Ocular Surface Failure in Urban Syndrome
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wrnberg, J.; Cunningham, K.; Romeo, J.; Marcos, A. Physical activity, exercise and low-grade systemic inflammation. Proc. Nutr. Soc. 2010, 69, 400–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazeer, S.; Jansonius, N.; Snieder, H.; Hammond, C.; Vehof, J. The relationship between occupation and dry eye. Ocul. Surf. 2019, 17, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Courtin, R.; Pereira, B.; Naughton, G.; Chamoux, A.; Chiambaretta, F.; Lanhers, C.; Dutheil, F. Prevalence of dry eye disease in visual display terminal workers: A systematic review and meta-analysis. BMJ Open 2016, 6, 6–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonardi, A.; Lanier, B. Urban eye allergy syndrome: A new clinical entity? Curr. Med. Res. Opin. 2008, 24, 2295–2302. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Vessichelli, M.; Mariggiò, S.; Varone, A.; Zizza, P.; Di Santo, A.; Amore, C.; Dell’Elba, G.; Cutignano, A.; Fontana, A.; Cacciapuoti, C.; et al. The natural phosphoinositide derivative glycerophosphoinositol inhibits the lipopolysaccharide-induced inflammatory and thrombotic responses. J. Biol. Chem. 2017, 292, 12828–12841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cucullo, L.; Hallene, K.; Dini, G.; Dal Toso, R.; Janigro, D. Glycerophosphoinositol and dexamethasone improve transendothelial electrical resistance in an in vitro study of the blood-brain barrier. Brain Res. 2004, 997, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Micera, A.; Jirsova, K.; Esposito, G.; Balzamino, B.O.; Di Zazzo, A.; Bonini, S. Mast cells populate the corneoscleral limbus: New insights for our understanding of limbal microenvironment. Investig. Ophthalmol. Vis. Sci. 2020, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacchetti, M.; Lambiase, A.; Aronni, S.; Griggi, T.; Ribatti, V.; Bonini, S.; Bonini, S. Hyperosmolar conjunctival provocation for the evaluation of nonspecific hyperreactivity in healthy patients and patients with allergy. J. Allergy Clin. Immunol. 2006, 118, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Di Zazzo, A.; Micera, A.; Coassin, M.; Varacalli, G.; Foulsham, W.; De Piano, M.; Bonini, S. Inflammaging at ocular surface: Clinical and biomolecular analyses in healthy volunteers. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1769–1775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Zazzo, A.; Gaudenzi, D.; Yin, J.; Coassin, M.; Fernandes, M.; Dana, R.; Bonini, S. Corneal angiogenic privilege and its failure. Exp. Eye Res. 2021, 204, 108457. [Google Scholar] [CrossRef] [PubMed]
- Micera, A.; Di Zazzo, A.; Esposito, G.; Longo, R.; Foulsham, W.; Sacco, R.; Sgrulletta, R.; Bonini, S. Age-related changes to human tear composition. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2024–2031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Zazzo, A.; Coassin, M.; Micera, A.; Mori, T.; De Piano, M.; Scartozzi, L.; Sgrulletta, R.; Bonini, S. Ocular surface diabetic disease: A neurogenic condition? Ocul. Surf. 2021, 19, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Di Zazzo, A.; Yang, W.; Coassin, M.; Micera, A.; Antonini, M.; Piccinni, F.; De Piano, M.; Kohler, I.; Harms, A.C.; Hankemeier, T.; et al. Signaling lipids as diagnostic biomarkers for ocular surface cicatrizing conjunctivitis. J. Mol. Med. 2020, 98, 751–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuang, J.; Shih, K.C.; Chan, T.C.; Wan, K.H.; Jhanji, V.; Tong, L. Preoperative optimization of ocular surface disease before cataract surgery. J. Cataract Refract. Surg. 2017, 43, 1596–1607. [Google Scholar] [CrossRef] [PubMed]
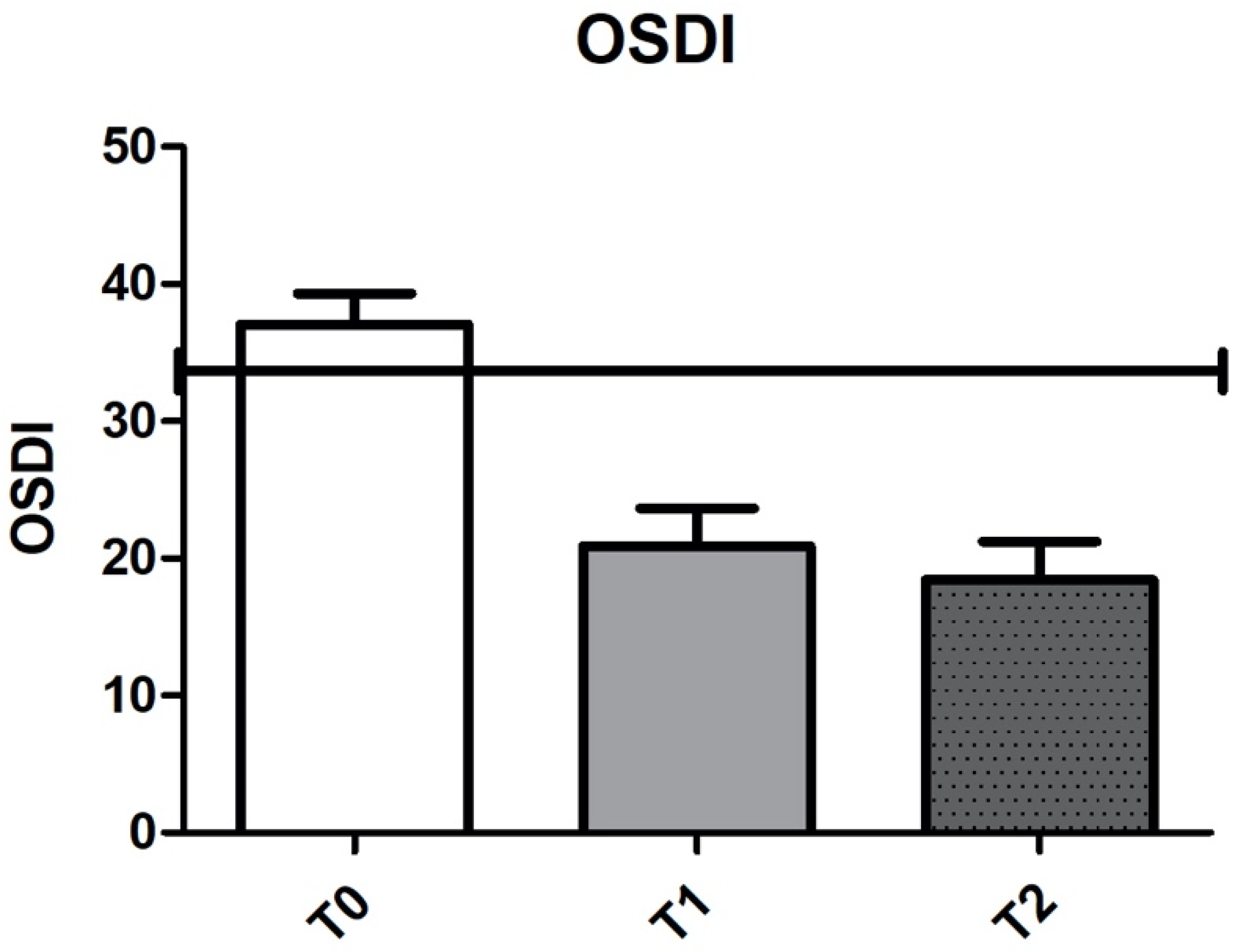

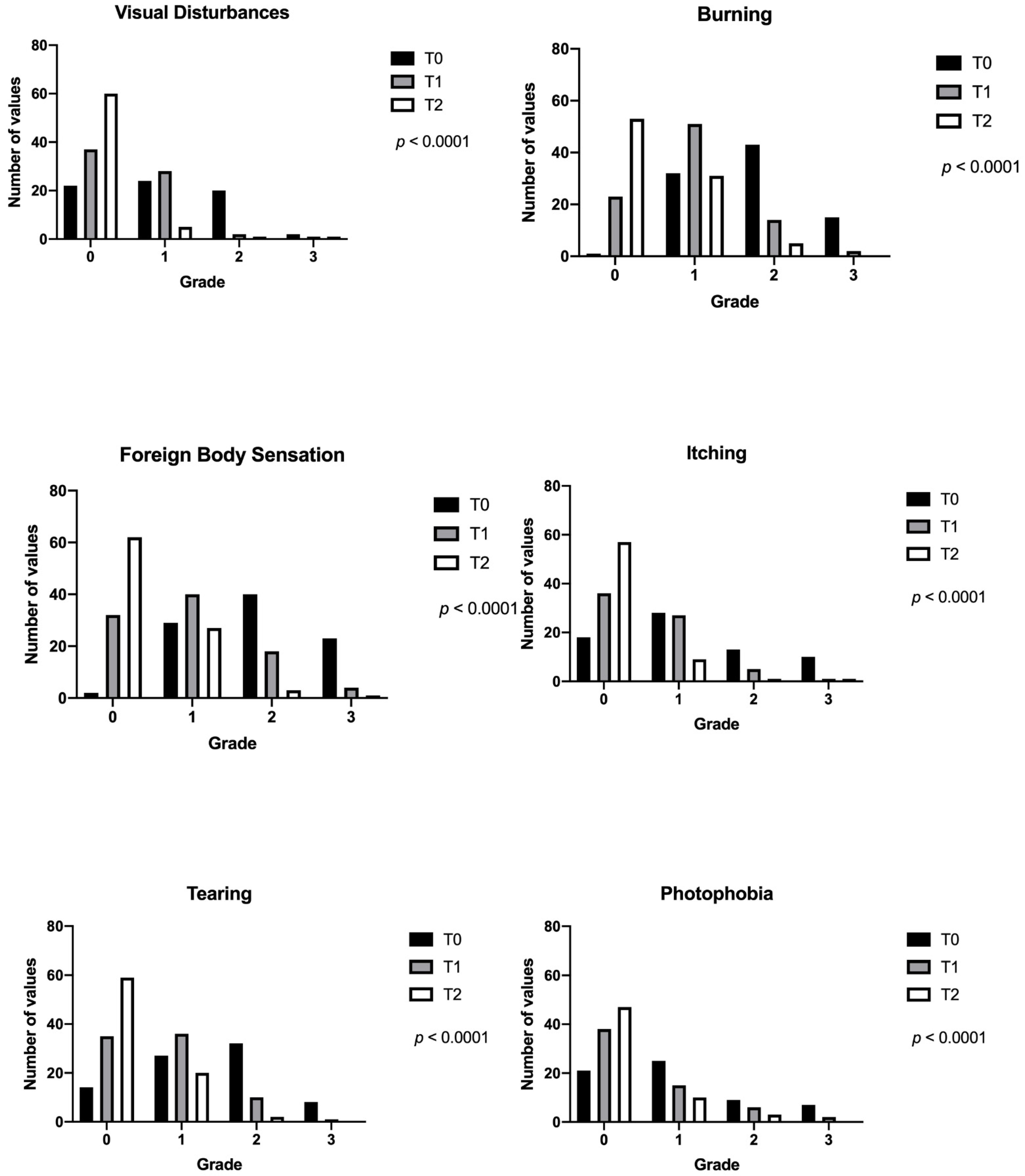
| Enrolment Visit (T0) | 1 Week (T1) | 1 Month (T2) | p | |
|---|---|---|---|---|
| OSDI | 39.9 ± 19 | 20.8 ± 17.9 | 18.4 ± 15.6 | <0.0001 |
| T-BUT | 5.2 ± 2 | 7.7 ± 2.2 | 9.7 ± 1.8 | <0.0001 |
| Schirmer test type 1 | 6.6 ± 2.4 | 9.7 ± 2.7 | 12.6 ± 2.6 | <0.0001 |
| Oxford score | 4.8 ± 3.6 | 4 ± 3.9 | 2.7 ± 3.5 | <0.0001 |
| Hyperemia score | 1.8 ± 0.8 | 1.2 ± 0.8 | 0.5 ± 0.7 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonini, M.; Gaudenzi, D.; Spelta, S.; Sborgia, G.; Poddi, M.; Micera, A.; Sgrulletta, R.; Coassin, M.; Di Zazzo, A. Ocular Surface Failure in Urban Syndrome. J. Clin. Med. 2021, 10, 3048. https://doi.org/10.3390/jcm10143048
Antonini M, Gaudenzi D, Spelta S, Sborgia G, Poddi M, Micera A, Sgrulletta R, Coassin M, Di Zazzo A. Ocular Surface Failure in Urban Syndrome. Journal of Clinical Medicine. 2021; 10(14):3048. https://doi.org/10.3390/jcm10143048
Chicago/Turabian StyleAntonini, Marco, Daniele Gaudenzi, Sara Spelta, Giancarlo Sborgia, Maria Poddi, Alessandra Micera, Roberto Sgrulletta, Marco Coassin, and Antonio Di Zazzo. 2021. "Ocular Surface Failure in Urban Syndrome" Journal of Clinical Medicine 10, no. 14: 3048. https://doi.org/10.3390/jcm10143048
APA StyleAntonini, M., Gaudenzi, D., Spelta, S., Sborgia, G., Poddi, M., Micera, A., Sgrulletta, R., Coassin, M., & Di Zazzo, A. (2021). Ocular Surface Failure in Urban Syndrome. Journal of Clinical Medicine, 10(14), 3048. https://doi.org/10.3390/jcm10143048








