The Role of Neuropeptides in Pathogenesis of Dry Eye
Abstract
1. Introduction
2. The Role of the Immune and Nervous Systems in the Pathogenesis of Dry Eye Disease
2.1. Immunopathogenesis of Dry Eye Disease
2.2. Role of Sensory Nerves in the Pathogenesis of Dry Eye Disease
3. Role of Neuropeptides and Its Distribution in Ocular Surface and Lacrimal Gland
3.1. Representative Neuropeptides and Their Receptors
3.2. Role of Neuropeptides in Neurogenic Inflammation
3.2.1. Concept of Neurogenic Inflammation
3.2.2. Role of Substance P (SP) in Neurogenic Inflammation
3.2.3. Role of Calcitonin Gene-Related Peptide (CGRP) in Neurogenic Inflammation
3.2.4. Role of Vasoactive Intestinal Polypeptide (VIP) and Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) in Neurogenic Inflammation
3.2.5. Role of Neuropeptide Y (NPY) in Neurogenic Inflammation
3.2.6. Role of Other Neuropeptides in Neurogenic Inflammation
3.3. Distribution of Nerves and Expression of Neuropeptides in Ocular Surface and Lacrimal Gland
3.3.1. Sensory and Autonomic Nervous Systems of Eye
3.3.2. Sensory and Autonomic Innervation of Cornea
3.3.3. Sensory and Autonomic Innervation of Conjunctiva and Eyelid Margin
3.3.4. Sensory and Autonomic Innervation of Lacrimal Gland
3.3.5. Sensory and Autonomic Innervation of Meibomian Gland
4. Recent Results Showing the Roles of Neuropeptides in the Pathogenesis of Dry Eye Disease
4.1. Preclinical Study
4.2. Clinical Study
4.2.1. Dry Eye Disease
4.2.2. Dry Eye after Refractive Surgery and Neuropeptides
4.2.3. Dry Eye Related to Wearing Contact Lens Wear and Neuropeptides
5. Limitations of the Research Performed to Date: What Is Missing?
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiu, I.; Von Hehn, C.A.; Woolf, C.J. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci. 2012, 15, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Beuerman, R.W.; Stern, M.E. Neurogenic Inflammation: A First Line of Defense for the Ocular Surface. Ocul. Surf. 2005, 3, S-203–S-206. [Google Scholar] [CrossRef]
- Sabatino, F.; Di Zazzo, A.; De Simone, L.; Bonini, S. The Intriguing Role of Neuropeptides at the Ocular Surface. Ocul. Surf. 2017, 15, 2–14. [Google Scholar] [CrossRef]
- Mashaghi, A.; Marmalidou, A.; Tehrani, M.; Grace, P.; Pothoulakis, C.; Dana, R. Neuropeptide substance P and the immune response. Cell. Mol. Life Sci. 2016, 73, 4249–4264. [Google Scholar] [CrossRef]
- Vitar, R.M.L.; Rama, P.; Ferrari, G. The two-faced effects of nerves and neuropeptides in corneal diseases. Prog. Retin. Eye Res. 2021, 100974. [Google Scholar] [CrossRef] [PubMed]
- Mantelli, F.; Micera, A.; Sacchetti, M.; Bonini, S. Neurogenic inflammation of the ocular surface. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Meseguer, V.; Alpizar, Y.A.; Luis, E.; Tajada, S.; Denlinger, B.; Fajardo, O.; Manenschijn, J.-A.; Fernandez-Peña, C.; Talavera, A.; Kichko, T.; et al. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat. Commun. 2014, 5, 3125. [Google Scholar] [CrossRef]
- Corrigan, F.; Mander, K.A.; Leonard, A.V.; Vink, R. Neurogenic inflammation after traumatic brain injury and its potentiation of classical inflammation. J. Neuroinflamm. 2016, 13, 1–12. [Google Scholar] [CrossRef]
- Choi, J.E.; Di Nardo, A. Skin neurogenic inflammation. Semin. Immunopathol. 2018, 40, 249–259. [Google Scholar] [CrossRef]
- Littlejohn, G.; Guymer, E. Neurogenic inflammation in fibromyalgia. Semin. Immunopathol. 2018, 40, 291–300. [Google Scholar] [CrossRef]
- Ramachandran, R. Neurogenic inflammation and its role in migraine. Semin. Immunopathol. 2018, 40, 301–314. [Google Scholar] [CrossRef]
- Matsuda, M.; Huh, Y.; Ji, R.-R. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J. Anesth. 2019, 33, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- DEWS. The Definition and Classification of Dry Eye Disease: Report of the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007). Ocul. Surf. 2007, 5, 75–92. [Google Scholar] [CrossRef]
- Bron, A.J.; de Paiva, C.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, W.; Chauhan, S.K.; Dana, R. Dry eye disease: An immune-mediated ocular surface disorder. Arch. Ophthalmol. 2012, 130, 90–100. [Google Scholar] [CrossRef]
- Moss, S.E.; Klein, R.; Klein, B.E. Prevalence of and Risk Factors for Dry Eye Syndrome. Arch. Ophthalmol. 2000, 118, 1264–1268. [Google Scholar] [CrossRef]
- Lemp, M.A. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. Eye Contact Lens 1995, 21, 221–232. [Google Scholar]
- Gipson, I.K.; Argüeso, P.; Beuerman, R.; Bonini, S.; Butovich, I.; Dana, R.; Dartt, D.; Gamache, D.; Ham, B.; Jumblatt, M.; et al. Research in Dry Eye: Report of the Research Subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf. 2007, 5, 179–193. [Google Scholar] [CrossRef]
- Stern, M.E.; Gao, J.; Siemasko, K.F.; Beuerman, R.W.; Pflugfelder, S.C. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp. Eye Res. 2004, 78, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C.; Irkeç, M.; Messmer, E.M.; Benítez-Del-Castillo, J.M.; Bonini, S.; Figueiredo, F.C.; Geerling, G.; Labetoulle, M.; Lemp, M.; Rolando, M.; et al. Clinical impact of inflammation in dry eye disease: Proceedings of the ODISSEY group meeting. Acta Ophthalmol. 2018, 96, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K.; Pflugfelder, S.C.; Liu, Z.; Baudouin, C.; Kim, H.M.; Messmer, E.M.; Kruse, F.; Liang, L.; Carreno-Galeano, J.T.; Rolando, M.; et al. Defining Dry Eye from a Clinical Perspective. Int. J. Mol. Sci. 2020, 21, 9271. [Google Scholar] [CrossRef] [PubMed]
- Barabino, S.; Chen, Y.; Chauhan, S.; Dana, R. Ocular surface immunity: Homeostatic mechanisms and their disruption in dry eye disease. Prog. Retin. Eye Res. 2012, 31, 271–285. [Google Scholar] [CrossRef]
- Luo, L.; Li, D.-Q.; Corrales, R.M.; Pflugfelder, S.C. Hyperosmolar Saline Is a Proinflammatory Stress on the Mouse Ocular Surface. Eye Contact Lens Sci. Clin. Pract. 2005, 31, 186–193. [Google Scholar] [CrossRef] [PubMed]
- De Paiva, C.S.; Pangelinan, S.B.; Chang, E.; Yoon, K.C.; Farley, W.J.; Li, D.Q.; Pflugfelder, S.C. Essential Role for c-Jun N-Terminal Kinase 2 in Corneal Epithelial Response to Desiccating Stress. Arch. Ophthalmol. 2009, 127, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-Q.; Luo, L.; Chen, Z.; Kim, H.S.; Song, X.J.; Pflugfelder, S.C. JNK and ERK MAP kinases mediate induction of IL-1β, TNF-α and IL-8 following hyperosmolar stress in human limbal epithelial cells. Exp. Eye Res. 2006, 82, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Coursey, T.G.; Bohat, R.; Barbosa, F.L.; Pflugfelder, S.C.; De Paiva, C.S. Desiccating Stress–Induced Chemokine Expression in the Epithelium Is Dependent on Upregulation of NKG2D/RAE-1 and Release of IFN-γ in Experimental Dry Eye. J. Immunol. 2014, 193, 5264–5272. [Google Scholar] [CrossRef]
- Gao, J.; Morgan, G.; Tieu, D.; Schwalb, T.A.; Luo, J.Y.; Wheeler, L.A.; Stern, M.E. ICAM-1 expression predisposes ocular tissues to immune-based inflammation in dry eye patients and Sjögrens syndrome-like MRL/lpr mice. Exp. Eye Res. 2004, 78, 823–835. [Google Scholar] [CrossRef]
- Cursiefen, C.; Chen, L.; Saint-Geniez, M.; Hamrah, P.; Jin, Y.; Rashid, S.; Pytowski, B.; Persaud, K.; Wu, Y.; Streilein, J.W.; et al. Nonvascular VEGF receptor 3 expression by corneal epithelium maintains avascularity and vision. Proc. Natl. Acad. Sci. USA 2006, 103, 11405–11410. [Google Scholar] [CrossRef]
- Ji, Y.W.; Lee, J.L.; Kang, H.G.; Gu, N.; Byun, H.; Yeo, A.; Noh, H.; Kim, S.; Choi, E.Y.; Song, J.S.; et al. Corneal lymphangiogenesis facilitates ocular surface inflammation and cell trafficking in dry eye disease. Ocul. Surf. 2018, 16, 306–313. [Google Scholar] [CrossRef]
- El Annan, J.; Chauhan, S.K.; Ecoiffier, T.; Zhang, Q.; Saban, D.R.; Dana, R. Characterization of Effector T Cells in Dry Eye Disease. Investig. Opthalmol. Vis. Sci. 2009, 50, 3802–3807. [Google Scholar] [CrossRef]
- De Paiva, C.S.; Chotikavanich, S.; Pangelinan, S.B.; Pitcher, J.D.; Fang, B.; Zheng, X.; Ma, P.; Farley, W.J.; Siemasko, K.F.; Niederkorn, J.Y.; et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009, 2, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.K.; El Annan, J.; Ecoiffier, T.; Goyal, S.; Zhang, Q.; Saban, D.R.; Dana, R. Autoimmunity in Dry Eye is due to Resistance of Th17 to Treg Suppression. J. Immunol. 2009, 182, 1247–1252. [Google Scholar] [CrossRef]
- Yoon, K.-C.; de Paiva, C.; Qi, H.; Chen, Z.; Farley, W.J.; Li, D.-Q.; Pflugfelder, S.C. Expression of Th-1 Chemokines and Chemokine Receptors on the Ocular Surface of C57BL/6 Mice: Effects of Desiccating Stress. Investig. Opthalmol. Vis. Sci. 2007, 48, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.K.; Jin, Y.; Goyal, S.; Lee, H.S.; Fuchsluger, T.A.; Lee, H.K.; Dana, R. A novel pro-lymphangiogenic function for Th17/IL-17. Blood J. Am. Soc. Hematol. 2011, 118, 4630–4634. [Google Scholar] [CrossRef] [PubMed]
- Niederkorn, J.Y.; Stern, M.E.; Pflugfelder, S.C.; de Paiva, C.; Corrales, R.M.; Gao, J.; Siemasko, K. Desiccating Stress Induces T Cell-Mediated Sjögren’s Syndrome-Like Lacrimal Keratoconjunctivitis. J. Immunol. 2006, 176, 3950–3957. [Google Scholar] [CrossRef] [PubMed]
- Foulsham, W.; Mittal, S.K.; Taketani, Y.; Chen, Y.; Nakao, T.; Chauhan, S.K.; Dana, R. Aged Mice Exhibit Severe Exacerbations of Dry Eye Disease with an Amplified Memory Th17 Cell Response. Am. J. Pathol. 2020, 190, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.-W.; Dohlman, T.H.; Foulsham, W.; McSoley, M.; Singh, R.; Chen, Y.; Dana, R. The role of Th17 immunity in chronic ocular surface disorders. Ocul. Surf. 2021, 19, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chauhan, S.K.; Lee, H.S.; Saban, D.R.; Dana, R. Chronic dry eye disease is principally mediated by effector memory Th17 cells. Mucosal Immunol. 2014, 7, 38–45. [Google Scholar] [CrossRef]
- Chen, Y.; Shao, C.; Fan, N.-W.; Nakao, T.; Amouzegar, A.; Chauhan, S.K.; Dana, R. The functions of IL-23 and IL-2 on driving autoimmune effector T-helper 17 cells into the memory pool in dry eye disease. Mucosal Immunol. 2021, 14, 177–186. [Google Scholar] [CrossRef]
- Chen, Y.; Chauhan, S.K.; Tan, X.; Dana, R. Interleukin-7 and -15 maintain pathogenic memory Th17 cells in autoimmunity. J. Autoimmun. 2017, 77, 96–103. [Google Scholar] [CrossRef]
- Chen, L.; Cursiefen, C.; Barabino, S.; Zhang, Q.; Dana, M.R. Novel expression and characterization of lymphatic vessel endothelial hyaluronate receptor 1 (LYVE-1) by conjunctival cells. Investig. Opthalmol. Vis. Sci. 2005, 46, 4536–4540. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goyal, S.; Chauhan, S.K.; El Annan, J.; Nallasamy, N.; Zhang, Q.; Dana, R. Evidence of Corneal Lymphangiogenesis in Dry Eye Disease: A potential link to adaptive immunity? Arch. Ophthalmol. 2010, 128, 819–824. [Google Scholar] [CrossRef]
- Garcia-Hirschfeld, J.; Lopez-Briones, L.G.; Belmonte, C. Neurotrophic Influences on Corneal Epithelial Cells. Exp. Eye Res. 1994, 59, 597–605. [Google Scholar] [CrossRef]
- Baker, K.S.; Anderson, S.C.; Romanowski, E.G.; Thoft, R.A.; Sundarraj, N. Trigeminal ganglion neurons affect corneal epithelial phenotype. Influence on type VII collagen expression in vitro. Investig. Ophthalmol. Vis. Sci. 1993, 34, 137–144. [Google Scholar]
- Müller, L.J.; Marfurt, C.F.; Kruse, F.; Tervo, T.M. Corneal nerves: Structure, contents and function. Exp. Eye Res. 2003, 76, 521–542. [Google Scholar] [CrossRef]
- Del Castillo, J.M.B.; Wasfy, M.A.S.; Fernandez, C.; Garcia-Sanchez, J. An In Vivo Confocal Masked Study on Corneal Epithelium and Subbasal Nerves in Patients with Dry Eye. Investig. Opthalmol. Vis. Sci. 2004, 45, 3030–3035. [Google Scholar] [CrossRef]
- Bourcier, T.; Acosta, M.C.; Borderie, V.; Borrás, F.; Gallar, J.; Bury, T.; Laroche, L.; Belmonte, C. Decreased Corneal Sensitivity in Patients with Dry Eye. Investig. Opthalmol. Vis. Sci. 2005, 46, 2341–2345. [Google Scholar] [CrossRef] [PubMed]
- Hoşal, B.M.; Örnek, N.; Zilelioğlu, G.; Elhan, A.H. Morphology of corneal nerves and corneal sensation in dry eye: A preliminary study. Eye 2004, 19, 1276–1279. [Google Scholar] [CrossRef]
- Erdélyi, B.; Kraak, R.; Zhivov, A.; Guthoff, R.; Németh, J. In vivo confocal laser scanning microscopy of the cornea in dry eye. Graefes Arch. Clin. Exp. Ophthalmol. 2006, 245, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Dastjerdi, M.H.; Dana, R. Corneal Nerve Alterations in Dry Eye-associated Ocular Surface Disease. Int. Ophthalmol. Clin. 2009, 49, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Kheirkhah, A.; Dohlman, T.; Amparo, F.; Arnoldner, M.A.; Jamali, A.; Hamrah, P.; Dana, R. Effects of Corneal Nerve Density on the Response to Treatment in Dry Eye Disease. Ophthalmol. 2015, 122, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Tepelus, T.C.; Chiu, G.B.; Huang, J.; Huang, P.; Sadda, S.R.; Irvine, J.; Lee, O.L. Correlation between corneal innervation and inflammation evaluated with confocal microscopy and symptomatology in patients with dry eye syndromes: A preliminary study. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Mizerska, K.; Marfurt, C.F.; Rosenblatt, M.I. Hyperosmolar Tears Induce Functional and Structural Alterations of Corneal Nerves: Electrophysiological and Anatomical Evidence Toward Neurotoxicity. Investig. Opthalmol. Vis. Sci. 2015, 56, 8125–8140. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Rosenblatt, M.I. Hyperosmolar Tears Enhance Cooling Sensitivity of the Corneal Nerves in Rats: Possible Neural Basis for Cold-Induced Dry Eye Pain. Investig. Opthalmol. Vis. Sci. 2014, 55, 5821–5833. [Google Scholar] [CrossRef]
- Guzmán, M.; Miglio, M.; Keitelman, I.; Shiromizu, C.M.; Sabbione, F.; Fuentes, F.; Trevani, A.S.; Giordano, M.N.; Galletti, J.G. Transient tear hyperosmolarity disrupts the neuroimmune homeostasis of the ocular surface and facilitates dry eye onset. Immunology 2020, 161, 148–161. [Google Scholar] [CrossRef] [PubMed]
- M’Garrech, M.; Rousseau, A.; Kaswin, G.; Sauer, A.; Barreau, E.; Bourcier, T.; Labetoulle, M. Impairment of Lacrimal Secretion in the Unaffected Fellow Eye of Patients with Recurrent Unilateral Herpetic Keratitis. Ophthalmology 2013, 120, 1959–1967. [Google Scholar] [CrossRef]
- Meng, I.D.; Kurose, M. The role of corneal afferent neurons in regulating tears under normal and dry eye conditions. Exp. Eye Res. 2013, 117, 79–87. [Google Scholar] [CrossRef]
- Lee, H.K.; Ryu, I.H.; Seo, K.Y.; Hong, S.; Kim, H.C.; Kim, E.K. Topical 0.1% Prednisolone Lowers Nerve Growth Factor Expression in Keratoconjunctivitis Sicca Patients. Ophthalmology 2006, 113, 198–205. [Google Scholar] [CrossRef]
- Stern, M.E.; Beuerman, R.W.; Fox, R.I.; Gao, J.; Mircheff, A.K.; Pflugfelder, S.C. The Pathology of Dry Eye: The interaction between the ocular surface and lacrimal glands. Cornea 1998, 17, 584–589. [Google Scholar] [CrossRef]
- Craig, J.P.; Nelson, J.D.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Chauhan, S.K.; de Paiva, C.S.; Gomes, J.A.P.; Hammitt, K.M.; Jones, L.; et al. TFOS DEWS II Report Executive Summary. Ocul. Surf. 2017, 15, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Troger, J.; Kieselbach, G.; Teuchner, B.; Kralinger, M.; Nguyen, Q.A.; Haas, G.; Yayan, J.; Göttinger, W.; Schmid, E. Peptidergic nerves in the eye, their source and potential pathophysiological relevance. Brain Res. Rev. 2007, 53, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Rolando, M.; Barabino, S.; Mingari, C.; Moretti, S.; Giuffrida, S.; Calabria, G. Distribution of Conjunctival HLA-DR Expression and the Pathogenesis of Damage in Early Dry Eyes. Cornea 2005, 24, 951–954. [Google Scholar] [CrossRef] [PubMed]
- Lambiase, A.; Sacchetti, M.; Bonini, S. Nerve growth factor therapy for corneal disease. Curr. Opin. Ophthalmol. 2012, 23, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Dartt, D.A.; Kessler, T.L.; Chung, E.-H.; Zieske, J.D. Vasoactive Intestinal Peptide-Stimulated Glycoconjugate Secretion from Conjunctival Goblet Cells. Exp. Eye Res. 1996, 63, 27–33. [Google Scholar] [CrossRef]
- Kovács, I.; Ludány, A.; Kőszegi, T.; Fehér, J.; Kovács, B.; Szolcsányi, J.; Pintér, E. Substance P released from sensory nerve endings influences tear secretion and goblet cell function in the rat. Neuropeptides 2005, 39, 395–402. [Google Scholar] [CrossRef]
- Hosoi, J.; Murphy, G.F.; Egan, C.L.; Lerner, E.A.; Grabbe, S.; Asahina, A.; Granstein, R.D. Regulation of Langerhans cell function by nerves containing calcitonin gene-related peptide. Nature 1993, 363, 159–163. [Google Scholar] [CrossRef]
- O’Connor, T.M.; O’Connell, J.; O’Brien, D.I.; Goode, T.; Bredin, C.P.; Shanahan, F. The role of substance P in inflammatory disease. J. Cell. Physiol. 2004, 201, 167–180. [Google Scholar] [CrossRef]
- Kodali, S.; Ding, W.; Huang, J.; Seiffert, K.; Wagner, J.A.; Granstein, R.D. Vasoactive Intestinal Peptide Modulates Langerhans Cell Immune Function. J. Immunol. 2004, 173, 6082–6088. [Google Scholar] [CrossRef]
- Groneberg, D.A.; Folkerts, G.; Peiser, C.; Chung, K.F.; Fischer, A. Neuropeptide Y (NPY). Pulm. Pharmacol. Ther. 2004, 17, 173–180. [Google Scholar] [CrossRef]
- Parra, A.; Madrid, R.; Echevarria, D.; Del Olmo, S.; Morenilla-Palao, C.; Acosta, M.C.; Gallar, J.; Dhaka, A.; Viana, F.; Belmonte, C. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat. Med. 2010, 16, 1396–1399. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, C.; Gallar, J. Cold Thermoreceptors, Unexpected Players in Tear Production and Ocular Dryness Sensations. Investig. Opthalmol. Vis. Sci. 2011, 52, 3888–3892. [Google Scholar] [CrossRef]
- Belmonte, C.; Nichols, J.J.; Cox, S.M.; Brock, J.; Begley, C.G.; Bereiter, D.A.; Dartt, D.A.; Galor, A.; Hamrah, P.; Ivanusic, J.; et al. TFOS DEWS II pain and sensation report. Ocul. Surf. 2017, 15, 404–437. [Google Scholar] [CrossRef] [PubMed]
- Quallo, T.; Vastani, N.; Horridge, E.; Gentry, C.; Parra, A.; Moss, S.; Viana, F.; Belmonte, C.; Andersson, D.A.; Bevan, S. TRPM8 is a neuronal osmosensor that regulates eye blinking in mice. Nat. Commun. 2015, 6, 7150. [Google Scholar] [CrossRef] [PubMed]
- Parra, A.; Gonzalez-Gonzalez, O.; Gallar, J.; Belmonte, C. Tear fluid hyperosmolality increases nerve impulse activity of cold thermoreceptor endings of the cornea. Pain 2014, 155, 1481–1491. [Google Scholar] [CrossRef]
- McKay, T.B.; Seyed-Razavi, Y.; Ghezzi, C.E.; Dieckmann, G.; Nieland, T.J.; Cairns, D.; Pollard, R.E.; Hamrah, P.; Kaplan, D.L. Corneal pain and experimental model development. Prog. Retin. Eye Res. 2019, 71, 88–113. [Google Scholar] [CrossRef] [PubMed]
- Piña, R.; Ugarte, G.; Campos, M.; Íñigo-Portugués, A.; Olivares, E.; Orio, P.; Belmonte, C.; Bacigalupo, J.; Madrid, R. Role of TRPM8 Channels in Altered Cold Sensitivity of Corneal Primary Sensory Neurons Induced by Axonal Damage. J. Neurosci. 2019, 39, 8177–8192. [Google Scholar] [CrossRef]
- Belmonte, C. Pain, Dryness, and Itch Sensations in Eye Surface Disorders Are Defined By a Balance Between Inflammation and Sensory Nerve Injury. Cornea 2019, 38, S11–S24. [Google Scholar] [CrossRef]
- Hirata, H.; Mizerska, K.; Dallacasagrande, V.; Rosenblatt, M.I. Estimating the Osmolarities of Tears During Evaporation Through the “Eyes” of the Corneal Nerves. Investig. Opthalmol. Vis. Sci. 2017, 58, 168–178. [Google Scholar] [CrossRef]
- Launay, P.-S.; Reboussin, E.; Liang, H.; Kessal, K.; Godefroy, D.; Rostene, W.; Sahel, J.-A.; Baudouin, C.; Parsadaniantz, S.M.; Le Goazigo, A.R. Ocular inflammation induces trigeminal pain, peripheral and central neuroinflammatory mechanisms. Neurobiol. Dis. 2016, 88, 16–28. [Google Scholar] [CrossRef]
- Guerrero-Moreno, A.; Baudouin, C.; Parsadaniantz, S.M.; Goazigo, A.R.-L. Morphological and Functional Changes of Corneal Nerves and Their Contribution to Peripheral and Central Sensory Abnormalities. Front. Cell. Neurosci. 2020, 14, 610342. [Google Scholar] [CrossRef]
- Vitar, R.M.L.; Barbariga, M.; Fonteyne, P.; Bignami, F.; Rama, P.; Ferrari, G. Modulating Ocular Surface Pain Through Neurokinin-1 Receptor Blockade. Investig. Opthalmol. Vis. Sci. 2021, 62, 26. [Google Scholar] [CrossRef] [PubMed]
- Kulka, M.; Sheen, C.H.; Tancowny, B.P.; Grammer, L.C.; Schleimer, R.P. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology 2008, 123, 398–410. [Google Scholar] [CrossRef]
- Micera, A.; Lambiase, A.; Bonini, S. The role of neuromediators in ocular allergy. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 466–471. [Google Scholar] [CrossRef]
- Hegarty, D.M.; Tonsfeldt, K.; Hermes, S.M.; Helfand, H.; Aicher, S.A. Differential localization of vesicular glutamate transporters and peptides in corneal afferents to trigeminal nucleus caudalis. J. Comp. Neurol. 2010, 518, 3557–3569. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Bazan, H.E.P. Neuroanatomy and Neurochemistry of Mouse Cornea. Investig. Opthalmol. Vis. Sci. 2016, 57, 664–674. [Google Scholar] [CrossRef]
- Byun, Y.-S.; Mok, J.-W.; Chung, S.-H.; Kim, H.-S.; Joo, C.-K. Ocular surface inflammation induces de novo expression of substance P in the trigeminal primary afferents with large cell bodies. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Khawaja, A.M.; Rogers, D.F. Tachykinins: Receptor to effector. Int. J. Biochem. Cell Biol. 1996, 28, 721–738. [Google Scholar] [CrossRef]
- Suvas, S. Role of Substance P Neuropeptide in Inflammation, Wound Healing, and Tissue Homeostasis. J. Immunol. 2017, 199, 1543–1552. [Google Scholar] [CrossRef]
- Krause, J.E.; Macdonald, M.R.; Takeda, Y. The polyprotein nature of substance P precursors. BioEssays 1989, 10, 62–69. [Google Scholar] [CrossRef]
- Steinhoff, M.; Von Mentzer, B.; Geppetti, P.; Pothoulakis, C.; Bunnett, N.W. Tachykinins and Their Receptors: Contributions to Physiological Control and the Mechanisms of Disease. Physiol. Rev. 2014, 94, 265–301. [Google Scholar] [CrossRef] [PubMed]
- Gerard, N.P.; Garraway, L.A.; Eddy, R.L.; Shows, T.B.; Iijima, H.; Paquet, J.L.; Gerard, C. Human substance P receptor (NK-1): Organization of the gene, chromosome localization and functional expression of cDNA clones. Biochemistry 1991, 30, 10640–10646. [Google Scholar] [CrossRef]
- Maggi, C.A.; Patacchini, R.; Rovero, P.; Giachetti, A. Tachykinin receptors and tachykinin receptor antagonists. J. Auton. Pharmacol. 1993, 13, 23–93. [Google Scholar] [CrossRef]
- Regoli, D.; Boudon, A.; Fauchére, J.L. Receptors and antagonists for substance P and related peptides. Pharmacol. Rev. 1994, 46, 551–599. [Google Scholar] [PubMed]
- Garcia-Recio, S.; Gascón, P. Biological and Pharmacological Aspects of the NK1-Receptor. BioMed Res. Int. 2015, 2015, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Green, D.P.; Limjunyawong, N.; Gour, N.; Pundir, P.; Dong, X. A Mast-Cell-Specific Receptor Mediates Neurogenic Inflammation and Pain. Neuron 2019, 101, 412–420.e3. [Google Scholar] [CrossRef] [PubMed]
- Hay, D.L.; Garelja, M.L.; Poyner, D.R.; Walker, C.S. Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR Review . Br. J. Pharmacol. 2018, 175, 3–17. [Google Scholar] [CrossRef]
- Russo, A.F.; Nelson, C.; Roos, B.A.; Rosenfeld, M.G. Differential regulation of the coexpressed calcitonin/alpha-CGRP and beta-CGRP neuroendocrine genes. J. Biol. Chem. 1988, 263, 5–8. [Google Scholar] [CrossRef]
- Russell, F.A.; King, R.; Smillie, S.-J.; Kodji, X.; Brain, S.D. Calcitonin Gene-Related Peptide: Physiology and Pathophysiology. Physiol. Rev. 2014, 94, 1099–1142. [Google Scholar] [CrossRef]
- Alexander, S.; Christopoulos, A.; Davenport, A.P.; Kelly, E.; Marrion, N.V.; Peters, J.A.; Faccenda, E.; Harding, S.; Pawson, A.J.; Sharman, J.; et al. The Concise Guide to Pharmacology 2017/18: G protein-coupled receptors. Br. J. Pharmacol. 2017, 174, S17–S129. [Google Scholar] [CrossRef]
- Liang, Y.-L.; Khoshouei, M.; Deganutti, G.; Glukhova, A.; Koole, C.; Peat, T.; Radjainia, M.; Plitzko, J.M.; Baumeister, W.; Miller, L.J.; et al. Cryo-EM structure of the active, Gs-protein complexed, human CGRP receptor. Nat. Cell Biol. 2018, 561, 492–497. [Google Scholar] [CrossRef]
- Poyner, D.R.; Sexton, P.M.; Marshall, I.; Smith, D.M.; Quirion, R.; Born, W.; Muff, R.; Fischer, J.A.; Foord, S.M. International Union of Pharmacology. XXXII. The Mammalian Calcitonin Gene-Related Peptides, Adrenomedullin, Amylin, and Calcitonin Receptors. Pharmacol. Rev. 2002, 54, 233–246. [Google Scholar] [CrossRef]
- Walker, C.S.; Conner, A.C.; Poyner, D.R.; Hay, D.L. Regulation of signal transduction by calcitonin gene-related peptide receptors. Trends Pharmacol. Sci. 2010, 31, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Gozes, I.; Nakai, H.; Byers, M.; Avidor, R.; Weinstein, Y.; Shani, Y.; Shows, T.B. Sequential expression in the nervous system of C-MYB andVIP genes, located in human chromosomal region 6q24. Somat. Cell Mol. Genet. 1987, 13, 305–313. [Google Scholar] [CrossRef]
- Linder, S.; Barkhem, T.; Norberg, A.; Persson, H.; Schalling, M.; Hokfelt, T.; Magnusson, G. Structure and expression of the gene encoding the vasoactive intestinal peptide precursor. Proc. Natl. Acad. Sci. USA 1987, 84, 605–609. [Google Scholar] [CrossRef]
- Solés-Tarrés, I.; Cabezas-Llobet, N.; Vaudry, D.; Xifró, X. Protective Effects of Pituitary Adenylate Cyclase-Activating Polypeptide and Vasoactive Intestinal Peptide Against Cognitive Decline in Neurodegenerative Diseases. Front. Cell. Neurosci. 2020, 14, 221. [Google Scholar] [CrossRef]
- Harmar, A.J.; Fahrenkrug, J.; Gozes, I.; Laburthe, M.; May, V.; Pisegna, J.R.; Vaudry, D.; Vaudry, H.; Waschek, J.A.; Said, S.I. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR Review. Br. J. Pharmacol. 2012, 166, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhou, Q.; Labroska, V.; Qin, S.; Darbalaei, S.; Wu, Y.; Yuliantie, E.; Xie, L.; Tao, H.; Cheng, J.; et al. G protein-coupled receptors: Structure- and function-based drug discovery. Signal. Transduct. Target. Ther. 2021, 6, 1–27. [Google Scholar] [CrossRef]
- Tatemoto, K. Neuropeptide Y: Complete amino acid sequence of the brain peptide. Proc. Natl. Acad. Sci. USA 1982, 79, 5485–5489. [Google Scholar] [CrossRef]
- Tatemoto, K.; Carlquist, M.; Mutt, V. Neuropeptide Y—A novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nat. Cell Biol. 1982, 296, 659–660. [Google Scholar] [CrossRef]
- Gray, T.S.; Morley, J.E. Neuropeptide Y: Anatomical distribution and possible function in mammalian nervous system. Life Sci. 1986, 38, 389–401. [Google Scholar] [CrossRef]
- Pedragosa-Badia, X.; Stichel, J.; Beck-Sickinger, A.G. Neuropeptide Y receptors: How to get subtype selectivity. Front. Endocrinol. 2013, 4, 5. [Google Scholar] [CrossRef]
- Cabrele, C.; Beck-Sickinger, A.G. Molecular characterization of the ligand-receptor interaction of the neuropeptide Y family. J. Pept. Sci. 2000, 6, 97–122. [Google Scholar] [CrossRef]
- Sun, Q.; Huguenard, J.R.; Prince, D.A. Neuropeptide Y receptors differentially modulate G-protein-activated inwardly rectifying K+ channels and high-voltage-activated Ca2+ channels in rat thalamic neurons. J. Physiol. 2001, 531, 67–79. [Google Scholar] [CrossRef]
- Richardson, J.D.; Vasko, M.R. Cellular Mechanisms of Neurogenic Inflammation. J. Pharmacol. Exp. Ther. 2002, 302, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Al-Aqaba, M.A.; Dhillon, V.K.; Mohammed, I.; Said, D.G.; Dua, H.S. Corneal nerves in health and disease. Prog. Retin. Eye Res. 2019, 73, 100762. [Google Scholar] [CrossRef] [PubMed]
- Brain, S.; Williams, T.J. Interactions between the tachykinins and calcitonin gene-related peptide lead to the modulation of oedema formation and blood flow in rat skin. Br. J. Pharmacol. 1989, 97, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Carolan, E.; Casale, T. Effects of neuropeptides on neutrophil migration through noncellular and endothelial barriers. J. Allergy Clin. Immunol. 1993, 92, 589–598. [Google Scholar] [CrossRef]
- Saban, M.R.; Saban, R.; Bjorling, D.; Haak-Frendscho, M. Involvement of leukotrienes, TNF-α, and the LFA-1/ICAM-1 interaction in substance P-induced granulocyte infiltration. J. Leukoc. Biol. 1997, 61, 445–451. [Google Scholar] [CrossRef]
- Hood, V.C.; Cruwys, S.C.; Urban, L.; Kidd, B.L. Differential role of neurokinin receptors in human lymphocyte and monocyte chemotaxis. Regul. Pept. 2000, 96, 17–21. [Google Scholar] [CrossRef]
- Shaheen, B.S.; Bakir, M.; Jain, S. Corneal nerves in health and disease. Surv. Ophthalmol. 2014, 59, 263–285. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, C.; Acosta, M.C.; Gallar, J. Neural basis of sensation in intact and injured corneas. Exp. Eye Res. 2004, 78, 513–525. [Google Scholar] [CrossRef]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and Molecular Mechanisms of Pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef]
- Pongratz, G.; Straub, R.H. Role of peripheral nerve fibres in acute and chronic inflammation in arthritis. Nat. Rev. Rheumatol. 2012, 9, 117–126. [Google Scholar] [CrossRef]
- Steinman, L. Elaborate interactions between the immune and nervous systems. Nat. Immunol. 2004, 5, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Chernova, I.; Lai, J.-P.; Li, H.; Schwartz, L.; Tuluc, F.; Korchak, H.M.; Douglas, S.D.; Kilpatrick, L.E. Substance P (SP) enhances CCL5-induced chemotaxis and intracellular signaling in human monocytes, which express the truncated neurokinin-1 receptor (NK1R). J. Leukoc. Biol. 2008, 85, 154–164. [Google Scholar] [CrossRef]
- Castellani, M.L.; Vecchiet, J.; Salini, V.; Conti, P.; Theoharides, T.C.; Caraffa, A.; Antinolfi, P.; Teté, S.; Ciampoli, C.; Cuccurullo, C.; et al. Stimulation of CCL2 (MCP-1) and CCL2 mRNA by substance P in LAD2 human mast cells. Transl. Res. 2009, 154, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Bignami, F.; Rama, P.; Ferrari, G. Substance P and its Inhibition in Ocular Inflammation. Curr. Drug Targets 2016, 17, 1265–1274. [Google Scholar] [CrossRef]
- Lotz, M.; Vaughan, J.H.; Carson, D.A. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science 1988, 241, 1218–1221. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.X.; Qin, B.; Zhou, A.P.; Yu, Q.Y.; Huang, Q.J.; Liang, Z.F. Regulation of NK92-MI Cell Cytotoxicity by Substance P. Scand. J. Immunol. 2011, 74, 107–113. [Google Scholar] [CrossRef]
- Lim, J.E.; Chung, E.; Son, Y. A neuropeptide, Substance-P, directly induces tissue-repairing M2 like macrophages by activating the PI3K/Akt/mTOR pathway even in the presence of IFNγ. Sci. Rep. 2017, 7, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Janelsins, B.M.; Mathers, A.R.; Tkacheva, O.A.; Erdos, G.; Shufesky, W.J.; Morelli, A.E.; Larregina, A.T. Proinflammatory tachykinins that signal through the neurokinin 1 receptor promote survival of dendritic cells and potent cellular immunity. Blood 2009, 113, 3017–3026. [Google Scholar] [CrossRef]
- Cunin, P.; Caillon, A.; Corvaisier, M.; Garo, E.; Scotet, M.; Blanchard, S.; Delneste, Y.; Jeannin, P. The Tachykinins Substance P and Hemokinin-1 Favor the Generation of Human Memory Th17 Cells by Inducing IL-1β, IL-23, and TNF-Like 1A Expression by Monocytes. J. Immunol. 2011, 186, 4175–4182. [Google Scholar] [CrossRef] [PubMed]
- Payan, D.G.; Brewster, D.R.; Goetzl, E.J. Specific stimulation of human T lymphocytes by substance P. J. Immunol. 1983, 131, 1613–1615. [Google Scholar] [PubMed]
- Calvo, C.F.; Chavanel, G.; Senik, A. Substance P enhances IL-2 expression in activated human T cells. J. Immunol. 1992, 148, 3498–3504. [Google Scholar]
- Blum, A.M.; Metwali, A.; Elliott, D.; Weinstock, J.V. T cell substance P receptor governs antigen-elicited IFN-γ production. Am. J. Physiol. Liver Physiol. 2003, 284, G197–G204. [Google Scholar] [CrossRef]
- Morelli, A.E.; Sumpter, T.L.; Rojas-Canales, D.M.; Bandyopadhyay, M.; Chen, Z.; Tkacheva, O.; Shufesky, W.J.; Wallace, C.T.; Watkins, S.; Berger, A.; et al. Neurokinin-1 Receptor Signaling Is Required for Efficient Ca2+ Flux in T-Cell-Receptor-Activated T Cells. Cell Rep. 2020, 30, 3448–3465.e8. [Google Scholar] [CrossRef]
- Charles, A.; Pozo-Rosich, P. Targeting calcitonin gene-related peptide: A new era in migraine therapy. Lancet 2019, 394, 1765–1774. [Google Scholar] [CrossRef]
- Mikami, N.; Matsushita, H.; Kato, T.; Kawasaki, R.; Sawazaki, T.; Kishimoto, T.; Ogitani, Y.; Watanabe, K.; Miyagi, Y.; Sueda, K.; et al. Calcitonin Gene-Related Peptide Is an Important Regulator of Cutaneous Immunity: Effect on Dendritic Cell and T Cell Functions. J. Immunol. 2011, 186, 6886–6893. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, S.; Knopf, M.A.; Bjork, S.K.; McGillis, J.P. Bone Marrow-Derived Macrophages Express Functional CGRP Receptors and Respond to CGRP by Increasing Transcription of c-fos and IL-6 mRNA. Cell. Immunol. 2001, 209, 140–148. [Google Scholar] [CrossRef]
- Eftekhari, S.; Warfvinge, K.; Blixt, F.W.; Edvinsson, L. Differentiation of Nerve Fibers Storing CGRP and CGRP Receptors in the Peripheral Trigeminovascular System. J. Pain 2013, 14, 1289–1303. [Google Scholar] [CrossRef] [PubMed]
- Mikami, N.; Watanabe, K.; Hashimoto, N.; Miyagi, Y.; Sueda, K.; Fukada, S.-I.; Yamamoto, H.; Tsujikawa, K. Calcitonin gene-related peptide enhances experimental autoimmune encephalomyelitis by promoting Th17-cell functions. Int. Immunol. 2012, 24, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Levite, M. Nerve-driven immunity. The direct effects of neurotransmitters on T-cell function. Ann. N. Y. Acad. Sci. 2006, 917, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Stohl, L.L.; Wagner, J.A.; Granstein, R.D. Calcitonin Gene-related Peptide Biases Langerhans Cells Towards Th2-type Immunity. J. Immunol. 2008, 181, 6020–6026. [Google Scholar] [CrossRef] [PubMed]
- Assas, B.M.; Pennock, J.I.; Miyan, J.A. Calcitonin gene-related peptide is a key neurotransmitter in the neuro-immune axis. Front. Neurosci. 2014, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Tokoyoda, K.; Tsujikawa, K.; Matsushita, H.; Ono, Y.; Hayashi, T.; Harada, Y.; Abe, R.; Kubo, M.; Yamamoto, H. Up-regulation of IL-4 production by the activated cAMP/cAMP-dependent protein kinase (protein kinase A) pathway in CD3/CD28-stimulated naive T cells. Int. Immunol. 2004, 16, 643–653. [Google Scholar] [CrossRef]
- Carucci, J.A.; Ignatius, R.; Wei, Y.; Cypess, A.M.; Schaer, D.A.; Pope, M.; Steinman, R.M.; Mojsov, S. Calcitonin gene-related peptide decreases expression of HLA-DR and CD86 by human dendritic cells and dampens dendritic cell-driven T cell-proliferative responses via the type I calcitonin gene-related peptide receptor. J. Immunol. 2000, 164, 3494–3499. [Google Scholar] [CrossRef]
- Harzenetter, M.D.; Novotny, A.R.; Gais, P.; Molina, C.A.; Altmayr, F.; Holzmann, B. Negative Regulation of TLR Responses by the Neuropeptide CGRP Is Mediated by the Transcriptional Repressor ICER. J. Immunol. 2007, 179, 607–615. [Google Scholar] [CrossRef]
- Holzmann, B. Modulation of immune responses by the neuropeptide CGRP. Amino Acids 2013, 45, 1–7. [Google Scholar] [CrossRef]
- Holzmann, B. Antiinflammatory activities of CGRP modulating innate immune responses in health and disease. Curr. Protein Pept. Sci. 2013, 14, 268–274. [Google Scholar] [CrossRef]
- Aoki-Nagase, T.; Nagase, T.; Oh-Hashi, Y.; Shindo, T.; Kurihara, Y.; Yamaguchi, Y.; Yamamoto, H.; Tomita, T.; Ohga, E.; Nagai, R.; et al. Attenuation of antigen-induced airway hyperresponsiveness in CGRP-deficient mice. Am. J. Physiol. Cell. Mol. Physiol. 2002, 283, L963–L970. [Google Scholar] [CrossRef]
- Li, M.; Wetzel-Strong, S.E.; Hua, X.; Tilley, S.L.; Oswald, E.; Krummel, M.F.; Caron, K.M. Deficiency of RAMP1 Attenuates Antigen-Induced Airway Hyperresponsiveness in Mice. PLoS ONE 2014, 9, e102356. [Google Scholar] [CrossRef]
- Manning, B.M.; Gruba, S.M.; Meyer, A.F.; Haynes, C.L. Neuropeptide-Induced Mast Cell Degranulation and Characterization of Signaling Modulation in Response to IgE Conditioning. ACS Chem. Biol. 2016, 11, 3077–3083. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Padanilam, B.J. Renal Nerves Drive Interstitial Fibrogenesis in Obstructive Nephropathy. J. Am. Soc. Nephrol. 2013, 24, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Fahrenkrug, J. Transmitter Role of Vasoactive Intestinal Peptide. Pharmacol. Toxicol. 1993, 72, 354–363. [Google Scholar] [CrossRef]
- Delgado, M.; Pozo, D.; Ganea, D. The Significance of Vasoactive Intestinal Peptide in Immunomodulation. Pharmacol. Rev. 2004, 56, 249–290. [Google Scholar] [CrossRef] [PubMed]
- Ganea, D.; Hooper, K.M.; Kong, W. The neuropeptide vasoactive intestinal peptide: Direct effects on immune cells and involvement in inflammatory and autoimmune diseases. Acta Physiol. 2015, 213, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Pozo, D.; Gonzalez-Rey, E.; Chorny, A.; Anderson, P.; Varela, N.; Delgado, M. Tuning immune tolerance with vasoactive intestinal peptide: A new therapeutic approach for immune disorders. Peptides 2007, 28, 1833–1846. [Google Scholar] [CrossRef]
- Delgado, M.; Ganea, D. Vasoactive intestinal peptide: A neuropeptide with pleiotropic immune functions. Amino Acids 2013, 45, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rey, E.; Chorny, A.; Delgado, M. Regulation of immune tolerance by anti-inflammatory neuropeptides. Nat. Rev. Immunol. 2007, 7, 52–63. [Google Scholar] [CrossRef]
- Shioda, S.; Nakamachi, T. PACAP as a neuroprotective factor in ischemic neuronal injuries. Peptides 2015, 72, 202–207. [Google Scholar] [CrossRef]
- Hirabayashi, T.; Nakamachi, T.; Shioda, S. Discovery of PACAP and its receptors in the brain. J. Headache Pain 2018, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.; Herzog, H.; Shi, Y.-C. Regulation of energy homeostasis by the NPY system. Trends Endocrinol. Metab. 2015, 26, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Straub, R.H.; Schaller, T.; Miller, L.E.; Von Hörsten, S.; Jessop, D.S.; Falk, W.; Schölmerich, J. Neuropeptide Y Cotransmission with Norepinephrine in the Sympathetic Nerve-Macrophage Interplay. J. Neurochem. 2008, 75, 2464–2471. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijević, M.; Stanojević, S.; Vujić, V.; Beck-Sickinger, A.; von Hörsten, S. Neuropeptide Y and its receptor subtypes specifically modulate rat peritoneal macrophage functions in vitro: Counter regulation through Y1 and Y2/5 receptors. Regul. Pept. 2005, 124, 163–172. [Google Scholar] [CrossRef]
- Dimitrijević, M.; Stanojević, S. The intriguing mission of neuropeptide Y in the immune system. Amino Acids 2011, 45, 41–53. [Google Scholar] [CrossRef]
- Buttari, B.; Profumo, E.; Domenici, G.; Tagliani, A.; Ippoliti, F.; Bonini, S.; Businaro, R.; Elenkov, I.; Riganò, R. Neuropeptide Y induces potent migration of human immature dendritic cells and promotes a Th2 polarization. FASEB J. 2014, 28, 3038–3049. [Google Scholar] [CrossRef]
- Ferreira, R.; Santos, T.; Viegas, M.; Cortes, L.; Bernardino, L.; Vieira, O.V.; Malva, J.O. Neuropeptide Y inhibits interleukin-1β-induced phagocytosis by microglial cells. J. Neuroinflamm. 2011, 8, 169. [Google Scholar] [CrossRef]
- Ferreira, R.; Santos, T.; Cortes, L.; Cochaud, S.; Agasse, F.; Silva, A.P.; Xapelli, S.; Malva, J.O. Neuropeptide Y inhibits interleukin-1 beta-induced microglia motility. J. Neurochem. 2011, 120, 93–105. [Google Scholar] [CrossRef]
- Phan, T.A.; Taylor, A.W. The neuropeptides α-MSH and NPY modulate phagocytosis and phagolysosome activation in RAW 264.7 cells. J. Neuroimmunol. 2013, 260, 9–16. [Google Scholar] [CrossRef]
- Catania, A.; Delgado, R.; Airaghi, L.; Cutuli, M.; Garofalo, L.; Carlin, A.; Demitri, M.T.; Lipton, J.M. α-MSH in Systemic Inflammation: Central and Peripheral Actions. Ann. N. Y. Acad. Sci. 2006, 885, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Brzoska, T.; Luger, T.A.; Maaser, C.; Abels, C.; Böhm, M. α-Melanocyte-Stimulating Hormone and Related Tripeptides: Biochemistry, Antiinflammatory and Protective Effects In Vitro and In Vivo, and Future Perspectives for the Treatment of Immune-Mediated Inflammatory Diseases. Endocr. Rev. 2008, 29, 581–602. [Google Scholar] [CrossRef]
- Taylor, A.W.; Lee, D.J. The Alpha-Melanocyte Stimulating Hormone Induces Conversion of Effector T Cells into Treg Cells. J. Transplant. 2011, 2011, 246856. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Gundlach, A.; Kofler, B. The galanin peptide family: Receptor pharmacology, pleiotropic biological actions, and implications in health and disease. Pharmacol. Ther. 2007, 115, 177–207. [Google Scholar] [CrossRef] [PubMed]
- Green, P.; Basbaum, A.; Levine, J. Sensory neuropeptide interactions in the production of plasma extravasation in the rat. Neuroscience 1992, 50, 745–749. [Google Scholar] [CrossRef]
- Talero, E.; Sánchez-Fidalgo, S.; Calvo, J.R.; Motilva, V. Galanin in the trinitrobenzene sulfonic acid rat model of experimental colitis. Int. Immunopharmacol. 2006, 6, 1404–1412. [Google Scholar] [CrossRef]
- Schmidhuber, S.M.; Santic, R.; Tam, C.W.; Bauer, J.W.; Kofler, B.; Brain, S.D. Galanin-Like Peptides Exert Potent Vasoactive Functions In Vivo. J. Investig. Dermatol. 2007, 127, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Brunner, S.M.; Reichmann, F.; Leitner, J.; Wölfl, S.; Bereswill, S.; Farzi, A.; Schneider, A.-M.; Klieser, E.; Neureiter, D.; Emberger, M.; et al. Galanin receptor 3 attenuates inflammation and influences the gut microbiota in an experimental murine colitis model. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Koller, A.; Brunner, S.M.; Bianchini, R.; Ramspacher, A.; Emberger, M.; Locker, F.; Schlager, S.; Kofler, B. Galanin is a potent modulator of cytokine and chemokine expression in human macrophages. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ramspacher, A.; Neudert, M.; Koller, A.; Schlager, S.; Kofler, B.; Brunner, S.M. Influence of the regulatory peptide galanin on cytokine expression in human monocytes. Ann. N. Y. Acad. Sci. 2019, 1455, 185–195. [Google Scholar] [CrossRef]
- Marfurt, C.F.; Kingsley, R.E.; Echtenkamp, S.E. Sensory and sympathetic innervation of the mammalian cornea. A retrograde tracing study. Investig. Ophthalmol. Vis. Sci. 1989, 30, 461–472. [Google Scholar]
- De Felipe, C.; Gonzalez, G.G.; Gallar, J.; Belmonte, C. Quantification and immunocytochemical characteristics of trigeminal ganglion neurons projecting to the cornea: Effect of corneal wounding. Eur. J. Pain 1999, 3, 31–39. [Google Scholar] [CrossRef]
- Launay, P.-S.; Godefroy, D.; Khabou, H.; Rostene, W.; Sahel, J.-A.; Baudouin, C.; Parsadaniantz, S.M.; Goazigo, A.R.-L. Combined 3DISCO clearing method, retrograde tracer and ultramicroscopy to map corneal neurons in a whole adult mouse trigeminal ganglion. Exp. Eye Res. 2015, 139, 136–143. [Google Scholar] [CrossRef]
- Figueira, L.; Janeiro, C.; Ferreirinha, F.; Serrão, P.; Perestrelo, S.; Falcão-Reis, F.; Correia-De-Sá, P.; Moura, D. Regulation of corneal noradrenaline release and topography of sympathetic innervation: Functional implications for adrenergic mechanisms in the human cornea. Exp. Eye Res. 2018, 174, 121–132. [Google Scholar] [CrossRef] [PubMed]
- May, C.A. Description and Function of the Ciliary Nerves—Some Historical Remarks on Choroidal Innervation. Exp. Eye Res. 1997, 65, 1–5. [Google Scholar] [CrossRef]
- Zander, E.; Weddell, G. Observations on the innervation of the cornea. J. Anat. 1951, 85, 68–99. [Google Scholar] [PubMed]
- Tusscher, M.T.; Klooster, J.; Van Der Want, J.; Lamers, W.; Vrensen, G. The allocation of nerve fibres to the anterior eye segment and peripheral ganglia of rats. I. The sensory innervation. Brain Res. 1989, 494, 95–104. [Google Scholar] [CrossRef]
- Oduntan, A.O. Cellular inflammatory response induced by sensory denervation of the conjunctiva in monkeys. J. Anat. 2005, 206, 287–294. [Google Scholar] [CrossRef]
- Ruskell, G.L. Innervation of the conjunctiva. Trans. Ophthalmol. Soc. 1985, 104 (Pt 4), 390–395. [Google Scholar]
- Munger, B.L.; Halata, Z. The sensorineural apparatus of the human eyelid. Am. J. Anat. 1984, 170, 181–204. [Google Scholar] [CrossRef]
- Tiwari, S.; Ali, M.J.; Vemuganti, G.K. Human lacrimal gland regeneration: Perspectives and review of literature. Saudi J. Ophthalmol. 2014, 28, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.M.; Nichols, J.J. The neurobiology of the meibomian glands. Ocul. Surf. 2014, 12, 167–177. [Google Scholar] [CrossRef]
- Bonini, S.; Rama, P.; Olzi, D.; Lambiase, A. Neurotrophic keratitis. Eye 2003, 17, 989–995. [Google Scholar] [CrossRef] [PubMed]
- De Felipe, C.; Belmonte, C. c-Jun expression after axotomy of corneal trigeminal ganglion neurons is dependent on the site of injury. Eur. J. Neurosci. 1999, 11, 899–906. [Google Scholar] [CrossRef]
- Bron, R.; Wood, R.J.; Brock, J.A.; Ivanusic, J.J. Piezo2 expression in corneal afferent neurons. J. Comp. Neurol. 2014, 522, 2967–2979. [Google Scholar] [CrossRef]
- Muller, L.J.; Pels, L.; Vrensen, G.F. Ultrastructural organization of human corneal nerves. Investig. Ophthalmol. Vis. Sci. 1996, 37, 476–488. [Google Scholar]
- Marfurt, C.F.; Cox, J.; Deek, S.; Dvorscak, L. Anatomy of the human corneal innervation. Exp. Eye Res. 2010, 90, 478–492. [Google Scholar] [CrossRef]
- Ueda, S.; del Cerro, M.; LoCascio, J.A.; Aquavella, J.V. Peptidergic and catecholaminergic fibers in the human corneal epithelium. An immunohistochemical and electron microscopic study. Acta Ophthalmol. Suppl. 1989, 192, 80–90. [Google Scholar] [CrossRef] [PubMed]
- LaVail, J.H.; Johnson, W.E.; Spencer, L.C. Immunohistochemical identification of trigeminal ganglion neurons that innervate the mouse cornea: Relevance to intercellular spread of herpes simplex virus. J. Comp. Neurol. 1993, 327, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.A.; Marfurt, C.F. Peptidergic innervation of the rat cornea. Exp. Eye Res. 1998, 66, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Unger, W.G.; Butler, J.M.; Cole, D.F.; Bloom, S.R.; McGregor, G.P. Substance P, vasoactive intestinal polypeptide (VIP) and somatostatin levels in ocular tissue of normal and sensorily denervated rabbit eyes. Exp. Eye Res. 1981, 32, 797–801. [Google Scholar] [CrossRef]
- Stone, R.A.; Laties, A.M.; Brecha, N.C. Substance P-like immunoreactive nerves in the anterior segment of the rabbit, cat and monkey eye. Neuroscience 1982, 7, 2459–2468. [Google Scholar] [CrossRef]
- Stone, R.A.; McGlinn, A.M. Calcitonin gene-related peptide immunoreactive nerves in human and rhesus monkey eyes. Invest. Ophthalmol. Vis. Sci. 1988, 29, 305–310. [Google Scholar] [PubMed]
- Stone, R.A.; Laties, A.M.; Emson, P.C. Neuropeptide Y and the ocular innervation of rat, guinea pig, cat and monkey. Neuroscience 1986, 17, 1207–1216. [Google Scholar] [CrossRef]
- Stone, R.A.; Tervo, T.; Tervo, K.; Tarkkanen, A. Vasoactive intestinal polypeptide-like immunoreactive nerves to the human eye. Acta Ophthalmol. 1986, 64, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Elsas, T.; Edvinsson, L.; Sundler, F.; Uddman, R. Neuronal pathways to the rat conjunctiva revealed by retrograde tracing and immunocytochemistry. Exp. Eye Res. 1994, 58, 117–126. [Google Scholar] [CrossRef]
- Luhtala, J.; Palkama, A.; Uusitalo, H. Calcitonin gene-related peptide immunoreactive nerve fibers in the rat conjunctiva. Investig. Ophthalmol. Vis. Sci. 1991, 32, 640–645. [Google Scholar] [PubMed]
- Diebold, Y.; Rios, J.D.; Hodges, R.R.; Rawe, I.; Dartt, D.A. Presence of nerves and their receptors in mouse and human conjunctival goblet cells. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2270–2282. [Google Scholar]
- Rios, J.D.; Zoukhri, D.; Rawe, I.M.; Hodges, R.R.; Zieske, J.D.; Dartt, D.A. Immunolocalization of muscarinic and VIP receptor subtypes and their role in stimulating goblet cell secretion. Invest. Ophthalmol. Vis. Sci. 1999, 40, 1102–1111. [Google Scholar] [PubMed]
- Laboisse, C.; Jarry, A.; Branka, J.E.; Merlin, D.; Bou-Hanna, C.; Vallette, G. Regulation of mucin exocytosis from intestinal goblet cells. Biochem. Soc. Trans. 1995, 23, 810–813. [Google Scholar] [CrossRef]
- Nikkinen, A.; Lehtosalo, J.I.; Uusitalo, H.; Palkama, A.; Panula, P. The lacrimal glands of the rat and the guinea pig are innervated by nerve fibers containing immunoreactivities for substance P and vasoactive intestinal polypeptide. Histochemistry 1984, 81, 23–27. [Google Scholar] [CrossRef]
- Seifert, P.; Stuppi, S.; Spitznas, M.; Weihe, E. Differential distribution of neuronal markers and neuropeptides in the human lacrimal gland. Graefes Arch. Clin. Exp. Ophthalmol. 1996, 234, 232–240. [Google Scholar] [CrossRef]
- Dartt, D.A. Neural regulation of lacrimal gland secretory processes: Relevance in dry eye diseases. Prog. Retin. Eye Res. 2009, 28, 155–177. [Google Scholar] [CrossRef]
- Cavallotti, C.; Frati, A.; Sagnelli, P.; Pescosolido, N. Re-evaluation and quantification of the different sources of nerve fibres supplying the rat eye. J. Anat. 2005, 206, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.W.; Tigges, M.; Stone, R.A. Peptidergic innervation of the primate meibomian gland. Investig. Ophthalmol. Vis. Sci. 1996, 37, 238–245. [Google Scholar]
- Luhtala, J.; Uusitalo, H. The distribution and origin of substance P immunoreactive nerve fibres in the rat conjunctiva. Exp. Eye Res. 1991, 53, 641–646. [Google Scholar] [CrossRef]
- Hodges, R.R.; Dartt, D.A. Regulatory pathways in lacrimal gland epithelium. Int. Rev. Cytol. 2003, 231, 129–196. [Google Scholar] [CrossRef]
- Yu, M.; Lee, S.M.; Lee, H.; Amouzegar, A.; Nakao, T.; Chen, Y.; Dana, R. Neurokinin-1 Receptor Antagonism Ameliorates Dry Eye Disease by Inhibiting Antigen-Presenting Cell Maturation and T Helper 17 Cell Activation. Am. J. Pathol. 2020, 190, 125–133. [Google Scholar] [CrossRef]
- Liu, L.; Dana, R.; Yin, J. Sensory neurons directly promote angiogenesis in response to inflammation via substance P signaling. FASEB J. 2020, 34, 6229–6243. [Google Scholar] [CrossRef]
- Taketani, Y.; Marmalidou, A.; Dohlman, T.H.; Singh, R.B.; Amouzegar, A.; Chauhan, S.K.; Chen, Y.; Dana, R. Restoration of Regulatory T-Cell Function in Dry Eye Disease by Antagonizing Substance P/Neurokinin-1 Receptor. Am. J. Pathol. 2020, 190, 1859–1866. [Google Scholar] [CrossRef]
- Lee, S.J.; Im, S.T.; Wu, J.; Cho, C.S.; Jo, D.H.; Chen, Y.; Dana, R.; Kim, J.H.; Lee, S.M. Corneal lymphangiogenesis in dry eye disease is regulated by substance P/neurokinin-1 receptor system through controlling expression of vascular endothelial growth factor receptor 3. Ocul. Surf. 2021, 22, 72–79. [Google Scholar] [CrossRef]
- Tatematsu, Y.; Khan, Q.; Blanco, T.; Bair, J.A.; Hodges, R.R.; Masli, S.; Dartt, D.A. Thrombospondin-1 Is Necessary for the Development and Repair of Corneal Nerves. Int. J. Mol. Sci. 2018, 19, 3191. [Google Scholar] [CrossRef] [PubMed]
- Ru, Y.; Huang, Y.; Liu, H.; Du, J.; Meng, Z.; Dou, Z.; Liu, X.; Wei, R.H.; Zhang, Y.; Zhao, S. alpha-Melanocyte-stimulating hormone ameliorates ocular surface dysfunctions and lesions in a scopolamine-induced dry eye model via PKA-CREB and MEK-Erk pathways. Sci. Rep. 2015, 5, 18619. [Google Scholar] [CrossRef] [PubMed]
- Nakamachi, T.; Ohtaki, H.; Seki, T.; Yofu, S.; Kagami, N.; Hashimoto, H.; Shintani, N.; Baba, A.; Mark, L.; Lanekoff, I.; et al. PACAP suppresses dry eye signs by stimulating tear secretion. Nat. Commun. 2016, 7, 12034. [Google Scholar] [CrossRef]
- Yang, L.; Sui, W.; Li, Y.; Qi, X.; Wang, Y.; Zhou, Q.; Gao, H. Substance P Inhibits Hyperosmotic Stress-Induced Apoptosis in Corneal Epithelial Cells through the Mechanism of Akt Activation and Reactive Oxygen Species Scavenging via the Neurokinin-1 Receptor. PLoS ONE 2016, 11, e0149865. [Google Scholar] [CrossRef]
- Chu, C.; Huang, Y.; Ru, Y.; Lu, X.; Zeng, X.; Liu, K.; Gan, L.; Zhang, Y.; Zhao, S. alpha-MSH ameliorates corneal surface dysfunction in scopolamine-induced dry eye rats and human corneal epithelial cells via enhancing EGFR expression. Exp. Eye Res. 2021, 210, 108685. [Google Scholar] [CrossRef]
- Lv, Y.; Chu, C.; Liu, K.; Ru, Y.; Zhang, Y.; Lu, X.; Gao, Y.; Zhang, C.; Zhao, S. A combination of CMC and alpha-MSH inhibited ROS activated NLRP3 inflammasome in hyperosmolarity stressed HCECs and scopolamine-induced dry eye rats. Sci. Rep. 2021, 11, 1184. [Google Scholar] [CrossRef]
- Kheirkhah, A.; Saboo, U.S.; Abud, T.B.; Dohlman, T.H.; Arnoldner, M.A.; Hamrah, P.; Dana, R. Reduced Corneal Endothelial Cell Density in Patients With Dry Eye Disease. Am. J. Ophthalmol. 2015, 159, 1022–1026. [Google Scholar] [CrossRef]
- Konttinen, Y.T.; Hukkanen, M.; Kemppinen, P.; Segerberg, M.; Sorsa, T.; Malmstrom, M.; Rose, S.; Itescu, S.; Polak, J.M. Peptide-containing nerves in labial salivary glands in Sjogren’s syndrome. Arthritis Rheum. 1992, 35, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Tornwall, J.; Uusitalo, H.; Hukkanen, M.; Sorsa, T.; Konttinen, Y.T. Distribution of vasoactive intestinal peptide (VIP) and its binding sites in labial salivary glands in Sjogren’s syndrome and in normal controls. Clin. Exp. Rheumatol. 1994, 12, 287–292. [Google Scholar]
- Batbayar, B.; Nagy, G.; Kovesi, G.; Zelles, T.; Feher, E. Morphological basis of sensory neuropathy and neuroimmunomodulation in minor salivary glands of patients with Sjogren’s syndrome. Arch. Oral Biol. 2004, 49, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; Jones, D.; Ji, Z.; Afonso, A.; Monroy, D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren’s syndrome keratoconjunctivitis sicca. Curr. Eye Res. 1999, 19, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Lambiase, A.; Micera, A.; Sacchetti, M.; Cortes, M.; Mantelli, F.; Bonini, S. Alterations of tear neuromediators in dry eye disease. Arch. Ophthalmol. 2011, 129, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Ogata, M.; Kawai, M.; Mashima, Y. Decreased substance P concentrations in tears from patients with corneal hypesthesia. Am. J. Ophthalmol. 2000, 129, 671–672. [Google Scholar] [CrossRef]
- Yamada, M.; Ogata, M.; Kawai, M.; Mashima, Y.; Nishida, T. Substance P and its metabolites in normal human tears. Invest. Ophthalmol. Vis. Sci. 2002, 43, 2622–2625. [Google Scholar]
- Tummanapalli, S.S.; Willcox, M.D.P.; Issar, T.; Yan, A.; Pisarcikova, J.; Kwai, N.; Poynten, A.M.; Krishnan, A.V.; Markoulli, M. Tear film substance P: A potential biomarker for diabetic peripheral neuropathy. Ocul. Surf. 2019, 17, 690–698. [Google Scholar] [CrossRef]
- Colorado, L.H.; Markoulli, M.; Edwards, K. The Relationship Between Corneal Dendritic Cells, Corneal Nerve Morphology and Tear Inflammatory Mediators and Neuropeptides in Healthy Individuals. Curr. Eye Res. 2019, 44, 840–848. [Google Scholar] [CrossRef]
- Markoulli, M.; Colorado, L.H.; Edwards, K. The Relationship between Corneal Nerve Morphology and Inflammatory Mediators and Neuropeptides in Healthy Individuals. Optom. Vis. Sci. 2020, 97, 145–153. [Google Scholar] [CrossRef]
- Marfurt, C.F.; Murphy, C.J.; Florczak, J.L. Morphology and neurochemistry of canine corneal innervation. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2242–2251. [Google Scholar]
- Yang, L.W.Y.; Mehta, J.S.; Liu, Y.C. Corneal neuromediator profiles following laser refractive surgery. Neural. Regen. Res. 2021, 16, 2177–2183. [Google Scholar] [CrossRef]
- Gao, S.; Li, S.; Liu, L.; Wang, Y.; Ding, H.; Li, L.; Zhong, X. Early changes in ocular surface and tear inflammatory mediators after small-incision lenticule extraction and femtosecond laser-assisted laser in situ keratomileusis. PLoS ONE 2014, 9, e107370. [Google Scholar] [CrossRef] [PubMed]
- Mertaniemi, P.; Ylatupa, S.; Partanen, P.; Tervo, T. Increased release of immunoreactive calcitonin gene-related peptide (CGRP) in tears after excimer laser keratectomy. Exp. Eye Res. 1995, 60, 659–665. [Google Scholar] [CrossRef]
- Chao, C.; Stapleton, F.; Zhou, X.; Chen, S.; Zhou, S.; Golebiowski, B. Structural and functional changes in corneal innervation after laser in situ keratomileusis and their relationship with dry eye. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 2029–2039. [Google Scholar] [CrossRef]
- Chao, C.; Golebiowski, B.; Zhao, X.; Chen, S.; Zhou, S.; Stapleton, F. Long-term Effects of LASIK on Corneal Innervation and Tear Neuropeptides and the Associations With Dry Eye. J. Refract. Surg. 2016, 32, 518–524. [Google Scholar] [CrossRef]
- Lopez-de la Rosa, A.; Garcia-Vazquez, C.; Fernandez, I.; Arroyo-Del Arroyo, C.; Enriquez-de-Salamanca, A.; Gonzalez-Garcia, M.J. Substance P Level in Tears as a Potential Biomarker for Contact Lens Discomfort. Ocul. Immunol. Inflamm. 2021, 29, 43–56. [Google Scholar] [CrossRef]
- Gomes, J.A.P.; Azar, D.T.; Baudouin, C.; Efron, N.; Hirayama, M.; Horwath-Winter, J.; Kim, T.; Mehta, J.S.; Messmer, E.M.; Pepose, J.S.; et al. TFOS DEWS II iatrogenic report. Ocul. Surf. 2017, 15, 511–538. [Google Scholar] [CrossRef]
- Golebiowski, B.; Chao, C.; Stapleton, F.; Jalbert, I. Corneal Nerve Morphology, Sensitivity, and Tear Neuropeptides in Contact Lens Wear. Optom. Vis. Sci. 2017, 94, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Gitter, B.D.; Waters, D.C.; Threlkeld, P.G.; Lovelace, A.M.; Matsumoto, K.; Bruns, R.F. Cyclosporin A is a substance P (tachykinin NK1) receptor antagonist. Eur. J. Pharmacol. 1995, 289, 439–446. [Google Scholar] [CrossRef]
- Mak, T.W.; Penninger, J.M.; Ohashi, P.S. Knockout mice: A paradigm shift in modern immunology. Nat. Rev. Immunol. 2001, 1, 11–19. [Google Scholar] [CrossRef]
- Goshi, N.; Morgan, R.K.; Lein, P.J.; Seker, E. A primary neural cell culture model to study neuron, astrocyte, and microglia interactions in neuroinflammation. J. Neuroinflamm. 2020, 17, 155. [Google Scholar] [CrossRef]
- Li, Z. In Vitro Micro Tissue and Organ Models for Toxicity Testing. In Comprehensive Biotechnology; Moo-Young, M., Ed.; Pergamon: Oxford, UK, 2019; pp. 535–549. [Google Scholar]
- Feuilloley, M.G.J. Antidromic neurogenic activity and cutaneous bacterial flora. Semin. Immunopathol. 2018, 40, 281–289. [Google Scholar] [CrossRef]
- Moon, J.; Yoon, C.H.; Choi, S.H.; Kim, M.K. Can Gut Microbiota Affect Dry Eye Syndrome? Int. J. Mol. Sci. 2020, 21, 8443. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Choi, S.H.; Yoon, C.H.; Kim, M.K. Gut dysbiosis is prevailing in Sjogren’s syndrome and is related to dry eye severity. PLoS ONE 2020, 15, e0229029. [Google Scholar] [CrossRef]
- Trujillo-Vargas, C.M.; Schaefer, L.; Alam, J.; Pflugfelder, S.C.; Britton, R.A.; de Paiva, C.S. The gut-eye-lacrimal gland-microbiome axis in Sjogren Syndrome. Ocul. Surf. 2020, 18, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, A.; Rimmer, V.; Walkden, A.; Brahma, A.; Carley, F.; McBain, A.J.; Radhakrishnan, H. Next-Generation Sequencing of the Ocular Surface Microbiome: In Health, Contact Lens Wear, Diabetes, Trachoma, and Dry Eye. Eye Contact Lens 2020, 46, 254–261. [Google Scholar] [CrossRef]
- Zilliox, M.J.; Gange, W.S.; Kuffel, G.; Mores, C.R.; Joyce, C.; de Bustros, P.; Bouchard, C.S. Assessing the ocular surface microbiome in severe ocular surface diseases. Ocul. Surf. 2020, 18, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Willis, K.A.; Postnikoff, C.K.; Freeman, A.; Rezonzew, G.; Nichols, K.; Gaggar, A.; Lal, C.V. The closed eye harbours a unique microbiome in dry eye disease. Sci. Rep. 2020, 10, 12035. [Google Scholar] [CrossRef]
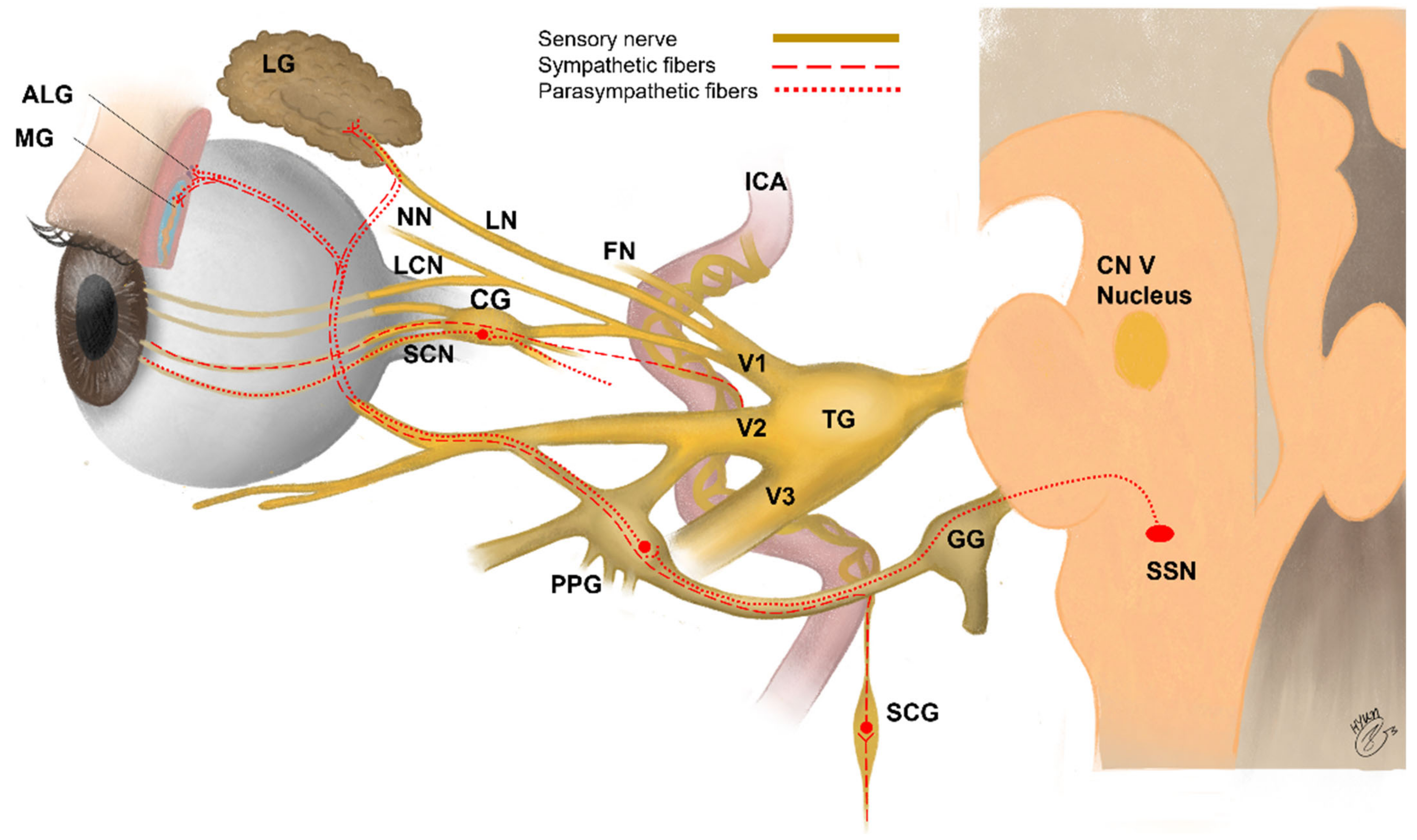
| Function | Nerve | Location of Nerve Cell Bodies | Main Distribution of Nerve | Minor Distribution of Nerve |
|---|---|---|---|---|
| Sensory nerves | Trigeminal nerve (CN V) Ophthalmic branch (CN V1) Maxillary branch (CN V2) | Trigeminal ganglion | Cornea (CN V1) [46] Conjunctiva (CN V1) [189] Eyelid (CN V1, V2) [190] | Lacrimal gland [191] Meibomian gland [192] |
| Parasympathetic nerves | Facial nerve (CN VII) | Pterygopalatine ganglion | Meibomian glands [192] Lacrimal gland [191] Conjunctiva [189] | Cornea [46] |
| Sympathetic nerves | Lateral horn to superior cervical ganglia | Superior cervical ganglion | Conjunctiva [189] Lacrimal gland [191] | Cornea [46] |
| Frequency of Nerve Fibers Expressing Each Neuropeptide According to Species | ||||
|---|---|---|---|---|
| SP (+) | CGRP (+) | NPY (+) | VIP (+) | |
| Cornea | Rabbit, cat, monkey: deep limbus/peripheral cornea > just beneath corneal epithelium, Rat: present in epithelium, stroma, and limbus | Rhesus monkey, human: around superficial limbal blood vessel/anterior stroma > epithelium, Rat: present in epithelium, stroma, and limbus | Rat, guinea pig, cat, rhesus monkey: around limbal blood vessel only, Rat: present in limbus and anterior stroma | Human: limited to limbal blood vessel (not in cornea proper), Rabbit: present *, Rat: present in limbus and anterior stroma |
| Ref. | [200,201,202] | [200,203] | [200,204] | [200,201,205] |
| Conjunctiva | Rat: present infrequently in epithelium, substantia propria close to vessels, and glands, Rabbit: present* | Rat: present moderately, mucocutaneous junction > epithelium/substantia propria/stroma in palpebral conjunctiva; present close to vessels and glands in bulbar conjunctiva | Rat: present moderately, scattered in parenchyma close to vessels and glands | Rat: present numerously, Human, mouse, rat, rabbit: present in epithelium and adjacent to goblet cells |
| Ref. | [201,206] | [206,207] | [206] | [201,206,208,209,210] |
| Lacrimal gland | Rat, guinea pig: duct system/blood vessel > interstitial space | Human, mouse: present rarely, Human: present in interlobular connective tissue, around blood vessels, and duct system | Human: present rarely, wall of arterioles > interlobular connective tissue | Human, mouse, rat, guinea pig: acini/around vessel > duct system |
| Ref. | [211] | [212,213] | [212] | [211,212,213] |
| Meibomian gland | Human: present sparsely, vessel > acini, Rhesus/cynomolgus monkeys, guinea pig: present in acini and vessel Rat: present sparsely, acini > vessel | Human: acini > vessel, rhesus/cynomolgus monkeys, rat, guinea pig: vessel > acini | Human, rhesus/cynomolgus monkeys, rat, guinea pig: present in vessel and acini | Human, rhesus/cynomolgus monkeys, rat: acini > vessels, Guinea pig: present in acini and vessels |
| Ref. | [192] | [192] | [192] | [192] |
| Related Neuropeptide | Species/Models | Administration Agent | Route of Administration | Highlights | Ref. |
|---|---|---|---|---|---|
| SP | Mice CEC dry-eye model | NK1R antagonist (1 μg/μL) (1) CP-99,994 (2) L-733,060 | Topical | NK1R antagonist decreased the frequency of mature APCs in cornea and draining LNs, where APCs prime naïve CD4+ T cells to differentiate into Th17 cells. | [218] |
| SP | Mice CEC dry-eye model | NK1R antagonist (36 μg/100 μL) Spantide I | Intraperitoneal | NK1R antagonist ameliorated dry eye through restoration of Treg function and suppression of Th17-mediated responses. | [220] |
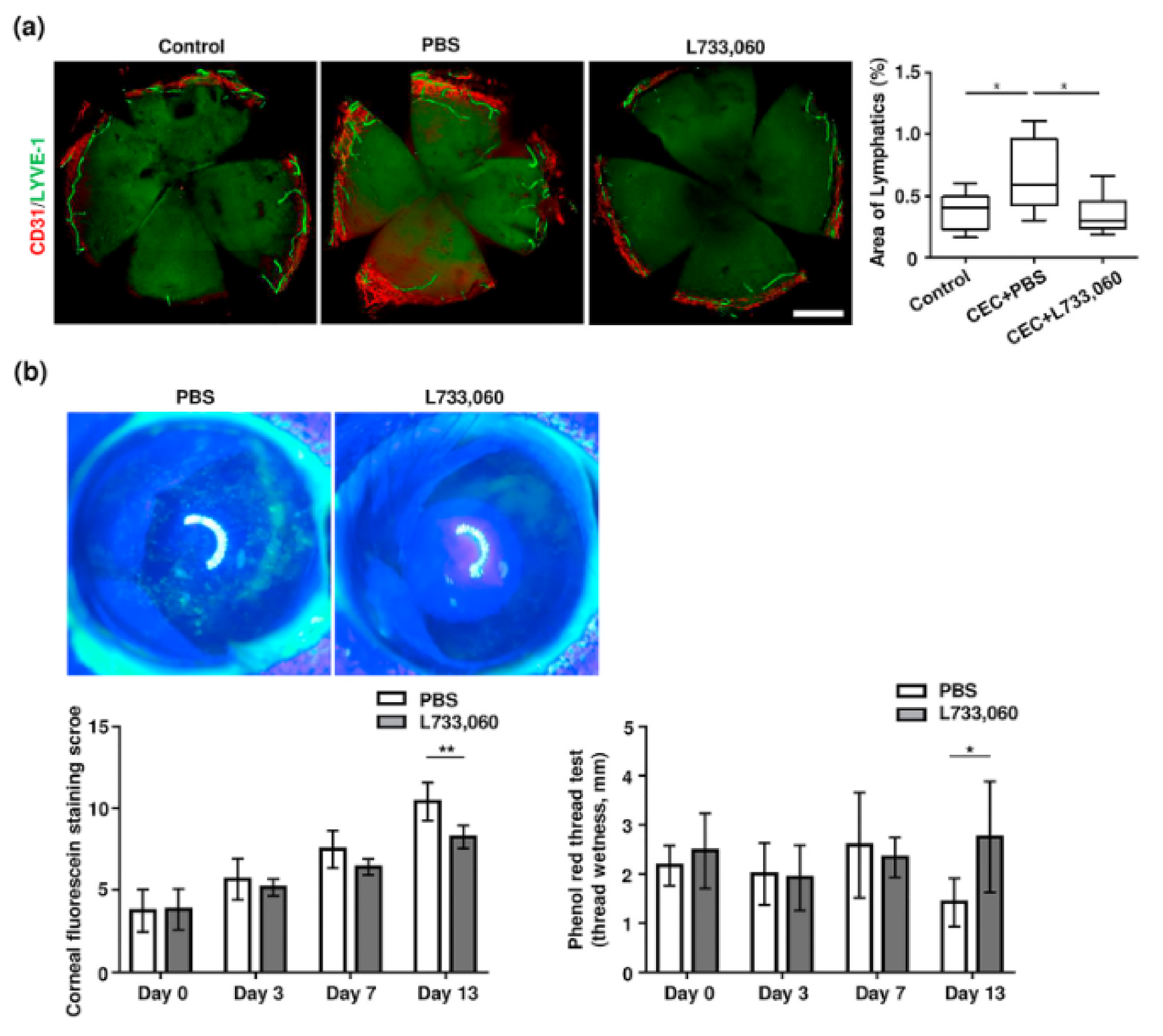
| SP | Mice CEC dry-eye model | NK1R antagonist (6.6 μg/5 μL) L-733,060 | Subconjunctival | NK1R antagonism reduced clinical signs and corneal lymphangiogenesis in dry eye. | [221] |
| SP | Mice CEC dry-eye model | - | - | Cultivated trigeminal ganglion neurons harvested from dry eye-induced mice expressed and secreted higher levels of SP. | [219] |
| SP, CGRP | Mice (TSP-1 deficient) Sjögren’s type aqueous-deficient dry-eye model | - | - | CGRP substantially decreased but SP did not change in the cornea of older TSP-1 deficient mice. However, it has not been established how loss of TSP-1 alters the level of corneal neuropeptides. | [222] |
| α-MSH | Rat Scopolamine-induced dry-eye model | α-MSH (1, 10, 100 μg/mL) | Topical | α-MSH ameliorated ocular surface dysfunction and reduced proinflammatory cytokines by activating the PKA-ERK pathway in the scopolamine-induced dry eye rats. | [223] |
| PACAP, VIP | Mice (PACAP null) non-Sjogren’s type aqueous-deficient dry-eye model | PACAP38 (1 fM~1 nM) PACAP27 (0.1 nM) VIP (0.1 nmM or 10 nM) PACAP receptor antagonist (PACAP6-38) VIP receptor antagonist (VIP6-28) | Topical | PACAP eye drops stimulated tear secretion in the infraorbital lacrimal glands via the PAC1R/AC/cAMP/PKA/AQP5 cascade and suppressed dry eye signs. | [224] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, D.D.-J.; Lee, S.-J.; Kim, J.-H.; Lee, S.-M. The Role of Neuropeptides in Pathogenesis of Dry Eye. J. Clin. Med. 2021, 10, 4248. https://doi.org/10.3390/jcm10184248
Hwang DD-J, Lee S-J, Kim J-H, Lee S-M. The Role of Neuropeptides in Pathogenesis of Dry Eye. Journal of Clinical Medicine. 2021; 10(18):4248. https://doi.org/10.3390/jcm10184248
Chicago/Turabian StyleHwang, Daniel Duck-Jin, Seok-Jae Lee, Jeong-Hun Kim, and Sang-Mok Lee. 2021. "The Role of Neuropeptides in Pathogenesis of Dry Eye" Journal of Clinical Medicine 10, no. 18: 4248. https://doi.org/10.3390/jcm10184248
APA StyleHwang, D. D.-J., Lee, S.-J., Kim, J.-H., & Lee, S.-M. (2021). The Role of Neuropeptides in Pathogenesis of Dry Eye. Journal of Clinical Medicine, 10(18), 4248. https://doi.org/10.3390/jcm10184248







