y.; Okabe, T.; Gotoh, A.; Katsuyama, T. In vitro activities of rabeprazole, a novel proton pump inhibitor, and its thioether derivative alone and in combination with other antimicrobials against recent clinical isolates of Helicobacter pylori. Antimicrob. Agents Chemother. 2000, 44, 458–461. [Google Scholar] [CrossRef] [Green Version]
- Graham, D.Y.; Shiotani, A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat. Clin. Pract. Gastroenterol. Hepatol. 2008, 5, 331. [Google Scholar] [CrossRef] [PubMed]
- Van der Hulst, R.W.; Keller, J.J.; Rauws, E.A.; Tytgat, G.N. Treatment of Helicobacter pylori infection: A review of the world literature. Helicobacter 1996, 1, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Bayerdorffer, E.; Miehlke, S.; Mannes, G.A.; Sommer, A.; Höchter, W.; Weingart, J.; Heldwein, W.; Klann, H.; Simon, T.; Schmitt, W.; et al. Double-blind trial of omeprazole and amoxicillin to cure Helicobacter pylori infection in patients with duodenal ulcers. Gastroenterology 1995, 108, 1412–1417. [Google Scholar] [CrossRef]
- Yang, J.C.; Lu, C.W.; Lin, C.J. Treatment of Helicobacter pylori infection: Current status and future concepts. World J. Gastroenterol. 2014, 20, 5283–5293. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Lin, C.J.; Wang, H.L.; Chen, J.D.; Kao, J.Y.; Shun, C.T.; Lu, C.W.; Lin, B.R.; Shieh, M.J.; Chang, M.C.; et al. High-dose dual therapy Is superior to standard first-line or rescue therapy for Helicobacter pylori infection. Clin. Gastroenterol. Hepatol. 2015, 13, 895–905. [Google Scholar] [CrossRef] [Green Version]
- De Boer, W.A.; Thys, J.C.; Borody, T.J.; Graham, D.Y.; O’Morain, C.; Tytgat, G.N. Proposal for use of a standard side effect scoring system in studies exploring Helicobacter pylori treatment regimens. Eur. J. Gastroenterol. Hepatol. 1996, 8, 641–643. [Google Scholar] [PubMed]
- Labenz, J.; Stolte, M.; Blum, A.L.; Jorias, I.; Leverkus, F.; Sollbohmer, M.; Bertrams, J.; Borsch, G. Intragastric acidity as a predictor of the success of Helicobacter pylori eradication: A study in peptic ulcer patients with omeprazole and amoxicillin. Gut 1995, 37, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.M.; Chang, C.Y.; Chen, M.J.; Chen, C.C.; Fang, Y.J.; Lee, J.Y.; Wu, J.Y.; Luo, J.C.; Liou, T.C.; Chang, W.H.; et al. The primary resistance of Helicobacter pylori in Taiwan after the national policy to restrict antibiotic consumption and its relation to virulence factors—A nationwide study. PLoS ONE 2015, 10, e0124199. [Google Scholar] [CrossRef]
- Savoldi, A.; Carrara, E.; Graham, D.Y.; Conti, M.; Tacconelli, E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-analysis in World Health Organization Regions. Gastroenterology 2018, 155, 1372–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atherton, J.C.; Cullen, D.J.; Kirk, G.E.; Hawkey, C.J.; Spiller, R.C. Enhanced eradication of Helicobacter pylori by pre- versus postprandial amoxycillin suspension with omeprazole: Implications for antibiotic delivery. Aliment Pharmacol. Ther. 1996, 10, 631–635. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Fan, L.; Zhu, Y.J.; Wang, T.Y.; Wang, X.W.; Chen, D.F.; Lan, C.H. Eradication efficacy of modified dual therapy compared with bismuth-containing quadruple therapy as a first-line treatment of Helicobacter pylori. Am. J. Gastroenterol. 2019, 114, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.P.; Zhang, D.; Zhang, T.; Wang, J.X.; Han, S.X.; Graham, D.Y.; Lu, H. PPI-amoxicillin dual therapy for Helicobacter pylori infection: An update based on a systematic review and meta-analysis. Helicobacter 2020, 25, e12692. [Google Scholar] [CrossRef] [Green Version]
- Furuta, T.; Shirai, N.; Kodaira, M.; Sugimoto, M.; Nogaki, A.; Kuriyama, S.; Iwaizumi, M.; Yamade, M.; Terakawa, I.; Ohashi, K.; et al. Pharmacogenomics-based tailored versus standard therapeutic regimen for eradication of H. pylori. Clin. Pharmacol. Ther. 2007, 81, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Gotoda, T.; Kusano, C.; Ikehara, H.; Ichijima, R.; Ohyauchi, M.; Ito, H.; Kawamura, M.; Ogata, Y.; Ohtaka, M.; et al. Seven-day vonoprazan and low-dose amoxicillin dual therapy as first-line Helicobacter pylori treatment: A multicentre randomised trial in Japan. Gut 2020, 69, 1019–1026. [Google Scholar] [CrossRef] [Green Version]
| Group | HDDT | CATT | p Value |
|---|---|---|---|
| Patient number | 36 | 35 | |
| Age (y/o) ‡ | 59.6 ± 10.1 | 58.0 ± 11.0 | 0.13 |
| Gender (F/M) | 20/16 | 21/14 | 0.89 |
| Smoking/alcohol | 7/5 | 2/4 | 0.14 |
| Endoscopic study | |||
| Duodenal ulcer | 8 | 4 | 0.53 |
| Gastric ulcer | 4 | 3 | |
| Gastritis | 27 | 25 | |
| Reflux esophagitis | 16 | 13 | |
| Clinical presentations | |||
| Epigastric pain | 32 | 30 | 0.21 |
| Abdomen fullness | 15 | 16 | |
| GERD § | 22 | 11 | |
| H. pylori eradicated success rate ‖ | |||
| Intention to treat ‖ | 33/36 (91.7%) | 27/35 (77.1%) | 0.75 |
| 95% CI | 78–97% | 61–87% | |
| Per-protocol | 33/35 (94.3%) | 27/32 (84.3%) | 0.89 |
| 95% CI | 79–99% | 66–94% | |
| Group | HDDT (n =) | CATT (n =) | p Value |
|---|---|---|---|
| Taste distortion | 3 (3.3%) | 4 (4.0%) | 0.89 |
| Diarrhea | 1(1.1%) | 1 (1.0%) | 0.52 |
| Dizziness | 1(1.1%) | 0 (0) | 0.96 |
| Headache | 2 (2.2%) | 1 (1.0%) | 0.94 |
| Abdominal pain | 1 (1.1%) | 2 (2.0%) | 0.93 |
| Skin rash | 0 (0) | 2 (2.0%) | 0.53 |
| Total incidence | 8 (8.9%) | 10 (10.1%) | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.-W.; Chang, L.-C.; Hua, C.-C.; Liu, C.-J.; Chou, T.-S.; Lin, C.-L.; Chien, R.-N. The Application of High-Dose Proton Pump Inhibitor Induction Treatment before Dual Therapy for Helicobacter pylori Eradication: An Open-Label Random Trial. J. Clin. Med. 2021, 10, 4352. https://doi.org/10.3390/jcm10194352
Chen L-W, Chang L-C, Hua C-C, Liu C-J, Chou T-S, Lin C-L, Chien R-N. The Application of High-Dose Proton Pump Inhibitor Induction Treatment before Dual Therapy for Helicobacter pylori Eradication: An Open-Label Random Trial. Journal of Clinical Medicine. 2021; 10(19):4352. https://doi.org/10.3390/jcm10194352
Chicago/Turabian StyleChen, Li-Wei, Liang-Che Chang, Chung-Ching Hua, Ching-Jung Liu, Tien-Shin Chou, Chih-Lang Lin, and Rong-Nan Chien. 2021. "The Application of High-Dose Proton Pump Inhibitor Induction Treatment before Dual Therapy for Helicobacter pylori Eradication: An Open-Label Random Trial" Journal of Clinical Medicine 10, no. 19: 4352. https://doi.org/10.3390/jcm10194352
APA StyleChen, L.-W., Chang, L.-C., Hua, C.-C., Liu, C.-J., Chou, T.-S., Lin, C.-L., & Chien, R.-N. (2021). The Application of High-Dose Proton Pump Inhibitor Induction Treatment before Dual Therapy for Helicobacter pylori Eradication: An Open-Label Random Trial. Journal of Clinical Medicine, 10(19), 4352. https://doi.org/10.3390/jcm10194352







