Sepsis and Cognitive Assessment
Abstract
:1. Introduction
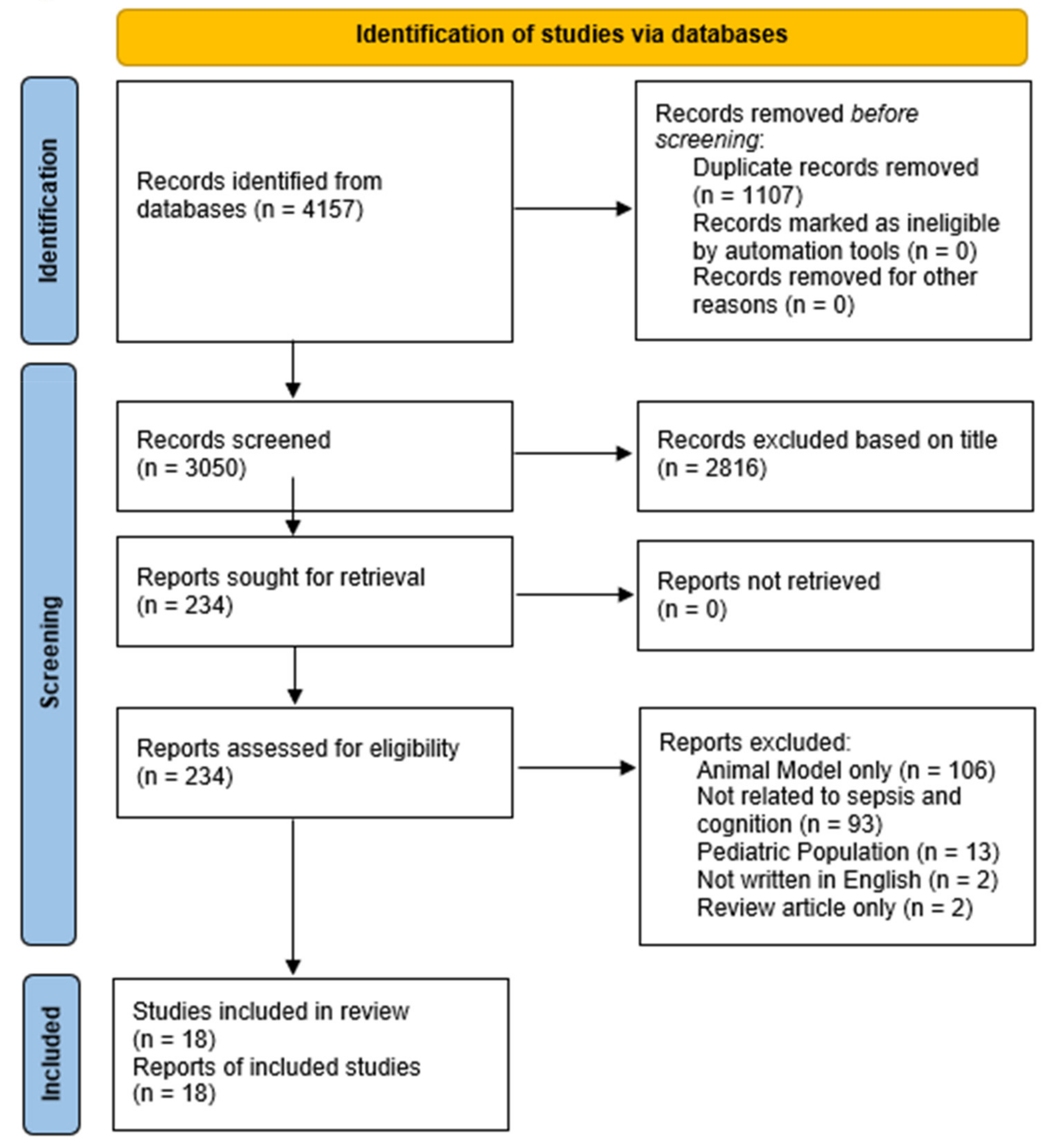
2. Materials and Methods
3. Results
3.1. Author Inclusion of Neuropsychology Expert and Study Design
3.2. Age, Education, Sex, Race and Ethnicity
3.3. Baseline and Time of Testing
3.4. Type of Measures, Congitive Domains, and Reported Scores
3.5. Statistical Analyses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gotts, J.; Matthay, M. Sepsis: Pathophysiology and clinical management. BMJ 2016, 353, i1585. [Google Scholar] [CrossRef] [Green Version]
- Kumar, G.; Kumar, N.; Taneja, A.; Kaleekal, T.; Tarima, S.; McGinley, E.; Jimenez, E.; Mohan, A.; Khan, R.A.; Whittle, J.; et al. Nationwide Trends of Severe Sepsis in the 21st Century (2000–2007). Chest 2011, 140, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.S.; Mannino, D.; Eaton, S.; Moss, M. The Epidemiology of Sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003, 348, 1546–1554. [Google Scholar] [CrossRef] [Green Version]
- Singer, M.; Deutschman, C.S.; Seymour, C.C.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Hajj, J.; Blaine, N.; Salavaci, J.; Jacoby, D. The “Centrality of Sepsis”: A Review on Incidence, Mortality, and Cost of Care. Healthcare 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhee, C.; Jones, T.M.; Hamad, Y.; Pande, A.; Varon, J.; O’Brien, C.; Anderson, D.J.; Warren, D.K.; Dantes, R.B.; Epstein, L.; et al. Prevalence, Underlying Causes, and Preventability of Sepsis-Associated Mortality in US Acute Care Hospitals. JAMA Netw. Open 2019, 2, e187571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brummel, N.E.; Balas, M.C.; Morandi, A.; Ferrante, L.; Gill, T.; Ely, E.W. Understanding and Reducing Disability in Older Adults Following Critical Illness. Crit. Care Med. 2015, 43, 1265–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkins, R.B.; Raymond, S.L.; Stortz, J.A.; Horiguchi, H.; Brakenridge, S.; Gardner, A.; Efron, P.A.; Bihorac, A.; Segal, M.; Moore, F.A.; et al. Chronic Critical Illness and the Persistent Inflammation, Immunosuppression, and Catabolism Syndrome. Front. Immunol. 2018, 9, 1511. [Google Scholar] [CrossRef]
- Satz, P. Brain reserve capacity on symptom onset after brain injury: A formulation and review of evidence for threshold theory. Neuropsychologia 1993, 7, 273–295. [Google Scholar] [CrossRef]
- Shen, H.-N.; Lu, C.-L.; Li, C.-Y. Dementia Increases the Risks of Acute Organ Dysfunction, Severe Sepsis and Mortality in Hospitalized Older Patients: A National Population-Based Study. PLoS ONE 2012, 7, e42751. [Google Scholar] [CrossRef] [Green Version]
- Wan, H.; Goodkind, D.; Kowal, P.U.S. Census Bureau, International Population Reports; P95/16-1, An Aging World: 2015; U.S. Government Publishing Office: Washington, DC, USA, 2016.
- Hebert, L.E.; Scherr, P.A.; Bienias, J.L.; Bennett, D.A.; Evans, D.A. Alzheimer Disease in the US Population Prevalence Estimates Using the 2000 Census. Arch. Neurol. 2003, 60, 1119–1122. Available online: https://jamanetwork.com (accessed on 30 July 2021). [CrossRef] [PubMed] [Green Version]
- Regazzoni, C.J.; Zamora, R.J.; Petrucci, E.; Pisarevsky, A.A.; Saad, A.K.; Mollein, D.D.; Luna, C.M.; Poderoso, J.J.; De Mollein, D. Hospital and 1-Year Outcomes of Septic Syndromes in Older People: A Cohort Study. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2008, 63, 210–212. [Google Scholar] [CrossRef] [Green Version]
- Girard, T.D.; Jackson, J.C.; Pandharipande, P.; Pun, B.T.; Thompson, J.L.; Shintani, A.K.; Gordon, S.M.; Canonico, A.E.; Dittus, R.S.; Bernard, G.R.; et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit. Care Med. 2010, 38, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Iwashyna, T.J.; Ely, E.W.; Smith, D.M.; Langa, K. Long-term Cognitive Impairment and Functional Disability Among Survivors of Severe Sepsis. JAMA 2010, 304, 1787–1794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davydow, D.S.; Hough, C.L.; Langa, K.; Iwashyna, T. Presepsis Depressive Symptoms Are Associated with Incident Cognitive Impairment in Survivors of Severe Sepsis: A Prospective Cohort Study of Older Americans. J. Am. Geriatr. Soc. 2012, 60, 2290–2296. [Google Scholar] [CrossRef] [PubMed]
- Merli, M.; Lucidi, C.; Pentassuglio, I.; Giannelli, V.; Giusto, M.; Di Gregorio, V.; Pasquale, C.; Nardelli, S.; Lattanzi, B.; Venditti, M.; et al. Increased risk of cognitive impairment in cirrhotic patients with bacterial infections. J. Hepatol. 2013, 59, 243–250. [Google Scholar] [CrossRef]
- Semmler, A.; Widmann, C.N.; Okulla, T.; Urbach, H.; Kaiser, M.; Widman, G.; Mormann, F.; Weide, J.; Fliessbach, K.; Hoeft, A.; et al. Persistent cognitive impairment, hippocampal atrophy and EEG changes in sepsis survivors. J. Neurol. Neurosurg. Psychiatry 2013, 84, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Götz, T.; Baumbach, P.; Huonker, R.; Kranczioch, C.; Witte, O.W.; Debener, S.; Klingner, C.; Brunkhorst, F.M.; Günther, A. Slowed peak resting frequency and MEG overactivation in survivors of severe sepsis and septic shock. Clin. Neurophysiol. 2015, 127, 1247–1253. [Google Scholar] [CrossRef]
- Götz, T.; Baumbach, P.; Reuken, P.; Huonker, R.; Kranczioch, C.; Debener, S.; Brunkhorst, F.M.; Witte, O.W.; Klingner, C.; Günther, A. The loss of neural synchrony in the post septic brain. Clin. Neurophysiol. 2016, 127, 2200–2207. [Google Scholar] [CrossRef]
- Needham, D.M.; Colantuoni, E.; Dinglas, V.D.; Hough, C.L.; Wozniak, A.W.; Jackson, J.C.; E Morris, P.; A Mendez-Tellez, P.; Ely, E.W.; O Hopkins, R. Rosuvastatin versus placebo for delirium in intensive care and subsequent cognitive impairment in patients with sepsis-associated acute respiratory distress syndrome: An ancillary study to a randomised controlled trial. Lancet Respir. Med. 2016, 4, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Pierrakos, C.; Attou, R.; Decorte, L.; Velissaris, D.; Cudia, A.; Gottignies, P.; Devriendt, J.; Tsolaki, M.; De Bels, D. Cerebral perfusion alterations and cognitive decline in critically ill sepsis survivors. Acta Clin. Belg. 2017, 72, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Collingridge, D.S.; Wilson, E.L.; Beesley, S.; Bose, S.; Orme, J.; Jackson, J.; Hopkins, R.O. Preliminary Validation of the Montreal Cognitive Assessment Tool among Sepsis Survivors: A Prospective Pilot Study. Ann. Am. Thorac. Soc. 2018, 15, 1108–1110. [Google Scholar] [CrossRef] [PubMed]
- Calsavara, A.J.C.; Costa, P.A.; Nobre, V.; Teixeira, A.L. Factors Associated With Short and Long Term Cognitive Changes in Patients With Sepsis. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.-Y.; Jee, S.J.; Kim, C.-S.; Suh, K.-S.; Wong, A.W.; Moon, J.Y. The feasibility study of Computer Cognitive Senior Assessment System-Screen (CoSAS-S) in critically ill patients with sepsis. J. Crit. Care 2018, 44, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Orhun, G.; Tuzun, E.; Ozcan, P.E.; Ulusoy, C.; Yildirim, E.; Küçükerden, M.; Gürvit, H.; Ali, A.; Esen, F. Association between inflammatory markers and cognitive outcome in patients with acute brain dysfunction due to sepsis. Arch. Neuropsychiatry 2018, 56, 63–70. [Google Scholar] [CrossRef]
- Seidel, G.; Gaser, C.; Götz, T.; Günther, A.; Hamzei, F. Accelerated brain ageing in sepsis survivors with cognitive long-term impairment. Eur. J. Neurosci. 2020, 52, 4395–4402. [Google Scholar] [CrossRef] [PubMed]
- Mankowski, R.T.; Anton, S.D.; Ghita, G.L.; Brumback, B.; Cox, M.C.; Mohr, A.M.; Leeuwenburgh, C.; Moldawer, L.L.; Efron, P.A.; Brakenridge, S.C.; et al. Older Sepsis Survivors Suffer Persistent Disability Burden and Poor Long-Term Survival. J. Am. Geriatr. Soc. 2020, 68, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.M.; Beesley, S.J.; Stubben, C.; Wilson, E.L.; Presson, A.P.; Grissom, C.; Maguire, C.; Rondina, M.T.; Hopkins, R.O. Postseptic Cognitive Impairment and Expression of APOE in Peripheral Blood: The Cognition After SepsiS (CASS) Observational Pilot Study. J. Intensiv. Care Med. 2021, 36, 262–270. [Google Scholar] [CrossRef]
- Wang, H.E.; Kabeto, M.M.; Gray, M.; Wadley, V.G.; Muntner, P.; Judd, S.E.; Safford, M.M.; Kempker, J.; Levine, D.A. Trajectory of Cognitive Decline After Sepsis. Crit. Care Med. 2021, 49, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Donders, J.; Stout, J. The Influence of Cognitive Reserve on Recovery from Traumatic Brain Injury. Arch. Clin. Neuropsychol. Off. J. Natl. Acad. Neuropsychol. 2019, 34, 206–213. [Google Scholar] [CrossRef] [Green Version]
- Krch, D.; Frank, L.E.; Chiaravalloti, N.D.; Vakil, E.; DeLuca, J. Cognitive reserve protects against memory decrements associated with neuropathology in traumatic brain injury. J. Head Trauma Rehabil. 2019, 34, E57–E65. [Google Scholar] [CrossRef] [PubMed]
- Steward, K.A.; Kennedy, R.; Novack, T.A.; Crowe, M.; Marson, D.C.; Triebel, K.L. The Role of Cognitive Reserve in Recovery From Traumatic Brain Injury. J. Head Trauma Rehabil. 2018, 33, E18–E27. [Google Scholar] [CrossRef]
- Monk, T.G.; Weldon, B.C.; Garvan, C.W.; Dede, D.E.; van der Aa, M.T.; Heilman, K.M.; Gravenstein, J.S. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology 2008, 108, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Funder, K.S.; Steinmetz, J.; Rasmussen, L.S. Methodological Issues of Postoperative Cognitive Dysfunction Research. Semin. Cardiothorac. Vasc. Anesthesia 2010, 14, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.S. Defining postoperative cognitive dysfunction. Eur. J. Anaesthesiol. 1998, 15, 761–764. [Google Scholar] [CrossRef]
- Selnes, O.A.; Gottesman, R.F.; Grega, M.A.; Baumgartner, W.A.; Zeger, S.L.; McKhann, G.M. Cognitive and Neurologic Outcomes after Coronary-Artery Bypass Surgery. N. Engl. J. Med. 2012, 366, 250–257. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, K.S.; Hsann, Y.M.; Lim, V.; Ong, B.C. The effect of comorbidity and age on hospital mortality and length of stay in patients with sepsis. J. Crit. Care 2010, 25, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Lamar, M.; Price, C.; Libon, D.J.; Penney, D.L.; Kaplan, E.; Grossman, M.; Heilman, K.M. Alterations in working memory as a function of leukoaraiosis in dementia. Neuropsychologia 2007, 45, 245–254. [Google Scholar] [CrossRef] [Green Version]
- Price, C.C.; Jefferson, A.L.; Merino, J.G.; Heilman, K.M.; Libon, D.J. Subcortical vascular dementia: Integrating neuropsychological and neuroradiologic data. Neurology 2005, 65, 376–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, C.C.; Tanner, J.J.; Monk, T.G.; Lydic, R. Postoperative Cognitive Disorders. Neurosci. Found. Anesthesiol. 2011, 19, 255–269. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, L.S.; Larsen, K.; Houx, P.; Skovgaard, L.T.; Hanning, C.D.; Moller, J.T.; ISPOCD Group. The assessment of postoperative cognitive function. Acta Anaesthesiol. Scand. 2001, 45, 275–289. [Google Scholar] [CrossRef]
- Jacobson, N.S.; Truax, P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. J. Consult. Clin. Psychol. 1991, 59, 12–19. [Google Scholar] [CrossRef]
- McQuail, J.A.; Dunn, A.R.; Stern, Y.; Barnes, C.A.; Kempermann, G.; Rapp, P.R.; Kaczorowski, C.C.; Foster, T.C. Cognitive Reserve in Model Systems for Mechanistic Discovery: The Importance of Longitudinal Studies. Front. Aging Neurosci. 2021, 12, 532. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y.; Arenaza-Urquiljo, E.M.; Bartrés-Faz, D.; Belleville, S.; Cantillon, M.; Chetelat, G.; Ewers, M.; Franzmeier, N.; Kempermann, G.; Kremen, W.S.; et al. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. J. Alzheimer’s Assoc. 2020, 16, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Lezak, M.D.; Howieson, D.B.; Loring, D.W.; Fischer, J.S. Neuropsychological Assessment; Oxford University Press: New York, NY, USA, 2004. [Google Scholar]
- Barter, J.; Kumar, A.; Stortz, J.A.; Hollen, M.; Nacionales, D.; Efron, P.A.; Moldawer, L.L.; Foster, T.C. Age and Sex Influence the Hippocampal Response and Recovery Following Sepsis. Mol. Neurobiol. 2019, 56, 8557–8572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chicco, D.; Jurman, G. Survival prediction of patients with sepsis from age, sex, and septic episode number alone. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Xu, J.; Tong, L.; Yao, J.; Guo, Z.; Lui, K.Y.; Hu, X.; Cao, L.; Zhu, Y.; Huang, F.; Guan, X.; et al. Association of Sex With Clinical Outcome in Critically Ill Sepsis Patients: A Retrospective Analysis of the Large Clinical Database MIMIC-III. Shock 2019, 52, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.J.; Johnson, A.J.; Terry, E.L.; Cardoso, J.; Garvan, C.; Staud, R.; Deutsch, G.; Deshpande, H.; Lai, S.; Addison, A.; et al. Resilience, pain, and the brain: Relationships differ by sociodemographics. J. Neurosci. Res. 2021, 99, 1207–1235. [Google Scholar] [CrossRef] [PubMed]
- Bagby, S.P.; Martin, D.; Chung, S.T.; Rajapakse, N. From the Outside In: Biological Mechanisms Linking Social and Environmental Exposures to Chronic Disease and to Health Disparities. Am. J. Public Health 2019, 109, S56–S63. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.V.; Pérez-Stable, E.J.; Anderson, N.A.; Bernard, M.A. The National Institute on Aging Health Disparities Research Framework. Ethn. Dis. 2015, 25, 245–254. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, J.B.; Bennett, T. Use of race and ethnicity in biomedical publication. JAMA 2003, 289, 2709–2716. [Google Scholar] [CrossRef] [PubMed]
- Loring, D.W. History of neuropsychology through epilepsy eyes. Arch. Clin. Neuropsychol. 2010, 25, 259–273. [Google Scholar] [CrossRef] [PubMed]
| 1st Author | Year | Sepsis Sample (n) | Control Sample (n) | Neuro Expert | Mean Age | Education Reported | Sex (male:female) | Race/Ethnicity |
|---|---|---|---|---|---|---|---|---|
| Regazzoni [13] | 2008 | 137 | No | No | 80.81 | NR | 67:70 | NR |
| Girard [14] | 2010 | 77 | No | Yes | 61 | Median = 12 (IQR = 10–13) years | 40:37 | NR |
| Iwashyna [15] | 2010 | 623 | No | No | 76.9 | NR | 281:362 | Black: 128 Hispanic: 44 White: 451 |
| Davydow [16] | 2012 | 517 | No | No | 76.1 | ≤HS = 38.5%; Some College = 34.8; >College degree = 26.7% | 235:282 | White: 416 Black: 95 Other: 6 |
| Merli [17] | 2013 | 31 | Yes (23) | No | NR | NR | NR | NR |
| Semmler [18] | 2013 | 25 | Yes (19) | Yes | 55.64 | NR | 13:12 | NR |
| Götz [19] | 2015 | 36 | Yes (30) | Yes | 59.8 | NR | 24:12 | NR |
| Götz [20] | 2016 | 36 | Yes (30) | Yes | 59.8 | NR | 24:12 | NR |
| Needham [21] | 2016 | 83 | Yes (106) | Yes | 52 | Mean = 13.9 ± 2.2 years | 39:44 | White: 68 Non-White: 15 |
| Pierrakos [22] | 2017 | 28 | No | Yes | 67.3 | NR | NR | NR |
| Brown [23] | 2018 | 40 | No | Yes | NR | <HS = 13%; HS = 20%; Associate degree = 18%; Higher education = 10% | 19:21 | NR |
| Calasavara [24] | 2018 | 33 | No | Yes | 49 | Mean = 7 (IQR = 4–8) years | 14:19 | NR |
| Kang [25] | 2018 | 36 | No | No | 67.8 | Mean = 7.4 ± 5.8 years | 29:7 | NR |
| Orhun [26] | 2019 | MMSE < 24 = 7 MMSE 24–30 = 14 | Yes (33) | Yes | MMSE < 24: 57.3 ± 3.1 MMSE 24–30: 53.2 ± 3 | MMSE < 24: Mean = 7.1 ± 1.1 years MMSE 24–30: Mean = 8.7 ± 1.0 years | MMSE < 24 = 4:3 MMSE 24–30 = 8:6 | NR |
| Seidel [27] | 2019 | 20 | Yes (44) | Yes | 53.8 | NR | 9:11 | NR |
| Mankowski [28] | 2020 | 328 | No | No | Young group = 35 Middle group = 58 Old group = 72 | NR | 176:152 | White: 293 African American: 30 American Indian: 1 Other: 1 Unknown: 1 |
| Brown [29] | 2021 | 30 | No | Yes | 56 | <HS = 10%; HS = 23%; Some College = 33%; Associate degree = 7%; Bachelor degree = 17%; >Bachelor degree = 10% | 13:17 | Asian: 1 Native Hawaiian/other pacific islander: 1 White: 28 |
| Wang [30] | 2021 | 840 | Yes (20,893) | No | 64.3 | <HS = 12.7%; HS = 26.8%; Some College = 29.3%; >College = 31.2% | NR in the final sample | NR in the final sample |
| 1st Author | Year | Baseline Testing | Time of Testing | Tests (Estimated Length) | Cognitive Domains | Reported Score |
|---|---|---|---|---|---|---|
| Regazzoni [13] | 2008 | No | Admit | MMSE (10’) | Global | Raw score |
| Girard [14] | 2010 | No | 3 months, 12 months post sepsis | MMSE, WAIS-III DS, TMT A&B, Coding, RAVLT, RCFT (Copy & Delay), VF (70’) | Global, ATT, DM, PS, VC, WM | T-scores (age and education adjusted based on specific population norms) |
| Iwashyna [15] | 2010 | Yes | 1998—death or 2006 | M-TICS (10’) | Global | % of cognitive impairment in sample |
| Davydow [16] | 2012 | Yes | Mean = 7y post sepsis | TICS or TICS-27 (10’) | Global | TICS raw score |
| Merli [17] | 2013 | No | Admit, 3 months post discharge | MMSE, TMT A & B, Digit Symbol (18’) | Global, ATT, PS, WM | Z-Scores (test specific population norms) |
| Semmler [18] | 2013 | No | 6–24 months post discharge | German Vocab. Test, NeuroCogFx, TMT A&B, AVLT, RCFT (70’) | ATT, DM, FM, PI, STM, VF | Mean unweighted score, (zDiff) = ((Cognitive Test z-score) − (Multiple Choice Word Test-B z-score))) (test specific population norms) |
| Götz [19] | 2015 | No | 0–2 months, 5–8 months, 10–15 months post ICU discharge | DemTect & CDT (15’) | Global | DemTect raw score |
| Götz [20] | 2016 | No | 0–2 months, 5–8 months, 10–15 months post ICU discharge | DemTect & CDT (15’) | Global | DemTect raw score |
| Needham [21] | 2016 | No | 6 months and 12 months post ICU discharge | Hayling Sentence Completion Test, VF, WAIS-III Similarities & DS, WMS-III LM (50’) | ATT, DM, EF, VR, WM | Percentage of scores 1.5 SD below the mean |
| Pierrakos [22] | 2017 | No | Sepsis discharge (~8d post sepsis onset) | MMSE, CDT (15’) | Global | MMSE raw score & MMSE recall sub-score |
| Brown [23] | 2018 | No | Sepsis discharge, 3 months, 6 months | MoCA, VF, WAIS-IV DS Similarities, WMS-IV LM, Hayling Sentence Completion Test (70’) | Global, ATT, DM, EF, VF, VR, WM | Percentage of scores 1.5 SD and 2.0 SD below the mean (test specific population norms) |
| Calasavara [24] | 2018 | No | 24 h post discharge, 1 year (median 393 days) | MMSE, CERAD (Verbal Fluency, TMT A&B, BNT, List Learning, Praxis, List Recall & Recognition, Praxis Recall) (70’) | Global, DM, EF, Language (naming, comprehension), PS, VF | MMSE raw score, CERAD mean scores |
| Kang [25] | 2018 | No | 48 hours after ICU admit | K-MoCA; K-MMSE; CoSAS-S (30’) | Global | Raw scores |
| Orhun [26] | 2019 | No | 0, 3 months, 12 months post sepsis | MMSE & ACE-R (Orientation-attention, Memory, VF, Language, Visuospatial Function) (35’) | Global | MMSE raw scores; ACE-R Sub-scores means |
| Seidel [27] | 2019 | No | 2.6 ± 1.9 years post sepsis | TAP, Go-No-Go paradigm, German version of AVLT (40’) | ATT, DM, WM | T-scores (age-adjusted based on test specific population norms) |
| Mankowski [28] | 2020 | No | 3, 6, 12 months post discharge | MMSE, HVLT-R (Total recall, Delayed recall, Retention), COWA (30’) | Global, DM, VF | Mean raw scores |
| Brown [29] | 2021 | No | 6 months post discharge | Hayling Sentence Completion Test (5’) | EF | Mean raw scores |
| Wang [30] | 2021 | Yes | 6 months post sepsis; every 2 years (2006–2017) | SIS, CERAD: WLL, WLD, AFT (20’) | Global | Mean raw scores |
| 1st Author | Year | Statistical Method | Education Correction | Sex and/or Race Correction | Findings |
|---|---|---|---|---|---|
| Regazzoni [13] | 2008 | Cox proportional hazard model and Kaplan-Meier test | No | No | Sepsis survivor MMSE mean = 20.14; scores predicted 1-year mortality. |
| Girard [14] | 2010 | Multiple nonlinear regression, propensity score matching | Yes | No | Cognitive impairment at 3 months: 79% (62% severe); 12 months: 79% (36% severe). As duration of delirium increased, cognition decreased. |
| Iwashyna [15] | 2010 | Latent growth curve models, random effects models, and logistic regressions. | No | No | Rate of moderate or severe cognitive impairment among survivors (pre-sepsis) increased from 6.1% (95% CI: 4.2%, 8.0%) before severe sepsis to 16.7% (95% CI: 13.8%, 19.7%) at the 1st assessment after severe sepsis. |
| Davydow [16] | 2012 | Paired t-test, Pearson’s correlation, logistic regression. | No | No | Cognitive impairment in 18% of severe sepsis survivors. Pre-sepsis depression was the greatest predictor of post-sepsis cognitive impairment. |
| Merli [17] | 2013 | Logistic regression. | Yes | No | No sepsis survivors were cognitively impaired, but 42% of sepsis survivors showed a decline in performance. |
| Semmler [18] | 2013 | Student t-tests, Pearson’s correlation, ANOVA, and MANCOVA. | No; Estimated premorbid verbal abilities instead. | No | Sepsis survivors impaired in 8 of 9 subtests (mainly learning and memory). Non-septic ICU survivors showed deficits in 6 subtests. |
| Götz [19] | 2015 | General estimating equations. | No | No | Sepsis survivors were impaired on periodic visual stimulation (familiar and unfamiliar pictures) and scored lower than HCs on the DemTect and CDT at all time points. |
| Götz [20] | 2016 | General estimating equations. | No | No | Sepsis survivors scored lower than HCs on the DemTect and CDT at all time points. |
| Needham [21] | 2016 | Joint survival models, linear regressions and logistic random intercept regression models. | No | No | 38% at 6m post-sepsis had cognitive impairment; 28% at 12m. |
| Pierrakos [22] | 2016 | Multivariable linear regression. | If education >12 years, no MMSE was given; CDT only. | No | 50% of sepsis survivors had cognitive decline, with greatest decline in information recall. |
| Brown [23] | 2018 | Concordance correlation coefficient and Fisher’s exact tests. | No | No | At discharge, 90% of survivors had MoCA< 25 (BNL). At 3 months, 70% BNL; 6 months, 57% BNL. Neuropsychology: 3 months, 43% impaired; 6 months, 57% impaired. WAIS-IV DS was the one test performance that did not improve from 3 to 6 months. |
| Calasavara [24] | 2018 | Student t-test, Mann-Whitney U, chi-square test, one sample t-test, marginal models, stepwise regression. | No | No | At discharge, survivors had lower MMSE and poor constructional praxis (p < 0.001). At 1 year, all performances normalized except for the BNT (p = 0.193) and constructional praxis (p < 0.001). |
| Kang [25] | 2018 | Receiver Operating Characteristics. | No | No | 53.1% of sepsis survivors were cognitively impaired on MMSE; 65.6% of sepsis survivors were cognitively impaired on MoCA |
| Orhun [26] | 2019 | Mann-Whitney U or Kruskal-Wallis tests, Dunn’s post-hoc test, Spearman correlation. | No | No | Initial mean MMSE = 25.4 ± 3.9; 3 months: 27.8 ± 2.8; 12 months: 28.4 ± 1.4 |
| Seidel [27] | 2019 | Two-tailed student t-test, Pearson correlation. | No | No | 55% of survivors had deficits in 1–2 domains and 20% in 3 or more domains. Primary difficulties in learning, alertness, working memory, and memory decay rate. |
| Mankowski [28] | 2020 | Fisher exact test and Kruskal-Wallis test. | No | No | Young adults performed better than middle-aged and older adults. No group differences between the middle-aged and older adults. |
| Brown [29] | 2020 | Weighted network analysis. | No | No | 20% of survivors had impairment in executive domain according to the Hayling Sentence Completion Test |
| Wang [30] | 2021 | Multivariable linear mixed-effects models. | Yes | No | SIS scores of sepsis survivors improved. AFT scores decreased, while WLD and WLL scores increased. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, L.C.; Dion, C.; Efron, P.A.; Price, C.C. Sepsis and Cognitive Assessment. J. Clin. Med. 2021, 10, 4269. https://doi.org/10.3390/jcm10184269
Jones LC, Dion C, Efron PA, Price CC. Sepsis and Cognitive Assessment. Journal of Clinical Medicine. 2021; 10(18):4269. https://doi.org/10.3390/jcm10184269
Chicago/Turabian StyleJones, Laura C., Catherine Dion, Philip A. Efron, and Catherine C. Price. 2021. "Sepsis and Cognitive Assessment" Journal of Clinical Medicine 10, no. 18: 4269. https://doi.org/10.3390/jcm10184269
APA StyleJones, L. C., Dion, C., Efron, P. A., & Price, C. C. (2021). Sepsis and Cognitive Assessment. Journal of Clinical Medicine, 10(18), 4269. https://doi.org/10.3390/jcm10184269





