Bacteria and Sepsis: Microbiome to the Rescue?
Abstract
:1. Introduction
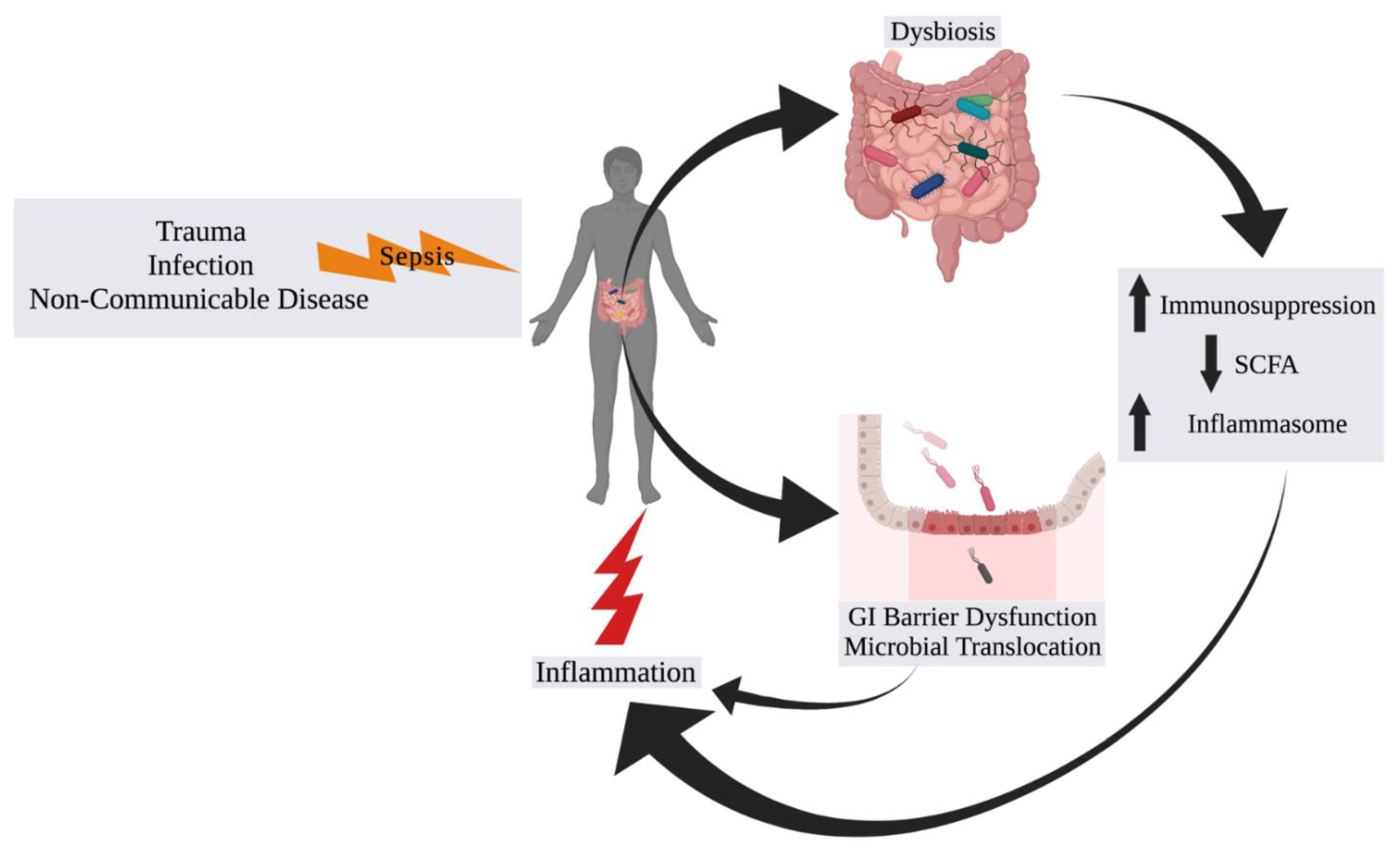
2. The Microbiome in Sepsis
2.1. Background and Associations
2.2. Progressing beyond Associations
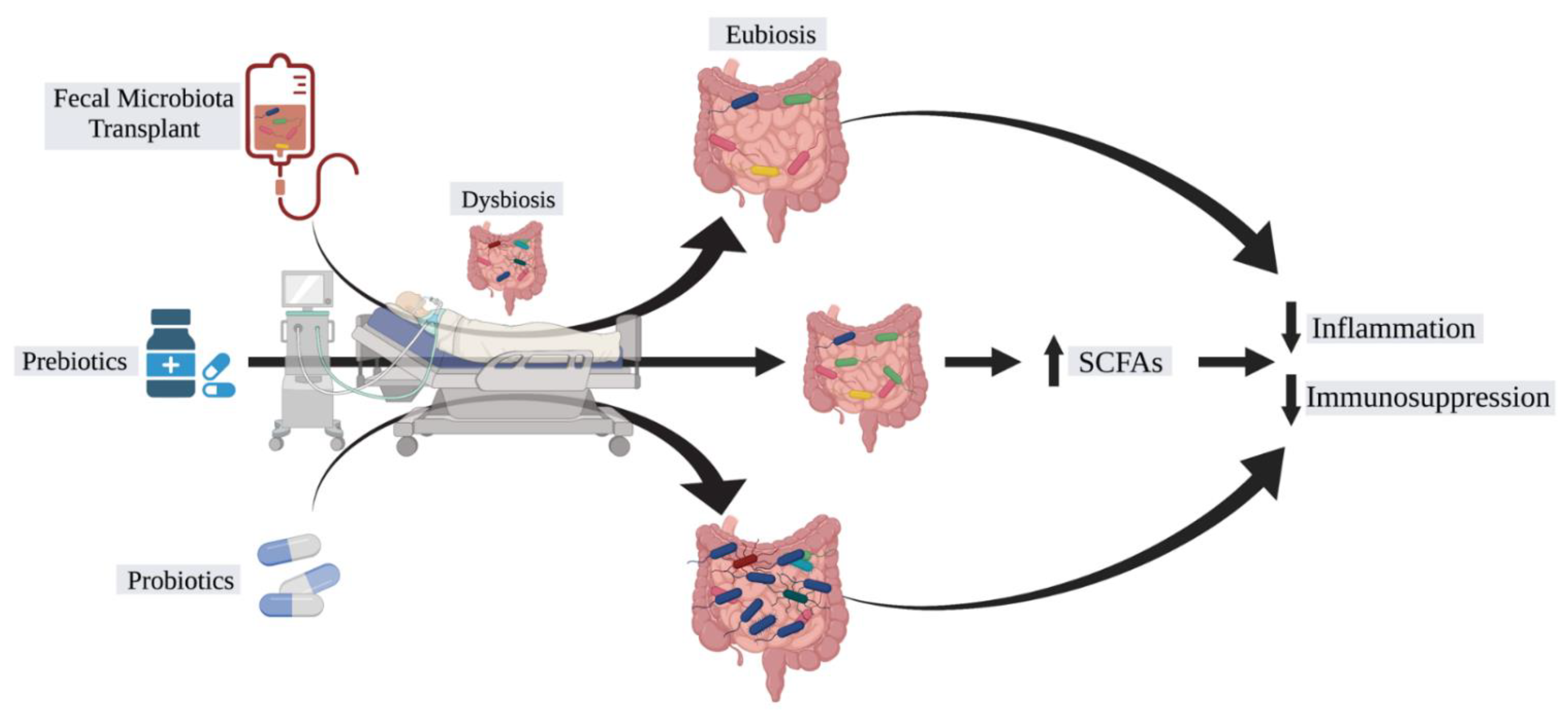
3. Manipulation of the Microbiome for Septic Patients
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rhee, C.; Dantes, R.; Epstein, L.; Murphy, D.J.; Seymour, C.W.; Iwashyna, T.J.; Kadri, S.S.; Angus, D.C.; Danner, R.L.; Fiore, A.E.; et al. Incidence and Trends of Sepsis in US Hospitals Using Clinical vs Claims Data, 2009-2014. JAMA 2017, 318, 1241–1249. [Google Scholar] [CrossRef]
- Torio, C.; Moore, B. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2013; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2016.
- Paoli, C.J.; Reynolds, M.A.; Sinha, M.; Gitlin, M.; Crouser, E. Epidemiology and Costs of Sepsis in the United States—An Analysis Based on Timing of Diagnosis and Severity Level*. Crit. Care Med. 2018, 46, 1889–1897. [Google Scholar] [CrossRef]
- Rudd, K.E.; Kissoon, N.; Limmathurotsakul, D.; Bory, S.; Mutahunga, B.; Seymour, C.W.; Angus, D.C.; West, T.E. The global burden of sepsis: Barriers and potential solutions. Crit. Care 2018, 22, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Nasa, P.; Juneja, D.; Singh, O.; Dang, R.; Arora, V. Severe Sepsis and its Impact on Outcome in Elderly and Very Elderly Patients Admitted in Intensive Care Unit. J. Intensiv. Care Med. 2011, 27, 179–183. [Google Scholar] [CrossRef]
- Martin, A.B.; Hartman, M.; Benson, J.; Catlin, A. National health expenditure accounts team national health spending in 2014: Faster growth driven by coverage expansion and prescription drug spending. Health Aff. 2016, 35, 150–160. [Google Scholar] [CrossRef]
- Martin, G.; Mannino, D.; Moss, M. The effect of age on the development and outcome of adult sepsis*. Crit. Care Med. 2006, 34, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Leung, R.K.-K.; Guan, W.; Au, W.W. Involvement of gut microbiome in human health and disease: Brief overview, knowledge gaps and research opportunities. Gut Pathog. 2018, 10, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, R.M.; Jobin, C. The Microbiome and Cancer: Is the ‘Oncobiome’ Mirage Real? Trends Cancer 2015, 1, 24–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen Recognition and Innate Immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.Y.; Nuñez, G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef] [Green Version]
- Seong, S.-Y.; Matzinger, P. Hydrophobicity: An ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 2004, 4, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-Like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [Green Version]
- Rivera, A.; Siracusa, M.C.; Yap, G.S.; Gause, W.C. Innate cell communication kick-starts pathogen-specific immunity. Nat. Immunol. 2016, 17, 356–363. [Google Scholar] [CrossRef]
- Gotts, J.E.; Matthay, M.A. Sepsis: Pathophysiology and clinical management. BMJ 2016, 353, i1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mogensen, T.H. Pathogen Recognition and Inflammatory Signaling in Innate Immune Defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, I.B. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA J. Am. Med. Assoc. 1995, 273, 59–65. [Google Scholar] [CrossRef]
- Horiguchi, H.; Loftus, T.; Hawkins, R.B.; Raymond, S.L.; Stortz, J.A.; Hollen, M.K.; Weiss, B.P.; Miller, E.S.; Bihorac, A.; Larson, S.; et al. Innate Immunity in the Persistent Inflammation, Immunosuppression, and Catabolism Syndrome and Its Implications for Therapy. Front. Immunol. 2018, 9, 595. [Google Scholar] [CrossRef]
- Sakr, Y.; Elia, C.; Mascia, L.; Barberis, B.; Cardellino, S.; Livigni, S.; Fiore, G.; Filippini, C.; Ranieri, V. The influence of gender on the epidemiology of and outcome from severe sepsis. Crit. Care 2013, 17, 9. [Google Scholar] [CrossRef] [Green Version]
- Wichmann, M.W.; Inthorn, D.; Andress, H.-J.; Schildberg, F.W. Incidence and mortality of severe sepsis in surgical intensive care patients: The influence of patient gender on disease process and outcome. Intensiv. Care Med. 2000, 26, 167–172. [Google Scholar] [CrossRef]
- Angus, D.C.; Linde-Zwirble, W.T.; Lidicker, J.; Clermont, G.; Carcillo, J.; Pinsky, M.R. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001, 29, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.S.; Mannino, D.; Eaton, S.; Moss, M. The Epidemiology of Sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003, 348, 1546–1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemay, A.C.; Anzueto, A.; Restrepo, M.I.; Mortensen, E.M. Predictors of Long-term Mortality After Severe Sepsis in the Elderly. Am. J. Med. Sci. 2014, 347, 282–288. [Google Scholar] [CrossRef] [Green Version]
- Barnato, A.E.; Alexander, S.L.; Linde-Zwirble, W.T.; Angus, D.C. Racial Variation in the Incidence, Care, and Outcomes of Severe Sepsis. Am. J. Respir. Crit. Care Med. 2008, 177, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.M.; Fingar, K.R.; Miller, M.A.; Coffey, R.; Barrett, M.; Flottemesch, T.; Moy, E. Racial disparities in sepsis-related in-hospital mortality: Using a broad case capture method and multivariate controls for clinical and hospital variables, 2004–2013. Crit. Care Med. 2017, 45, 9. [Google Scholar] [CrossRef]
- Dombrovskiy, V.Y.; Martin, A.A.; Sunderram, J.; Paz, H.L. Occurrence and outcomes of sepsis: Influence of race*. Crit. Care Med. 2007, 35, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Angele, M.K.; Pratschke, S.; Hubbard, W.J.; Chaudry, I.H. Gender differences in sepsis: Cardiovascular and immunological aspects. Virulence 2013, 5, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Kostic, A.; Xavier, R.J.; Gevers, D. The Microbiome in Inflammatory Bowel Disease: Current Status and the Future Ahead. Gastroenterology 2014, 146, 1489–1499. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.W.; Hazen, S.L. The contributory role of gut microbiota in cardiovascular disease. J. Clin. Investig. 2014, 124, 4204–4211. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800–812. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, P.; Curtis, N. The effect of antibiotics on the composition of the intestinal microbiota—A systematic review. J. Infect. 2019, 79, 471–489. [Google Scholar] [CrossRef]
- Wang, F.; Meng, J.; Zhang, L.; Johnson, T.; Chen, C.; Roy, S. Morphine induces changes in the gut microbiome and metabolome in a morphine dependence model. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.; Zhang, Z.; Guo, N.; Li, X.; Yang, M.; Peng, Y.; Ma, X.; Yu, K.; Wang, C. Effects of Sevoflurane Inhalation Anesthesia on the Intestinal Microbiome in Mice. Front. Cell. Infect. Microbiol. 2021, 11, 217. [Google Scholar] [CrossRef]
- Adelman, M.W.; Woodworth, M.H.; Langelier, C.; Busch, L.M.; Kempker, J.A.; Kraft, C.S.; Martin, G.S. The gut microbiome’s role in the development, maintenance, and outcomes of sepsis. Crit. Care 2020, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lankelma, J.M.; Van Vught, L.A.; Belzer, C.; Schultz, M.J.; Van Der Poll, T.; De Vos, W.M.; Wiersinga, W.J. Critically ill patients demonstrate large interpersonal variation in intestinal microbiota dysregulation: A pilot study. Intensiv. Care Med. 2016, 43, 59–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freedberg, D.E.; Zhou, M.J.; Cohen, M.E.; Annavajhala, M.; Khan, S.; Moscoso, D.I.; Brooks, C.; Whittier, S.; Chong, D.H.; Uhlemann, A.-C.; et al. Pathogen colonization of the gastrointestinal microbiome at intensive care unit admission and risk for subsequent death or infection. Intensiv. Care Med. 2018, 44, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Ravi, A.; Halstead, F.D.; Bamford, A.; Casey, A.; Thomson, N.M.; Van Schaik, W.; Snelson, C.; Goulden, R.; Foster-Nyarko, E.; Savva, G.M.; et al. Loss of microbial diversity and pathogen domination of the gut microbiota in critically ill patients. Microb. Genom. 2019, 5, e293. [Google Scholar] [CrossRef]
- Rao, K.; Patel, A.; Seekatz, A.; Bassis, C.; Sun, Y.; Bachman, M. Gut microbiome features are associated with sepsis onset and outcomes. bioRxiv 2021. [Google Scholar] [CrossRef]
- Valdés-Duque, B.E.; Giraldo-Giraldo, N.A.; Jaillier-Ramírez, A.M.; Giraldo-Villa, A.; Acevedo-Castaño, I.; Yepes-Molina, M.A.; Barbosa-Barbosa, J.; Barrera-Causil, C.J.; Agudelo-Ochoa, G.M. Stool Short-Chain Fatty Acids in Critically Ill Patients with Sepsis. J. Am. Coll. Nutr. 2020, 39, 706–712. [Google Scholar] [CrossRef]
- Yang, Y.; Jobin, C. Novel insights into microbiome in colitis and colorectal cancer. Curr. Opin. Gastroenterol. 2017, 33, 422–427. [Google Scholar] [CrossRef] [PubMed]
- MacFie, J.; Reddy, B.S.; Gatt, M.; Jain, P.K.; Sowdi, R.; Mitchell, C.J. Bacterial translocation studied in 927 patients over 13 years. Br. J. Surg. 2005, 93, 87–93. [Google Scholar] [CrossRef]
- Woodcock, N.P.; Sudheer, V.; El-Barghouti, N.; Perry, E.P.; MacFie, J. Bacterial translocation in patients undergoing abdominal aortic aneurysm repair. Br. J. Surg. 2000, 87, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. 2020, 6, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Seregin, S.S.; Yang, D.; Fukase, K.; Chamaillard, M.; Alnemri, E.S.; Inohara, N.; Chen, G.Y.; Núñez, G. The NLRP6 Inflammasome Recognizes Lipoteichoic Acid and Regulates Gram-Positive Pathogen Infection. Cell 2018, 175, 1651–1664.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-Planillo, R.; Kuffa, P.; Martínez-Colón, G.; Smith, B.L.; Rajendiran, T.M.; Núñez, G. K+ Efflux Is the Common Trigger of NLRP3 Inflammasome Activation by Bacterial Toxins and Particulate Matter. Immunity 2013, 38, 1142–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayward, J.; Mathur, A.; Ngo, C.; Man, S.M. Cytosolic Recognition of Microbes and Pathogens: Inflammasomes in Action. Microbiol. Mol. Biol. Rev. 2018, 82, e15-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathinam, V.A.; Vanaja, S.K.; Waggoner, L.; Sokolovska, A.; Becker, C.; Stuart, L.M.; Leong, J.M.; Fitzgerald, K.A. TRIF Licenses Caspase-11-Dependent NLRP3 Inflammasome Activation by Gram-Negative Bacteria. Cell 2012, 150, 606–619. [Google Scholar] [CrossRef] [Green Version]
- Lu, F.; Chen, H.; Hong, Y.; Lin, Y.; Liu, L.; Wei, N.; Wu, Q.; Liao, S.; Yang, S.; He, J.; et al. A gain-of-function NLRP3 3′-UTR polymorphism causes miR-146a-mediated suppression of NLRP3 expression and confers protection against sepsis progression. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Townsend, C.M.; Parker, C.E.; Macdonald, J.K.; Nguyen, T.M.; Jairath, V.; Feagan, B.G.; Khanna, R. Antibiotics for induction and maintenance of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2019, 2, CD012730. [Google Scholar] [CrossRef]
- Febvre, H.P.; Rao, S.; Gindin, M.; Goodwin, N.D.M.; Finer, E.; Vivanco, J.S.; Lu, S.; Manter, D.K.; Wallace, T.C.; Weir, T.L. PHAGE Study: Effects of Supplemental Bacteriophage Intake on Inflammation and Gut Microbiota in Healthy Adults. Nutrients 2019, 11, 666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; De Vos, W.M.; Visser, C.E.; Kuijper, E.; Bartelsman, J.F.W.M.; Tijssen, J.G.P.; et al. Duodenal Infusion of Donor Feces for RecurrentClostridium difficile. N. Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef] [Green Version]
- DeFilipp, Z.; Bloom, P.P.; Soto, M.T.; Mansour, M.K.; Sater, M.; Huntley, M.H.; Turbett, S.; Chung, R.T.; Chen, Y.-B.; Hohmann, E.L. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N. Engl. J. Med. 2019, 381, 2043–2050. [Google Scholar] [CrossRef]
- Haak, B.W.; Prescott, H.; Wiersinga, W.J.; Haak, B.W.; Prescott, H.; Wiersinga, W.J. Therapeutic Potential of the Gut Microbiota in the Prevention and Treatment of Sepsis. Front. Immunol. 2018, 9, 2042. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Wang, C.; Tang, C.; He, Q.; Zhao, X.; Li, N.; Li, J. Therapeutic Modulation and Reestablishment of the Intestinal Microbiota With Fecal Microbiota Transplantation Resolves Sepsis and Diarrhea in a Patient. Am. J. Gastroenterol. 2014, 109, 1832–1834. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Yang, J.; Wang, J.; Yang, Y.; Huang, J.; Gong, H.; Cui, H.; Chen, D. Successful treatment with fecal microbiota transplantation in patients with multiple organ dysfunction syndrome and diarrhea following severe sepsis. Crit. Care 2016, 20, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukovic, E.; Moitra, V.K.; Freedberg, D.E. The microbiome: Implications for perioperative and critical care. Curr. Opin. Anaesthesiol. 2019, 32, 412–420. [Google Scholar] [CrossRef]
- Manzanares, W.; Lemieux, M.; Langlois, P.L.; Wischmeyer, P.E. Probiotic and synbiotic therapy in critical illness: A systematic review and meta-analysis. Crit. Care 2016, 20, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Yelin, I.; Flett, K.B.; Merakou, C.; Mehrotra, P.; Stam, J.; Snesrud, E.; Hinkle, M.; Lesho, E.; McGann, P.; McAdam, A.J.; et al. Genomic and epidemiological evidence of bacterial transmission from probiotic capsule to blood in ICU patients. Nat. Med. 2019, 25, 1728–1732. [Google Scholar] [CrossRef] [PubMed]
- Kuczynski, J.; Lauber, C.L.; Walters, W.A.; Parfrey, L.W.; Clemente, J.C.; Gevers, D.; Knight, R. Experimental and analytical tools for studying the human microbiome. Nat. Rev. Genet. 2011, 13, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Banerjee, D. Metabolomics and the Microbiome as Biomarkers in Sepsis. Crit. Care Clin. 2020, 36, 105–113. [Google Scholar] [CrossRef]
- Sun, Y.V.; Hu, Y.-J. Integrative Analysis of Multi-omics Data for Discovery and Functional Studies of Complex Human Diseases. Adv. Genet. 2016, 93, 147–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.; Xu, F.; Su, C.; Li, H.; Lv, N.; Liu, Y.; Gao, Y.; Lan, Y.; Li, J. Alterations in the Gut Microbiome and Cecal Metabolome During Klebsiella pneumoniae-Induced Pneumosepsis. Front. Immunol. 2020, 11, 1331. [Google Scholar] [CrossRef] [PubMed]
| Study | Objective | Cohort | Intervention | Location | Status |
|---|---|---|---|---|---|
| Gut Microbiome Dysbiosis in Sepsis-Induced Coagulopathy | Analyze gut microbiome alternations and coagulation studies in ICU adult patients | ICU adult patients | Observational | China | Recruiting |
| Predicting EONS in PPROM Patients (PEONS) | Analyze microbiome via 16S rRNA sequencing of neonates with and without early-onset neonatal sepsis | Neonates with and without early-onset neonatal sepsis | Observational | Germany | Active, not recruiting |
| Novel Mechanisms and Approaches to Treat Neonatal Sepsis | Characterize immune genomic expression and microbiome in preterm and term neonates | Preterm and term neonates, healthy adult controls | Observational | United States | Recruiting |
| Molecular Diagnosis and Risk Stratification of Sepsis in India (MARS-India) | Characterize immunoinflammatory status and microbiome in septic patients | Septic patients, non-septic ICU patients, healthy controls | Observational | India | Recruiting |
| Study of Early Enteral Dextrose in Sepsis (SEEDS) | Study how early enteral dextrose infusion in septic patients impacts serum pro-inflammatory IL-6 levels | Septic patients | Enteral dextrose infusion vs. enteral water control | United States | Completed |
| Characterization of Intestinal Microbiota Stability in Preterm Born Neonates (NEC) | Analyze gastrointestinal microbiome in preterm infants for association with risk of developing NEC/LOS | Preterm infants with and without NEC/LOS | Observational | Switzerland | Recruiting |
| SEPSIS Observational Cohort Study in Young Infants in Bangladesh | Analyze gastrointestinal microbiome in young infants for association with risk of developing severe infection | Young infants | Observational | Bangladesh | Recruiting |
| Prebiotic Fiber to Prevent Pathogen Colonization in the ICU | Study how fiber supplementation in ICU patients impacts pathogen colonization/infection | ICU adult patients | High fiber diet vs. lower fiber diet | United States | Completed |
| Effect of Gut Microbiota on the Prognosis of Sepsis | Analyze relationship between gut microbiota and prognosis of sepsis | Adult patients with sepsis | Observational | China | Not yet recruiting |
| The Role of the Microbiota in the Systemic Immune Response (MISSION-1) | Study how depleting gut microbiota impacts systemic immune response | Healthy adults treated with antibiotics | Antibiotics (ciprofloxacin, vancomycin, metronidazole) | Netherlands | Completed |
| Human Milk Fortification in Extremely Preterm Infants (N-forte) | Study how bovine-milk based fortifier in extremely premature infants impacts incidence of NEC, culture-proven sepsis, and mortality | Extremely premature infants | Bovine milk-based fortifier vs. control fortifier | Sweden | Recruiting |
| Bovine Colostrum as a Human Milk Fortifier for Preterm Infants (FortiColos-Ⅱ) | Study how bovine colostrum in preterm infants impacts weight gain, NEC incidence, and late-onset sepsis incidence | Preterm infants | Bovine Colostrum fortifier vs. control fortifier | China | Recruiting |
| Oropharyngeal Administration of Mother’s Colostrum for Premature Infants | Study how mother’s colostrum in extremely premature infants impacts incidence of late-onset sepsis, NEC, and VAP | Extremely premature infants | Oropharyngeal mother’s milk vs. oropharyngeal water control | United States | Active, not recruiting |
| Bovine Colostrum as a Fortifier Added to Human Milk for Preterm Infants (FortiColos) | Study how bovine colostrum in preterm infants impacts weight gain, NEC incidence, and late-onset sepsis incidence | Preterm infants | Bovine Colostrum fortifier vs. control fortifier | Denmark | Recruiting |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.; Thomas, R.M. Bacteria and Sepsis: Microbiome to the Rescue? J. Clin. Med. 2021, 10, 3578. https://doi.org/10.3390/jcm10163578
Kang H, Thomas RM. Bacteria and Sepsis: Microbiome to the Rescue? Journal of Clinical Medicine. 2021; 10(16):3578. https://doi.org/10.3390/jcm10163578
Chicago/Turabian StyleKang, Hansol, and Ryan M. Thomas. 2021. "Bacteria and Sepsis: Microbiome to the Rescue?" Journal of Clinical Medicine 10, no. 16: 3578. https://doi.org/10.3390/jcm10163578
APA StyleKang, H., & Thomas, R. M. (2021). Bacteria and Sepsis: Microbiome to the Rescue? Journal of Clinical Medicine, 10(16), 3578. https://doi.org/10.3390/jcm10163578






