Role of Immune Cell Diversity and Heterogeneity in Corneal Graft Survival: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Database Retrieval and Search Strategy
2.2. Data Extraction
2.3. Statistical Analysis
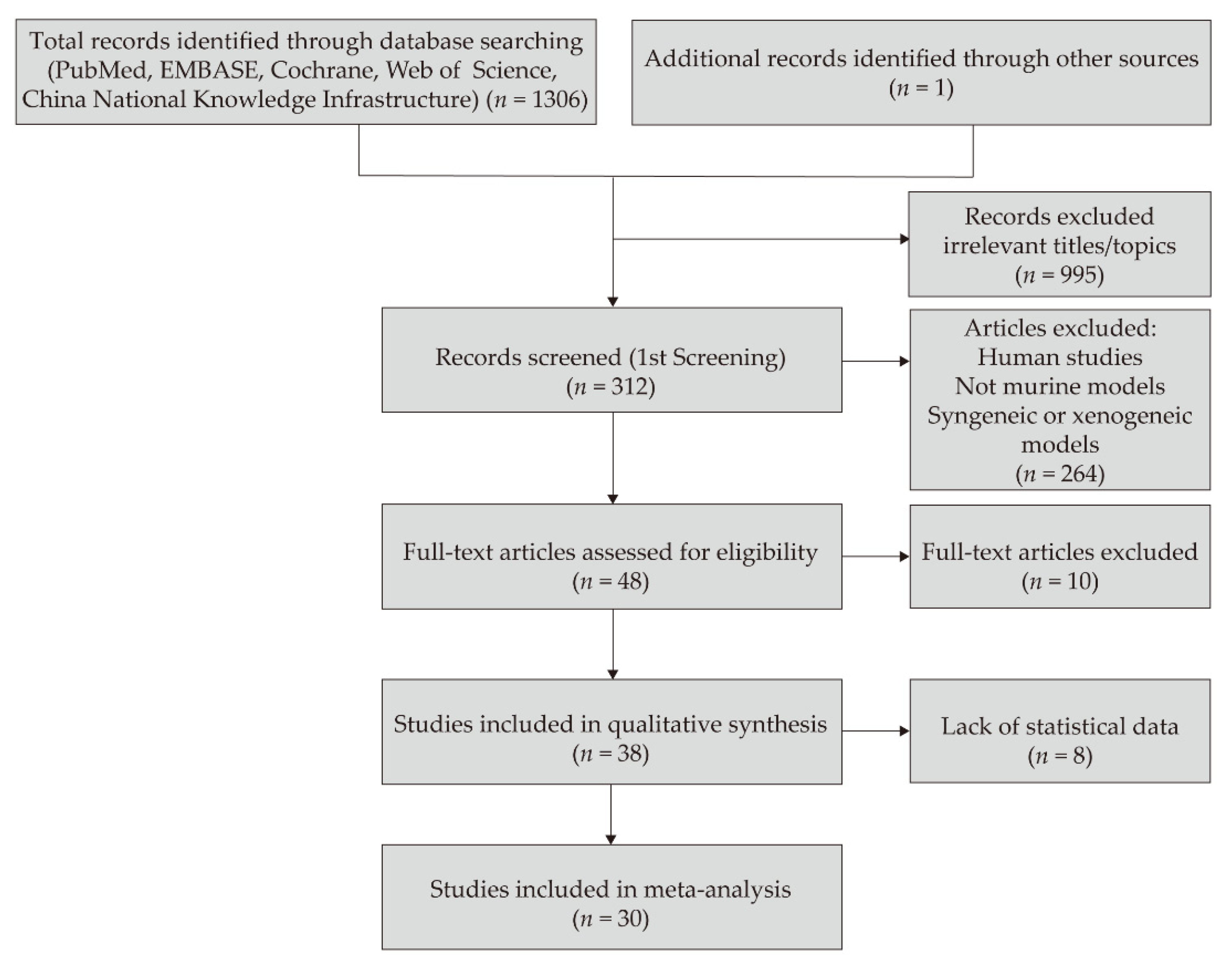
3. Results
3.1. Study Characteristics
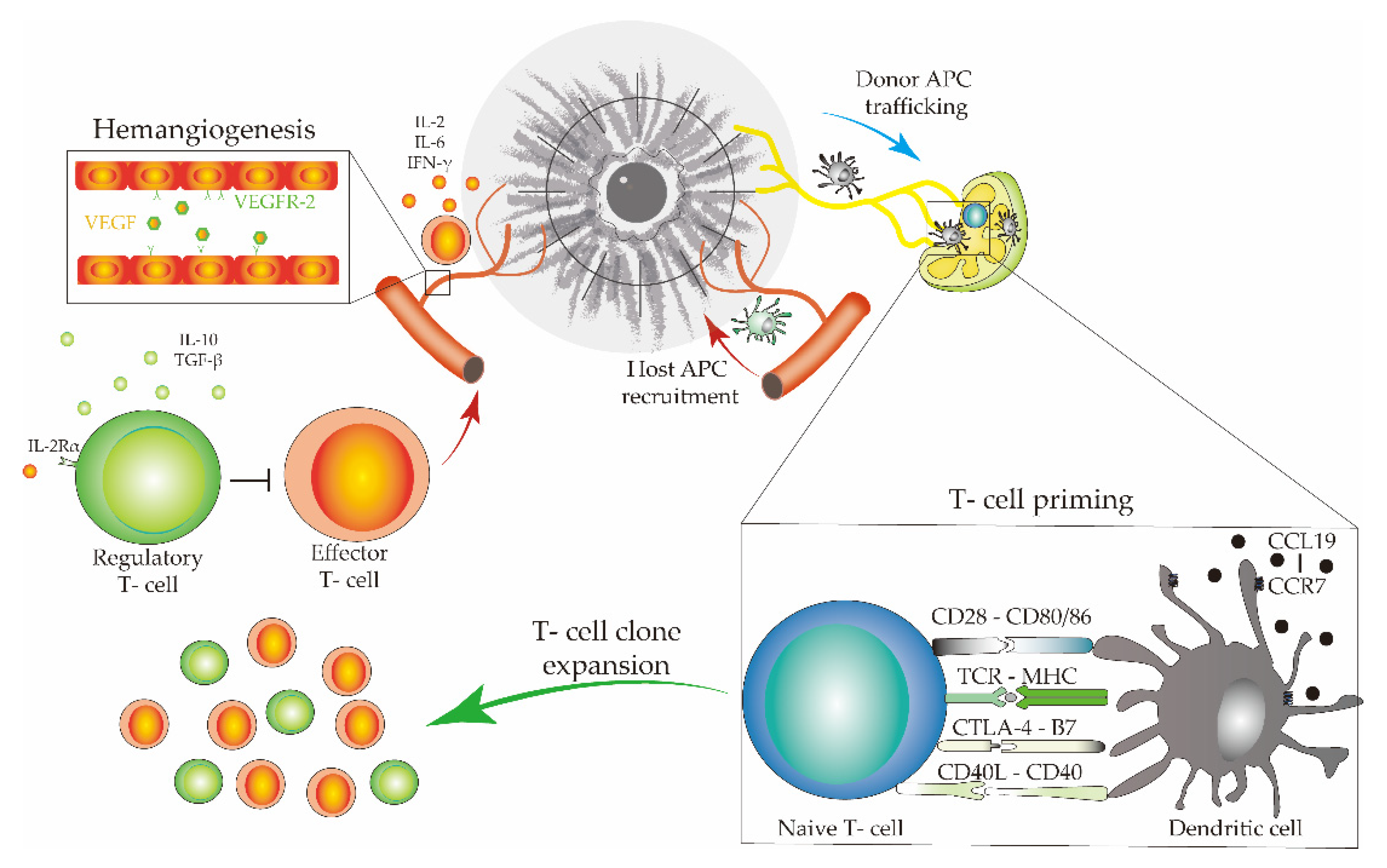
3.2. Immune Cells, Cytokines, and Pathways Associated with Allograft Rejection and Survival
3.2.1. T-Cell Subsets in Corneal-Allograft Rejection
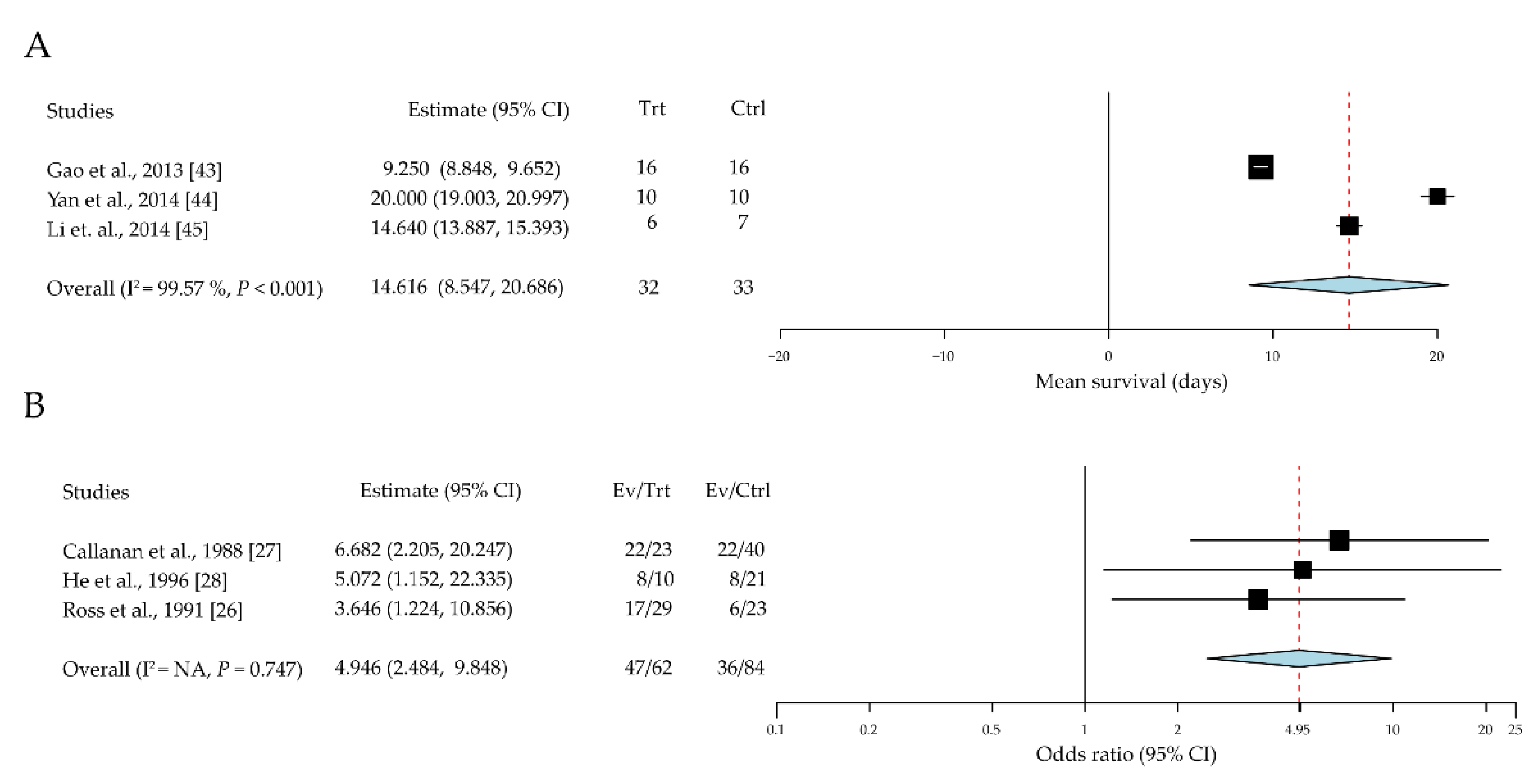
3.2.2. Dendritic Cell Heterogeneity in Different Roles of Corneal Allograft Tolerance
3.2.3. Macrophages Contribute to the Immunopathogenesis of Corneal-Graft Rejection
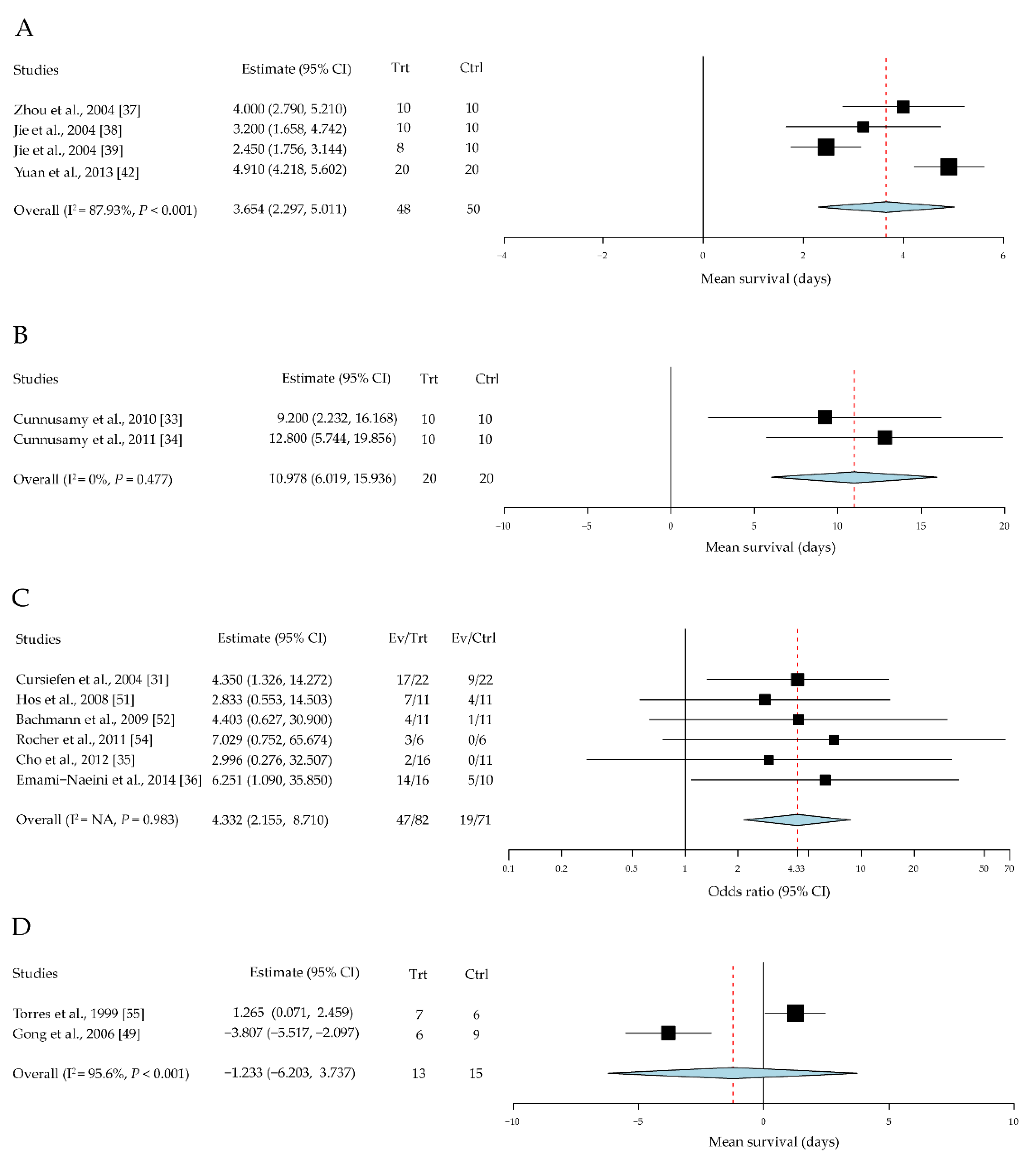
3.2.4. Cytokine Diversity in the Regulation of Corneal-Allograft Rejection
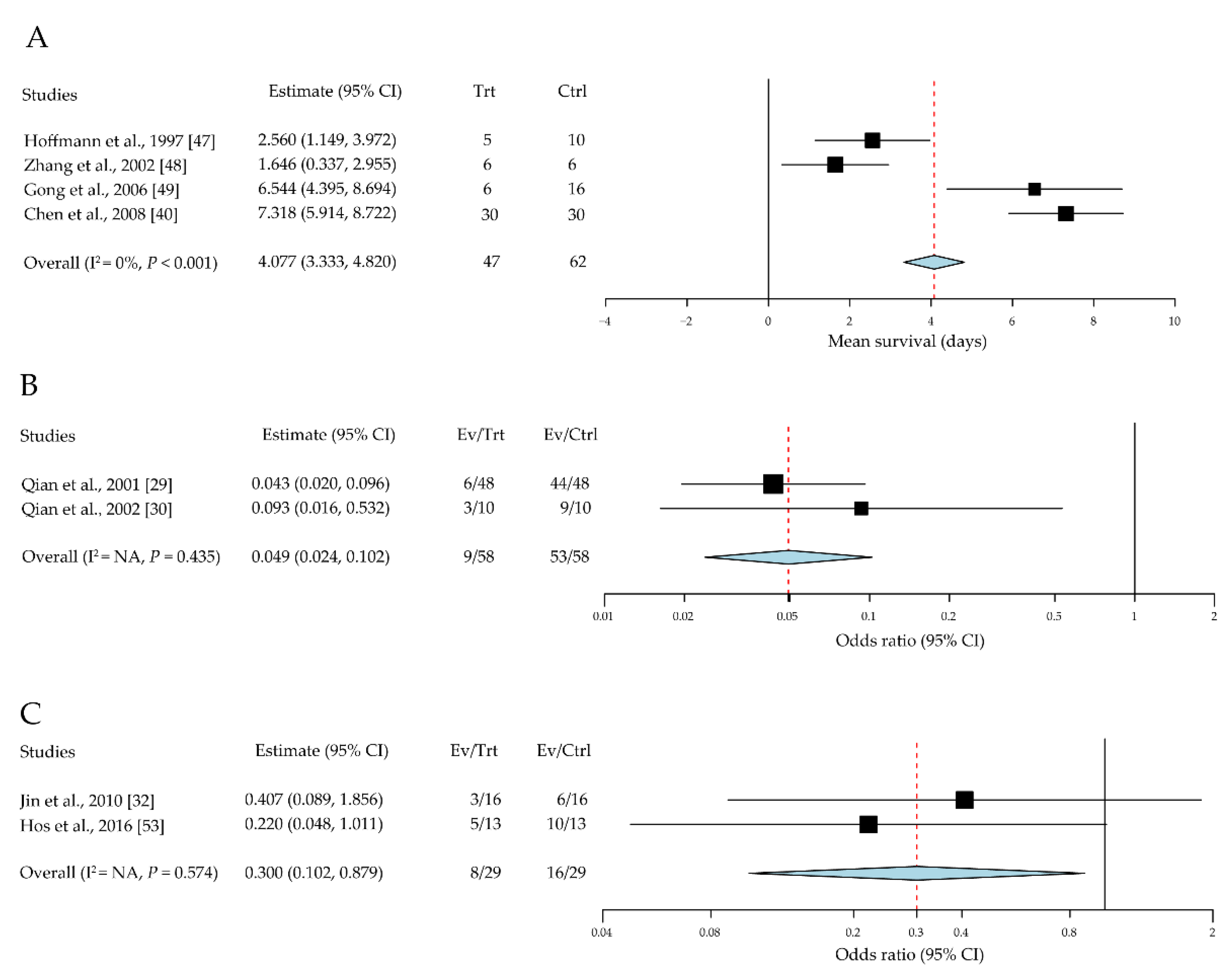
3.2.5. Co-Stimulatory Pathways in Corneal-Allograft Survival
3.2.6. Regulatory T Cells (Tregs) Promote Corneal Allograft Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mathews, P.M.; Lindsley, K.; Aldave, A.J.; Akpek, E.K. Etiology of Global Corneal Blindness and Current Practices of Corneal Transplantation: A Focused Review. Cornea 2018, 37, 1198–1203. [Google Scholar] [CrossRef]
- Williams, K.A.; Muehlberg, S.M.; Lewis, R.F.; Coster, D.J. How successful is corneal transplantation? A report from the Australian Corneal Graft Register. Eye 1995, 9 Pt 2, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Napoli, P.E.; Nioi, M.; d’Aloja, E.; Fossarello, M. The Bull’s Eye Pattern of the Tear Film in Humans during Visual Fixation on En-Face Optical Coherence Tomography. Sci. Rep. 2019, 9, 1413. [Google Scholar] [CrossRef] [PubMed]
- Napoli, P.E.; Nioi, M.; Gabiati, L.; Laurenzo, M.; De-Giorgio, F.; Scorcia, V.; Grassi, S.; d’Aloja, E.; Fossarello, M. Repeatability and reproducibility of post-mortem central corneal thickness measurements using a portable optical coherence tomography system in humans: A prospective multicenter study. Sci. Rep. 2020, 10, 14508. [Google Scholar] [CrossRef] [PubMed]
- Coster, D.J.; Williams, K.A. The impact of corneal allograft rejection on the long-term outcome of corneal transplantation. Am. J. Ophthalmol. 2005, 140, 1112–1122. [Google Scholar] [CrossRef]
- Naacke, H.G.; Borderie, V.M.; Bourcier, T.; Touzeau, O.; Moldovan, M.; Laroche, L. Outcome of Corneal transplantation rejection. Cornea 2001, 20, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Di Zazzo, A.; Gaudenzi, D.; Yin, J.; Coassin, M.; Fernandes, M.; Dana, R.; Bonini, S. Corneal angiogenic privilege and its failure. Exp. Eye Res. 2021, 204, 108457. [Google Scholar] [CrossRef] [PubMed]
- Hori, J.; Yamaguchi, T.; Keino, H.; Hamrah, P.; Maruyama, K. Immune privilege in corneal transplantation. Prog. Retin. Eye Res. 2019, 72, 100758. [Google Scholar] [CrossRef]
- Keino, H.; Horie, S.; Sugita, S. Immune Privilege and Eye-Derived T-Regulatory Cells. J. Immunol. Res. 2018, 2018. [Google Scholar] [CrossRef]
- Niederkorn, J.Y. Corneal Transplantation and Immune Privilege. Int. Rev. Immunol. 2013, 32, 57–67. [Google Scholar] [CrossRef]
- Subbannayya, Y.; Pinto, S.M.; Mohanty, V.; Dagamajalu, S.; Prasad, T.S.K.; Murthy, K.R. What Makes Cornea Immunologically Unique and Privileged? Mechanistic Clues from a High-Resolution Proteomic Landscape of the Human Cornea. Omics A J. Integr. Biol. 2020, 24, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Artifoni, M.; Rothschild, P.R.; Brezin, A.; Guillevin, L.; Puechal, X. Ocular inflammatory diseases associated with rheumatoid arthritis. Nat. Rev. Rheumatol. 2014, 10, 108–116. [Google Scholar] [CrossRef]
- Yazici, A.T.; Kara, N.; Yuksel, K.; Altinkaynak, H.; Baz, O.; Bozkurt, E.; Demirok, A. The biomechanical properties of the cornea in patients with systemic lupus erythematosus. Eye 2011, 25, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Hamrah, P.; Huq, S.O.; Liu, Y.; Zhang, Q.; Dana, M.R. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J. Leukoc. Biol. 2003, 74, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Randleman, J.B.; Stulting, R.D. Prevention and treatment of corneal graft rejection: Current practice patterns (2004). Cornea 2006, 25, 286–290. [Google Scholar] [CrossRef]
- Borel, J.F.; Feurer, C.; Magnee, C.; Stahelin, H. Effects of the new anti-lymphocytic peptide cyclosporin A in animals. Immunology 1977, 32, 1017–1025. [Google Scholar]
- Abudou, M.; Wu, T.; Evans, J.R.; Chen, X. Immunosuppressants for the prophylaxis of corneal graft rejection after penetrating keratoplasty. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Birnbaum, F.; Reis, A.; Bohringer, D.; Sokolowska, Y.; Mayer, K.; Voiculescu, A.; Oellerich, M.; Sundmacher, R.; Reinhard, T. An open prospective pilot study on the use of rapamycin after penetrating high-risk keratoplasty. Transplantation 2006, 81, 767–772. [Google Scholar] [CrossRef]
- Stanbury, R.M.; Graham, E.M. Systemic corticosteroid therapy—Side effects and their management. Br. J. Ophthalmol. 1998, 82, 704–708. [Google Scholar] [CrossRef]
- Tahvildari, M.; Emami-Naeini, P.; Omoto, M.; Mashaghi, A.; Chauhan, S.K.; Dana, R. Treatment of donor corneal tissue with immunomodulatory cytokines: A novel strategy to promote graft survival in high-risk corneal transplantation. Sci. Rep. 2017, 7, 971. [Google Scholar] [CrossRef]
- Sandhu, H.S.; Hemmati, H.D.; Dana, R. Immune checkpoint inhibitors and corneal transplant rejection: A call for awareness. Immunotherapy 2020, 12, 947–949. [Google Scholar] [CrossRef]
- Boisjoly, H.M.; Roy, R.; Dube, I.; Laughrea, P.A.; Michaud, R.; Douville, P.; Heebert, J. HLA-A,B and DR matching in corneal transplantation. Ophthalmology 1986, 93, 1290–1297. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Inomata, T.; Kitazawa, K.; Kuno, T.; Sung, J.; Nakamura, M.; Iwagami, M.; Takagi, H.; Midorikawa-Inomata, A.; Zhu, J.; Fujimoto, K.; et al. Clinical and Prodromal Ocular Symptoms in Coronavirus Disease: A Systematic Review and Meta-Analysis. Investig. Ophthalmol. Vis. Sci. 2020, 61, 29. [Google Scholar] [CrossRef]
- Ross, J.; He, Y.G.; Pidherney, M.; Mellon, J.; Niederkorn, J.Y. The differential effects of donor versus host Langerhans cells in the rejection of MHC-matched corneal allografts. Transplantation 1991, 52, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Callanan, D.; Peeler, J.; Niederkorn, J.Y. Characteristics of rejection of orthotopic corneal allografts in the rat. Transplantation 1988, 45, 437–443. [Google Scholar] [CrossRef] [PubMed]
- He, Y.G.; Niederkorn, J.Y. Depletion of donor-derived Langerhans cells promotes corneal allograft survival. Cornea 1996, 15, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Boisgerault, F.; Benichou, G.; Dana, M.R. Blockade of CD40-CD154 costimulatory pathway promotes survival of allogeneic corneal transplants. Investig. Ophthalmol. Vis. Sci. 2001, 42, 987–994. [Google Scholar]
- Qian, Y.; Dana, M.R. Effect of locally administered anti-CD154 (CD40 ligand) monoclonal antibody on survival of allogeneic corneal transplants. Cornea 2002, 21, 592–597. [Google Scholar] [CrossRef]
- Cursiefen, C.; Cao, J.; Chen, L.; Liu, Y.; Maruyama, K.; Jackson, D.; Kruse, F.E.; Wiegand, S.J.; Dana, M.R.; Streilein, J.W. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2666–2673. [Google Scholar] [CrossRef]
- Jin, Y.; Chauhan, S.K.; Saban, D.R.; Dana, R. Role of CCR7 in facilitating direct allosensitization and regulatory T-cell function in high-risk corneal transplantation. Investig. Ophthalmol. Vis. Sci. 2010, 51, 816–821. [Google Scholar] [CrossRef]
- Cunnusamy, K.; Chen, P.W.; Niederkorn, J.Y. IL-17 promotes immune privilege of corneal allografts. J. Immunol. 2010, 185, 4651–4658. [Google Scholar] [CrossRef]
- Cunnusamy, K.; Chen, P.W.; Niederkorn, J.Y. IL-17A-dependent CD4+CD25+ regulatory T cells promote immune privilege of corneal allografts. J. Immunol. 2011, 186, 6737–6745. [Google Scholar] [CrossRef]
- Cho, Y.K.; Zhang, X.; Uehara, H.; Young, J.R.; Archer, B.; Ambati, B. Vascular Endothelial Growth Factor Receptor 1 morpholino increases graft survival in a murine penetrating keratoplasty model. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8458–8471. [Google Scholar] [CrossRef]
- Emami-Naeini, P.; Dohlman, T.H.; Omoto, M.; Hattori, T.; Chen, Y.; Lee, H.S.; Chauhan, S.K.; Dana, R. Soluble vascular endothelial growth factor receptor-3 suppresses allosensitization and promotes corneal allograft survival. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 1755–1762. [Google Scholar] [CrossRef]
- Zhou, J.; Lu, X.H.; Dang, S.T.; Bai, L.; Zhang, Y.Q.; Xu, N. Subconjunctival interleukin-1 receptor antagonist inhibits graft rejection following high-risk penetrating keratoplasty in rats. Di Yi Jun Yi Da Xue Xue Bao 2004, 24, 539–541. [Google Scholar] [PubMed]
- Jie, Y.; Zhang, W.H.; Pan, Z.Q.; Wu, Y.Y.; Wang, Y. Interleukin-1 receptor antagonist eye drops promoting high-risk corneal allografts survival in rats. Chin. Med. J. 2004, 117, 711–716. [Google Scholar] [PubMed]
- Jie, Y.; Pan, Z.; Chen, Y.; Wei, Y.; Zhang, W.; Xu, L.; Wu, Y.; Peng, H. SEB combined with IL-1ra could prolong the survival of the rat allografts in high-risk corneal transplantation. Transpl. Proc. 2004, 36, 3267–3271. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Shi, W.Y.; Wang, F.H.; Xie, L.X. CTLA4-FasL protein for the prevention of immune rejection in mouse corneal transplantation. Zhonghua Yan Ke Za Zhi 2008, 44, 56–60. [Google Scholar] [PubMed]
- He, Y.; Jie, Y.; Wang, B.; Zeng, H.; Zhang, Y.; Pan, Z. Adoptive transfer of donor corneal antigen-specific regulatory T cells can prolong mice corneal grafts survival. Cornea 2010, 29 (Suppl. 1), S25–S31. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Liu, Y.; Huang, W.; Zhou, S.; Ling, S.; Chen, J. The experimental treatment of corneal graft rejection with the interleukin-1 receptor antagonist (IL-1ra) gene. PLoS ONE 2013, 8, e60714. [Google Scholar] [CrossRef]
- Gao, X.W.; Fu, Y.; Li, W.J.; Du, A.J.; Li, X.; Zhao, X.D. Mechanism of immune tolerance induced by donor derived immature dendritic cells in rat high-risk corneal transplantation. Int. J. Ophthalmol. 2013, 6, 269–275. [Google Scholar] [CrossRef]
- Yan, F.; Cai, L.; Hui, Y.; Chen, S.; Meng, H.; Huang, Z. Tolerogenic dendritic cells suppress murine corneal allograft rejection by modulating CD28/CTLA-4 expression on regulatory T cells. Cell Biol. Int. 2014, 38, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Tian, L.; Diao, Y.; Li, X.; Zhao, L.; Wang, X. Exogenous IL-10 induces corneal transplantation immune tolerance by a mechanism associated with the altered Th1/Th2 cytokine ratio and the increased expression of TGF-beta. Mol. Med. Rep. 2014, 9, 2245–2250. [Google Scholar] [CrossRef]
- Xu, Q.; Tan, X.; Zhang, Y.; Jie, Y.; Pan, Z. Subconjunctival injection of in vitro transforming growth factor-beta-induced regulatory T cells prolongs allogeneic corneal graft survival in mice. Int. J. Clin. Exp. Med. 2015, 8, 20271–20278. [Google Scholar]
- Hoffmann, F.; Zhang, E.P.; Pohl, T.; Kunzendorf, U.; Wachtlin, J.; Bulfone-Paus, S. Inhibition of corneal allograft reaction by CTLA4-Ig. Graefes Arch. Clin. Exp. Ophthalmol. 1997, 235, 535–540. [Google Scholar] [CrossRef]
- Zhang, E.P.; Bulfone-Paus, S.; Hoffmann, F. Immunomodulation after keratoplasty by CTL4-Ig and anti-CD154 antibodies. Ophthalmologe 2002, 99, 183–187. [Google Scholar] [CrossRef]
- Gong, N.; Pleyer, U.; Yang, J.; Vogt, K.; Hill, M.; Anegon, I.; Volk, H.D.; Ritter, T. Influence of local and systemic CTLA4Ig gene transfer on corneal allograft survival. J. Gene Med. 2006, 8, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Gong, N.; Pleyer, U.; Volk, H.D.; Ritter, T. Effects of local and systemic viral interleukin-10 gene transfer on corneal allograft survival. Gene 2007, 14, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Hos, D.; Bock, F.; Dietrich, T.; Onderka, J.; Kruse, F.E.; Thierauch, K.H.; Cursiefen, C. Inflammatory corneal (lymph)angiogenesis is blocked by VEGFR-tyrosine kinase inhibitor ZK 261991, resulting in improved graft survival after corneal transplantation. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1836–1842. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, B.O.; Luetjen-Drecoll, E.; Bock, F.; Wiegand, S.J.; Hos, D.; Dana, R.; Kruse, F.E.; Cursiefen, C. Transient postoperative vascular endothelial growth factor (VEGF)-neutralisation improves graft survival in corneas with partly regressed inflammatory neovascularisation. Br. J. Ophthalmol. 2009, 93, 1075–1080. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hos, D.; Dorrie, J.; Schaft, N.; Bock, F.; Notara, M.; Kruse, F.E.; Krautwald, S.; Cursiefen, C.; Bachmann, B.O. Blockade of CCR7 leads to decreased dendritic cell migration to draining lymph nodes and promotes graft survival in low-risk corneal transplantation. Exp. Eye Res. 2016, 146, 1–6. [Google Scholar] [CrossRef]
- Rocher, N.; Behar-Cohen, F.; Pournaras, J.A.; Naud, M.C.; Jeanny, J.C.; Jonet, L.; Bourges, J.L. Effects of rat anti-VEGF antibody in a rat model of corneal graft rejection by topical and subconjunctival routes. Mol. Vis. 2011, 17, 104–112. [Google Scholar] [PubMed]
- Torres, P.F.; de Vos, A.F.; Martins, B.; Kijlstra, A. Interleukin 10 treatment does not prolong experimental corneal allograft survival. Ophthalmic Res. 1999, 31, 297–303. [Google Scholar] [CrossRef]
- Krieger, N.R.; Fathman, C.G. The use of CD4 and CD8 knockout mice to study the role of T-cell subsets in allotransplant rejection. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 1997, 16, 263–267. [Google Scholar]
- Haskova, Z.; Usiu, N.; Pepose, J.S.; Ferguson, T.A.; Stuart, P.M. CD4+ T cells are critical for corneal, but not skin, allograft rejection. Transplantation 2000, 69, 483–487. [Google Scholar] [CrossRef]
- Appleby, S.L.; Jessup, C.F.; Mortimer, L.A.; Kirk, K.; Brereton, H.M.; Coster, D.J.; Tan, C.K.; Williams, K.A. Expression of an anti-CD4 single-chain antibody fragment from the donor cornea can prolong corneal allograft survival in inbred rats. Br. J. Ophthalmol. 2013, 97, 101–105. [Google Scholar] [CrossRef]
- Ayliffe, W.; Alam, Y.; Bell, E.B.; McLeod, D.; Hutchinson, I.V. Prolongation of rat corneal graft survival by treatment with anti-CD4 monoclonal antibody. Br. J. Ophthalmol. 1992, 76, 602–606. [Google Scholar] [CrossRef][Green Version]
- Coupland, S.E.; Krause, L.; Karow, A.C.; Bartlett, R.R.; Lehmann, M.; Hoffmann, F. Delay in corneal allograft rejection due to anti-CD4 antibody given alone and in combination with cyclosporin A and leflunomide. Ger. J. Ophthalmol. 1995, 4, 294–301. [Google Scholar]
- He, Y.G.; Ross, J.; Niederkorn, J.Y. Promotion of murine orthotopic corneal allograft survival by systemic administration of anti-CD4 monoclonal antibody. Investig. Ophthalmol. Vis. Sci. 1991, 32, 2723–2728. [Google Scholar]
- Li, S.; Lu, H.; Sella, R.; Zhang, W.; Dong, H.; Guo, C.; Afshari, N.A.; Pan, Z.; Jie, Y. The Effects of Anti-LAP Monoclonal Antibody Down-regulation of CD4+LAP+ T Cells on Allogeneic Corneal Transplantation in Mice. Sci. Rep. 2018, 8, 8021. [Google Scholar] [CrossRef] [PubMed]
- Pleyer, U.; Milani, J.K.; Dukes, A.; Chou, J.; Lutz, S.; Rückert, D.; Thiel, H.J.; Mondino, B.J. Effect of topically applied anti-CD4 monoclonal antibodies on orthotopic corneal allografts in a rat model. Investig. Ophthalmol. Vis. Sci. 1995, 36, 52–61. [Google Scholar]
- Hegde, S.; Niederkorn, J.Y. The role of cytotoxic T lymphocytes in corneal allograft rejection. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3341–3347. [Google Scholar]
- Niederkorn, J.Y.; Stevens, C.; Mellon, J.; Mayhew, E. Differential roles of CD8+ and CD8- T lymphocytes in corneal allograft rejection in ‘high-risk’ hosts. Am. J. Transpl. 2006, 6, 705–713. [Google Scholar] [CrossRef]
- Maruyama, K.; Nakazawa, T.; Cursiefen, C.; Maruyama, Y.; Van Rooijen, N.; D’Amore, P.A.; Kinoshita, S. The maintenance of lymphatic vessels in the cornea is dependent on the presence of macrophages. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3145–3153. [Google Scholar] [CrossRef]
- Oh, J.Y.; Lee, H.J.; Ko, A.Y.; Ko, J.H.; Kim, M.K.; Wee, W.R. Analysis of macrophage phenotype in rejected corneal allografts. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7779–7784. [Google Scholar] [CrossRef]
- Slegers, T.P.; Broersma, L.; van Rooijen, N.; Hooymans, J.M.; van Rij, G.; van der Gaag, R. Macrophages play a role in the early phase of corneal allograft rejection in rats. Transplantation 2004, 77, 1641–1646. [Google Scholar] [CrossRef]
- Slegers, T.P.; Torres, P.F.; Broersma, L.; van Rooijen, N.; van Rij, G.; van der Gaag, R. Effect of macrophage depletion on immune effector mechanisms during corneal allograft rejection in rats. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2239–2247. [Google Scholar]
- Slegers, T.P.; van der Gaag, R.; van Rooijen, N.; van Rij, G.; Streilein, J.W. Effect of local macrophage depletion on cellular immunity and tolerance evoked by corneal allografts. Curr. Eye Res. 2003, 26, 73–79. [Google Scholar] [CrossRef]
- Yamada, J.; Maruyama, K.; Sano, Y.; Kinoshita, S.; Murata, Y.; Hamuro, J. Promotion of corneal allograft survival by the induction of oxidative macrophages. Investig. Ophthalmol. Vis. Sci. 2004, 45, 448–454. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tahvildari, M.; Inomata, T.; Amouzegar, A.; Dana, R. Regulatory T cell modulation of cytokine and cellular networks in corneal graft rejection. Curr. Ophthalmol. Rep. 2018, 6, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Di Zazzo, A.; Tahvildari, M.; Subbarayal, B.; Yin, J.; Dohlman, T.H.; Inomata, T.; Mashaghi, A.; Chauhan, S.K.; Dana, R. Proangiogenic Function of T Cells in Corneal Transplantation. Transplantation 2017, 101, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Artis, D.; Spits, H. The biology of innate lymphoid cells. Nature 2015, 517, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.P.; Mills, K.H. Dendritic cells and other innate determinants of T helper cell polarisation. Trends Immunol. 2013, 34, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Inomata, T.; Fujimoto, K.; Uchida, K.; Fujio, K.; Nagino, K.; Miura, M.; Negishi, N.; Okumura, Y.; Akasaki, Y.; et al. Ex Vivo-Induced Bone Marrow-Derived Myeloid Suppressor Cells Prevent Corneal Allograft Rejection in Mice. Investig. Ophthalmol. Vis. Sci. 2021, 62, 3. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Steinman, R.M. Dendritic cells: Specialized and regulated antigen processing machines. Cell 2001, 106, 255–258. [Google Scholar] [CrossRef]
- Inomata, T.; Hua, J.; Nakao, T.; Shiang, T.; Chiang, H.; Amouzegar, A.; Dana, R. Corneal Tissue From Dry Eye Donors Leads to Enhanced Graft Rejection. Cornea 2018, 37, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Inomata, T.; Fujimoto, K.; Okumura, Y.; Zhu, J.; Fujio, K.; Shokirova, H.; Miura, M.; Okano, M.; Funaki, T.; Sung, J.; et al. Novel immunotherapeutic effects of topically administered ripasudil (K-115) on corneal allograft survival. Sci. Rep. 2020, 10, 19817. [Google Scholar] [CrossRef]
- Hua, J.; Jin, Y.; Chen, Y.; Inomata, T.; Lee, H.; Chauhan, S.K.; Petasis, N.A.; Serhan, C.N.; Dana, R. The resolvin D1 analogue controls maturation of dendritic cells and suppresses alloimmunity in corneal transplantation. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5944–5951. [Google Scholar] [CrossRef]
- Sim, W.J.; Malinarich, F.; Fairhurst, A.M.; Connolly, J.E. Generation of Immature, Mature and Tolerogenic Dendritic Cells with Differing Metabolic Phenotypes. J. Vis. Exp. JoVE 2016, 10.3791/54128. [Google Scholar] [CrossRef] [PubMed]
- Collin, M.; Milne, P. Langerhans cell origin and regulation. Curr. Opin. Hematol. 2016, 23, 28–35. [Google Scholar] [CrossRef]
- Sano, Y.; Ksander, B.R.; Streilein, J.W. Fate of orthotopic corneal allografts in eyes that cannot support anterior chamber-associated immune deviation induction. Investig. Ophthalmol. Vis. Sci. 1995, 36, 2176–2185. [Google Scholar]
- Williamson, J.S.; DiMarco, S.; Streilein, J.W. Immunobiology of Langerhans cells on the ocular surface. I. Langerhans cells within the central cornea interfere with induction of anterior chamber associated immune deviation. Investig. Ophthalmol. Vis. Sci. 1987, 28, 1527–1532. [Google Scholar]
- Peeler, J.; Niederkorn, J.; Matoba, A. Corneal allografts induce cytotoxic T cell but not delayed hypersensitivity responses in mice. Investig. Ophthalmol. Vis. Sci. 1985, 26, 1516–1523. [Google Scholar]
- Yamaguchi, T.; Higa, K.; Yagi-Yaguchi, Y.; Ueda, K.; Noma, H.; Shibata, S.; Nagai, T.; Tomida, D.; Yasu-Mimura, R.; Ibrahim, O.; et al. Pathological processes in aqueous humor due to iris atrophy predispose to early corneal graft failure in humans and mice. Sci. Adv. 2020, 6, eaaz5195. [Google Scholar] [CrossRef]
- Fust, A.; Csuka, D.; Imre, L.; Bausz, M.; Nagymihaly, A.; Fust, G.; Csorvasi, A.; Nemeth, J.; Varga, L. The role of complement activation in the pathogenesis of Fuchs’ dystrophy. Mol. Immunol. 2014, 58, 177–181. [Google Scholar] [CrossRef]
- Kerenyi, A.; Nagy, G.; Veres, A.; Varga, L.; Fust, A.; Nagymihany, A.; Czumbel, N.; Suveges, I.; Fust, G. C1r-C1s-C1inhibitor (C1rs-C1inh) complex measurements in tears of patients before and after penetrating keratoplasty. Curr. Eye Res. 2002, 24, 99–104. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, L.; Jin, Y.; Saban, D.R.; Chauhan, S.K.; Dana, R. Depletion of passenger leukocytes from corneal grafts: An effective means of promoting transplant survival? Investig. Ophthalmol. Vis. Sci. 2009, 50, 3137–3144. [Google Scholar] [CrossRef]
- Islam, R.; Islam, M.M.; Nilsson, P.H.; Mohlin, C.; Hagen, K.T.; Paschalis, E.I.; Woods, R.L.; Bhowmick, S.C.; Dohlman, C.H.; Espevik, T.; et al. Combined blockade of complement C5 and TLR co-receptor CD14 synergistically inhibits pig-to-human corneal xenograft induced innate inflammatory responses. Acta Biomater. 2021, 127, 169–179. [Google Scholar] [CrossRef]
- Hua, J.; Stevenson, W.; Dohlman, T.H.; Inomata, T.; Tahvildari, M.; Calcagno, N.; Pirmadjid, N.; Sadrai, Z.; Chauhan, S.K.; Dana, R. Graft Site Microenvironment Determines Dendritic Cell Trafficking Through the CCR7-CCL19/21 Axis. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1457–1467. [Google Scholar] [CrossRef]
- Abe, M.; Zahorchak, A.F.; Colvin, B.L.; Thomson, A.W. Migratory responses of murine hepatic myeloid, lymphoid-related, and plasmacytoid dendritic cells to CC chemokines. Transplantation 2004, 78, 762–765. [Google Scholar] [CrossRef]
- Forster, R.; Schubel, A.; Breitfeld, D.; Kremmer, E.; Renner-Muller, I.; Wolf, E.; Lipp, M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 1999, 99, 23–33. [Google Scholar] [CrossRef]
- Bretscher, P.A. A two-step, two-signal model for the primary activation of precursor helper T cells. Proc. Natl. Acad. Sci. USA 1999, 96, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Iwata, M.; Soya, K.; Sawa, M.; Sakimoto, T.; Hwang, D.G. CD40 expression in normal human cornea and regulation of CD40 in cultured human corneal epithelial and stromal cells. Investig. Ophthalmol. Vis. Sci. 2002, 43, 348–357. [Google Scholar]
- van Kooten, C.; Banchereau, J. Functions of CD40 on B cells, dendritic cells and other cells. Curr. Opin. Immunol. 1997, 9, 330–337. [Google Scholar] [CrossRef]
- Graf, D.; Muller, S.; Korthauer, U.; van Kooten, C.; Weise, C.; Kroczek, R.A. A soluble form of TRAP (CD40 ligand) is rapidly released after T cell activation. Eur. J. Immunol. 1995, 25, 1749–1754. [Google Scholar] [CrossRef]
- Quezada, S.A.; Jarvinen, L.Z.; Lind, E.F.; Noelle, R.J. CD40/CD154 interactions at the interface of tolerance and immunity. Annu. Rev. Immunol. 2004, 22, 307–328. [Google Scholar] [CrossRef]
- Walker, L.S.; Sansom, D.M. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat. Rev. Immunol. 2011, 11, 852–863. [Google Scholar] [CrossRef] [PubMed]
- Nosov, M.; Wilk, M.; Morcos, M.; Cregg, M.; O’Flynn, L.; Treacy, O.; Ritter, T. Role of lentivirus-mediated overexpression of programmed death-ligand 1 on corneal allograft survival. Am. J. Transpl. 2012, 12, 1313–1322. [Google Scholar] [CrossRef]
- Shen, L.; Jin, Y.; Freeman, G.J.; Sharpe, A.H.; Dana, M.R. The function of donor versus recipient programmed death-ligand 1 in corneal allograft survival. J. Immunol. 2007, 179, 3672–3679. [Google Scholar] [CrossRef] [PubMed]
- Hori, J.; Wang, M.; Miyashita, M.; Tanemoto, K.; Takahashi, H.; Takemori, T.; Okumura, K.; Yagita, H.; Azuma, M. B7-H1-induced apoptosis as a mechanism of immune privilege of corneal allografts. J. Immunol. 2006, 177, 5928–5935. [Google Scholar] [CrossRef] [PubMed]
- Sugita, S.; Usui, Y.; Horie, S.; Futagami, Y.; Yamada, Y.; Ma, J.; Kezuka, T.; Hamada, H.; Usui, T.; Mochizuki, M.; et al. Human corneal endothelial cells expressing programmed death-ligand 1 (PD-L1) suppress PD-1+ T helper 1 cells by a contact-dependent mechanism. Investig. Ophthalmol. Vis. Sci. 2009, 50, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Dutra, C.; Rocha Garcia, B.; Vieira, R.; Figueiredo, A.C.; Néron, Y. EP1.04-32 Successful Corneal Transplantation in a Patient Treated with Nivolumab for Metastatic Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2019, 14, S981. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef]
- Rider, P.; Carmi, Y.; Guttman, O.; Braiman, A.; Cohen, I.; Voronov, E.; White, M.R.; Dinarello, C.A.; Apte, R.N. IL-1alpha and IL-1beta recruit different myeloid cells and promote different stages of sterile inflammation. J. Immunol. 2011, 187, 4835–4843. [Google Scholar] [CrossRef]
- Dinarello, C.A.; van der Meer, J.W. Treating inflammation by blocking interleukin-1 in humans. Semin. Immunol. 2013, 25, 469–484. [Google Scholar] [CrossRef]
- Solomon, A.; Rosenblatt, M.; Monroy, D.; Ji, Z.; Pflugfelder, S.C.; Tseng, S.C. Suppression of interleukin 1alpha and interleukin 1beta in human limbal epithelial cells cultured on the amniotic membrane stromal matrix. Br. J. Ophthalmol. 2001, 85, 444–449. [Google Scholar] [CrossRef]
- Iwakura, Y.; Ishigame, H.; Saijo, S.; Nakae, S. Functional specialization of interleukin-17 family members. Immunity 2011, 34, 149–162. [Google Scholar] [CrossRef]
- Subbarayal, B.; Chauhan, S.K.; Di Zazzo, A.; Dana, R. IL-17 Augments B Cell Activation in Ocular Surface Autoimmunity. J. Immunol. 2016, 197, 3464–3470. [Google Scholar] [CrossRef]
- Schofield, C.; Fischer, S.K.; Townsend, M.J.; Mosesova, S.; Peng, K.; Setiadi, A.F.; Song, A.; Baruch, A. Characterization of IL-17AA and IL-17FF in rheumatoid arthritis and multiple sclerosis. Bioanalysis 2016, 8, 2317–2327. [Google Scholar] [CrossRef] [PubMed]
- Chae, W.J.; Gibson, T.F.; Zelterman, D.; Hao, L.; Henegariu, O.; Bothwell, A.L. Ablation of IL-17A abrogates progression of spontaneous intestinal tumorigenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 5540–5544. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.T.; Zobell, S.; Jarosz, J.G.; Stuart, P.M. Anti-IL-17 therapy restricts and reverses late-term corneal allorejection. J. Immunol. 2015, 194, 4029–4038. [Google Scholar] [CrossRef]
- Kwan, T.; Chadban, S.J.; Ma, J.; Bao, S.; Alexander, S.I.; Wu, H. IL-17 deficiency attenuates allograft injury and prolongs survival in a murine model of fully MHC-mismatched renal allograft transplantation. Am. J. Transpl. 2015, 15, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Inomata, T.; Mashaghi, A.; Di Zazzo, A.; Lee, S.M.; Chiang, H.; Dana, R. Kinetics of Angiogenic Responses in Corneal Transplantation. Cornea 2017, 36, 491–496. [Google Scholar] [CrossRef]
- Shokirova, H.; Inomata, T.; Saitoh, T.; Zhu, J.; Fujio, K.; Okumura, Y.; Yanagawa, A.; Fujimoto, K.; Sung, J.; Eguchi, A.; et al. Topical administration of the kappa opioid receptor agonist nalfurafine suppresses corneal neovascularization and inflammation. Sci. Rep. 2021, 11, 8647. [Google Scholar] [CrossRef]
- Ji, Y.W.; Lee, J.L.; Kang, H.G.; Gu, N.; Byun, H.; Yeo, A.; Noh, H.; Kim, S.; Choi, E.Y.; Song, J.S.; et al. Corneal lymphangiogenesis facilitates ocular surface inflammation and cell trafficking in dry eye disease. Ocul. Surf. 2018, 16, 306–313. [Google Scholar] [CrossRef]
- Nebbioso, M.; Iannaccone, A.; Duse, M.; Aventaggiato, M.; Bruscolini, A.; Zicari, A.M. Vascular Endothelial Growth Factor (VEGF) Serological and Lacrimal Signaling in Patients Affected by Vernal Keratoconjunctivitis (VKC). J. Ophthalmol. 2018, 2018, 3850172. [Google Scholar] [CrossRef]
- Di Zazzo, A.; Kheirkhah, A.; Abud, T.B.; Goyal, S.; Dana, R. Management of high-risk corneal transplantation. Surv. Ophthalmol. 2017, 62, 816–827. [Google Scholar] [CrossRef]
- Zhong, W.; Montana, M.; Santosa, S.M.; Isjwara, I.D.; Huang, Y.H.; Han, K.Y.; O’Neil, C.; Wang, A.; Cortina, M.S.; de la Cruz, J.; et al. Angiogenesis and lymphangiogenesis in corneal transplantation—A review. Surv. Ophthalmol. 2018, 63, 453–479. [Google Scholar] [CrossRef]
- Inomata, T.; Hua, J.; Di Zazzo, A.; Dana, R. Impaired Function of Peripherally Induced Regulatory T Cells in Hosts at High Risk of Graft Rejection. Sci. Rep. 2016, 6, 39924. [Google Scholar] [CrossRef]
- You, I.C.; Kang, I.S.; Lee, S.H.; Yoon, K.C. Therapeutic effect of subconjunctival injection of bevacizumab in the treatment of corneal neovascularization. Acta Ophthalmol. 2009, 87, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Doctor, P.P.; Bhat, P.V.; Foster, C.S. Subconjunctival bevacizumab for corneal neovascularization. Cornea 2008, 27, 992–995. [Google Scholar] [CrossRef]
- Buznyk, O.; Azharuddin, M.; Islam, M.M.; Fagerholm, P.; Pasyechnikova, N.; Patra, H.K. Collagen-based scaffolds with infused anti-VEGF release system as potential cornea substitute for high-risk keratoplasty: A preliminary in vitro evaluation. Heliyon 2020, 6, e05105. [Google Scholar] [CrossRef]
- Lam, A.J.; Hoeppli, R.E.; Levings, M.K. Harnessing Advances in T Regulatory Cell Biology for Cellular Therapy in Transplantation. Transplantation 2017, 101, 2277–2287. [Google Scholar] [CrossRef] [PubMed]
- Swart, J.F.; Delemarre, E.M.; van Wijk, F.; Boelens, J.J.; Kuball, J.; van Laar, J.M.; Wulffraat, N.M. Haematopoietic stem cell transplantation for autoimmune diseases. Nat. Rev. Rheumatol. 2017, 13, 244–256. [Google Scholar] [CrossRef]
- Tahvildari, M.; Omoto, M.; Chen, Y.; Emami-Naeini, P.; Inomata, T.; Dohlman, T.H.; Kaye, A.E.; Chauhan, S.K.; Dana, R. In Vivo Expansion of Regulatory T Cells by Low-Dose Interleukin-2 Treatment Increases Allograft Survival in Corneal Transplantation. Transplantation 2016, 100, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Inomata, T. A New Immunotherapy Using Regulatory T-Cells for High-Risk Corneal Transplantation. Juntendo Med. J. 2017, 63, 2–7. [Google Scholar] [CrossRef][Green Version]
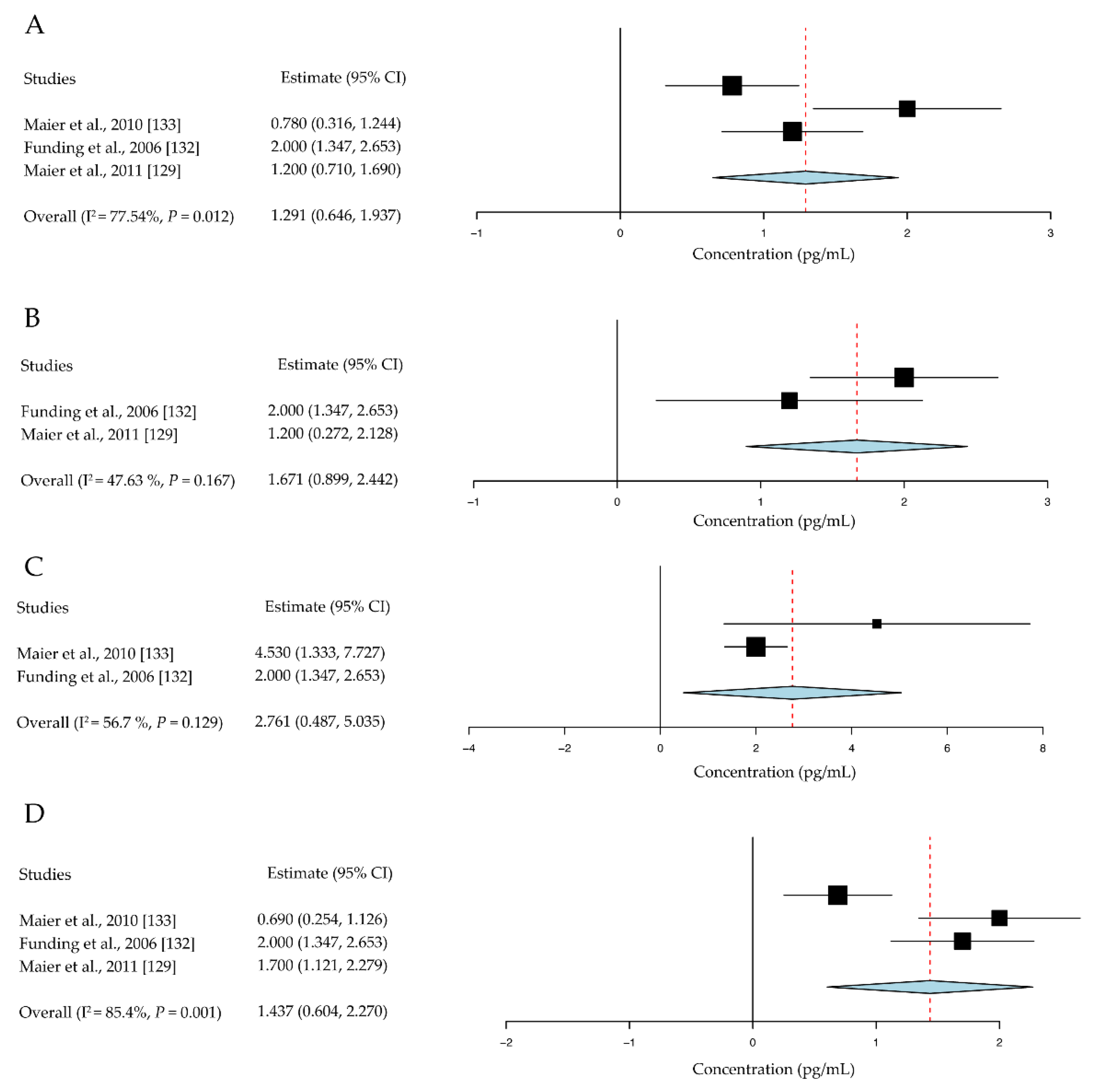
- Maier, P.; Heizmann, U.; Bohringer, D.; Kern, Y.; Reinhard, T. Predicting the risk for corneal graft rejection by aqueous humor analysis. Mol. Vis. 2011, 17, 1016–1023. [Google Scholar]
- Hayashi, T.; Takahashi, H.; Inoda, S.; Shimizu, T.; Kobayashi, A.; Kawashima, H.; Yamaguchi, T.; Yamagami, S. Aqueous humour cytokine profiles after Descemet’s membrane endothelial keratoplasty. Sci. Rep. 2021, 11, 17064. [Google Scholar] [CrossRef]
- Funding, M.; Vorum, H.; Nexo, E.; Moestrup, S.K.; Ehlers, N.; Moller, H.J. Soluble CD163 and interleukin-6 are increased in aqueous humour from patients with endothelial rejection of corneal grafts. Acta Ophthalmol. Scand. 2005, 83, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Funding, M.; Hansen, T.K.; Gjedsted, J.; Ehlers, N. Simultaneous quantification of 17 immune mediators in aqueous humour from patients with corneal rejection. Acta Ophthalmol. Scand. 2006, 84, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Maier, P.; Heizmann, U.; Bohringer, D.; Kern, Y.; Reinhard, T. Distinct cytokine pattern in aqueous humor during immune reactions following penetrating keratoplasty. Mol. Vis. 2010, 16, 53–60. [Google Scholar] [PubMed]
- Lohan, P.; Murphy, N.; Treacy, O.; Lynch, K.; Morcos, M.; Chen, B.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Third-Party Allogeneic Mesenchymal Stromal Cells Prevent Rejection in a Pre-sensitized High-Risk Model of Corneal Transplantation. Front. Immunol. 2018, 9, 2666. [Google Scholar] [CrossRef]
- Dekaris, I.; Gabric, N.; Draca, N.; Pauk-Gulic, M.; Milicic, N. Three-year corneal graft survival rate in high-risk cases treated with subconjunctival and topical bevacizumab. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 287–294. [Google Scholar] [CrossRef]
- Vassileva, P.I.; Hergeldzhieva, T.G. Avastin use in high risk corneal transplantation. Graefes Arch. Clin. Exp. Ophthalmol. 2009, 247, 1701–1706. [Google Scholar] [CrossRef]
- Trufanov, S.V.; Malozhen, S.A.; Krakhmaleva, D.A.; Surnina, Z.V.; Pivin, E.A.; Kasparova, E.A. Antiangiogenic therapy in high-risk keratoplasty. Vestn Oftalmol. 2020, 136, 11–18. [Google Scholar] [CrossRef]
- Di Zazzo, A.; Micera, A.; Coassin, M.; Varacalli, G.; Foulsham, W.; De Piano, M.; Bonini, S. InflammAging at Ocular Surface: Clinical and Biomolecular Analyses in Healthy Volunteers. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1769–1775. [Google Scholar] [CrossRef]
- Hua, J.; Inomata, T.; Chen, Y.; Foulsham, W.; Stevenson, W.; Shiang, T.; Bluestone, J.A.; Dana, R. Pathological conversion of regulatory T cells is associated with loss of allotolerance. Sci. Rep. 2018, 8, 7059. [Google Scholar] [CrossRef]
- Di Zazzo, A.; Lee, S.-M.; Sung, J.; Niutta, M.; Coassin, M.; Mashaghi, A.; Inomata, T. Variable Responses to Corneal Grafts: Insights from Immunology and Systems Biology. J. Clin. Med. 2020, 9, 586. [Google Scholar] [CrossRef]
| Inclusion criteria |
| Study objective: corneal survival or rejection data from murine allogeneic corneal transplantation experiment; various intervention factors were investigated |
| Study design: experimental research using the murine (mice or rat) corneal allograft model, studied using the valid data of experimental and control groups |
| Outcome: evaluation of allograft survival from innate and adaptive immunity perspective, case number, mean survival or rejection days with standard deviation, survival or rejection rate, and follow-up duration |
| Exclusion criteria |
| Experimental methods and protocols, reviews, systematic reviews, and conference proceedings |
| Studies only involving syngeneic transplantation; allogeneic models with animals other than mice or rats and the xenograft animal model |
| Preprinted articles |
| Conference abstracts |
| Source | Publication Date | Country | Species and Allograft Model | No. (Treated/Control) | Intervention Factors | Intervention Method | Evaluation Parameter | Follow-Up Duration |
|---|---|---|---|---|---|---|---|---|
| Gao et al. [43] | 18 June 2013 | China | Rats DA into F344 | 16/16 | Immature DCs | Tail vein | Mean survival days | All rejected on day 25 |
| Yan et al. [44] | 14 March 2014 | China | Mouse BALB/c into B6 | 10/10 | Bone marrow derived DCs | Tail vein | Mean survival days | 8 weeks |
| Li et al. [45] | 27 March 2014 | China | Rats SD into Wistar | 6/7 | Bone marrow derived DCs | Intravenous | Mean survival days | Not mentioned |
| Callanan et al. [27] | February 1988 | United States | Rats Wistar into LEW | 23/40 | LCs | LCs-containing graft | Rejection rate | >6 weeks |
| He et al. [28] | January 1996 | United States | Mouse NZB into CB6F1 | 10/21 | LCs | LCs-containing graft | Rejection rate | 8 weeks |
| Ross et al. [26] | November 1991 | United States | Rats LEW into F344 | 29/23 | LCs | LCs-containing graft | Rejection rate | >6 weeks |
| Hoffmann et al. [47] | August 1997 | Germany | Mouse C3H into BALB/c | 5/10 | The recombinant fusion protein, CTLA4-Ig | Intraperitoneal injection | Mean rejection days | All rejected on day 24 |
| Zhang et al. [48] | March 2002 | Germany | Mouse C3H into BALB/c | 6/6 | The recombinant fusion protein, CTLA4-Ig | Intraperitoneal injection | Immunoreaction days | 40 days |
| Gong et al. [49] | April 2006 | Germany | Rats DA into LEW | 6/16 | Adenovirus type 5 encoding CTLA-Ig | Intraperitoneal injection | Mean survival days | 40 days |
| Chen et al. [40] | January 2008 | China | Mouse B6 into BALB/c | 30/30 | CTLA4-FasL protein | Protein-immersed graft | Mean survival days | Not mentioned |
| Qian et al. [29] | April 2001 | United States | Mouse B10.D2 into BALB/c | 48/48 | Anti-CD154 antibody | Intraperitoneal injection | Survival rate | 8 weeks |
| Qian et al. [30] | August 2002 | United States | Mouse B10.D2 into BALB/c | 10/10 | Anti-CD154 antibody | Subconjunctival injection | Survival rate | 8 weeks |
| Jin et al. [32] | February 2010 | United States | Mouse B6 into BALB/c | 16/16 | CCR7 (-/-) | Gene knock-out donor grafts | Survival rate | 8 weeks |
| Hos et al. [53] | May 2016 | Germany | Mouse B6 into BALB/c | 13/13 | CCL19-Ig | Eye drops | Survival rate | 8 weeks |
| Zhou et al. [37] | May 2004 | China | Rats Wistar into SD | 10/10 | IL-1RA | Eye drops | Mean survival days | 4 weeks |
| Jie et al. [38] | May 2004 | China | Rats SD into Wistar | 10/10 | IL-1RA | Eye drops | Mean survival days | Until all rejected |
| Jie et al. [39] | December 2004 | China | Rats F344 into LEW | 8/10 | IL-1RA | Subconjunctival injection | Mean survival days | Until all rejected |
| Yuan et al. [42] | May 2013 | China | Rats SD into Wistar | 20/20 | IL-1RA | Subconjunctival injection | Mean rejection days | Until all rejected |
| Torres et al. [55] | 18 August 1998 | Portugal | Rats PVG into AO | 7/6 | Interleukin-10 | Subconjunctival injection | Mean rejection days | Until all rejected |
| Gong et al. [50] | 9 November 2006 | Germany | Rats Wistar to LEW | 6/9 | Interleukin-10 | IL-10 gene transfer graft | Mean rejection days | Until all rejected |
| Cunnusamy et al. [33] | 15 October 2010 | United States | Mouse B6 into BALB/c | 10/10 | Anti-IL-17A antibody | Intraperitoneal injection | Mean rejection days | 8 weeks |
| Cunnusamy et al. [34] | 15 June 2011 | United States | Mouse B6 into BALB/c | 10/10 | Anti-IL-17A antibody | Intraperitoneal injection | Mean rejection days | 8 weeks |
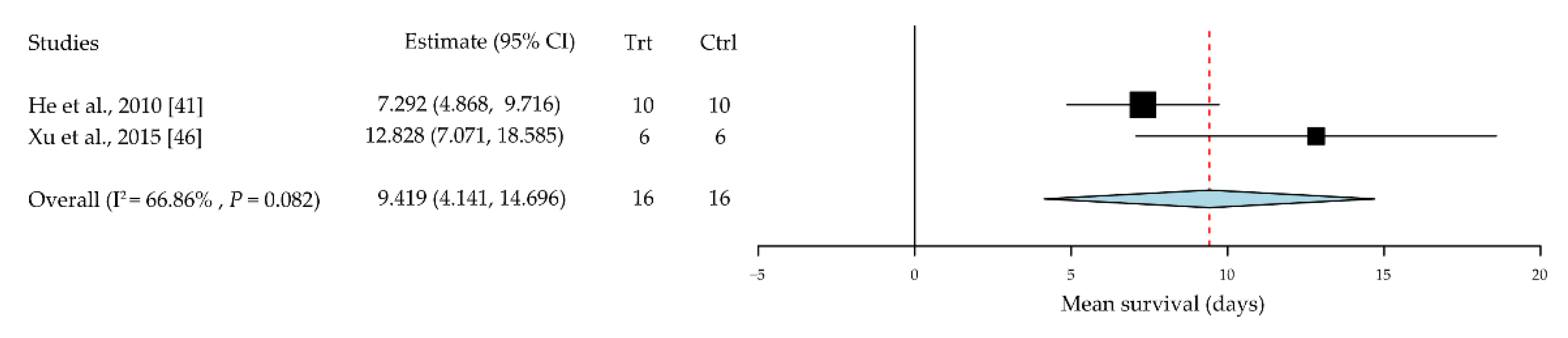
| He et al. [41] | November 2010 | China | Mouse B6 into BALB/c | 10/10 | CD4+CD25+ T cells | Retroorbital injection | Median survival days | All rejected on day 29 |
| Xu et al. [46] | 15 November 2015 | China | Mouse B6 into BALB/c | 6/6 | TGF-β-induced regulatory T cells | Subconjunctival injection | Mean survival days | 8 weeks |
| Cursiefen et al. [31] | August 2004 | United States | Mouse B6 into BALB/c | 22/22 | Molecular trap for VEGF-A | Intraperitoneal injection | Survival rate | 8 weeks |
| Hos et al. [51] | May 2008 | Germany | Mouse B6 into BALB/c | 11/11 | VEGFR-Tyrosine kinase inhibitor | Intraperitoneal injection | Survival rate | 8 weeks |
| Bachmann et al. [52] | August 2009 | Germany | Mouse B6 into BALB/c | 11/11 | Molecular trap for VEGF-A | Intraperitoneal injection | Survival rate | 8 weeks |
| Rocher et al. [54] | January 2011 | France | Rats BN into LEW | 6/6 | Anti-VEGF antibody | Subconjunctival injection | Survival rate | Day 21 * |
| Cho et al. [35] | December 2012 | United States | Mouse B6 into BALB/c | 16/11 | VEGR-1-morpholino | Subconjunctival injection | Survival rate | 8 weeks |
| Emami-Naeini et al. [36] | November 2014 | United States | Mouse B6 into BALB/c | 16/10 | Soluble VEGFR-3 | Intraperitoneal injection | Survival rate | 8 weeks |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Inomata, T.; Di Zazzo, A.; Kitazawa, K.; Okumura, Y.; Coassin, M.; Surico, P.L.; Fujio, K.; Yanagawa, A.; Miura, M.; et al. Role of Immune Cell Diversity and Heterogeneity in Corneal Graft Survival: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 4667. https://doi.org/10.3390/jcm10204667
Zhu J, Inomata T, Di Zazzo A, Kitazawa K, Okumura Y, Coassin M, Surico PL, Fujio K, Yanagawa A, Miura M, et al. Role of Immune Cell Diversity and Heterogeneity in Corneal Graft Survival: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2021; 10(20):4667. https://doi.org/10.3390/jcm10204667
Chicago/Turabian StyleZhu, Jun, Takenori Inomata, Antonio Di Zazzo, Koji Kitazawa, Yuichi Okumura, Marco Coassin, Pier Luigi Surico, Kenta Fujio, Ai Yanagawa, Maria Miura, and et al. 2021. "Role of Immune Cell Diversity and Heterogeneity in Corneal Graft Survival: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 10, no. 20: 4667. https://doi.org/10.3390/jcm10204667
APA StyleZhu, J., Inomata, T., Di Zazzo, A., Kitazawa, K., Okumura, Y., Coassin, M., Surico, P. L., Fujio, K., Yanagawa, A., Miura, M., Akasaki, Y., Fujimoto, K., Nagino, K., Midorikawa-Inomata, A., Hirosawa, K., Kuwahara, M., Huang, T., Shokirova, H., Eguchi, A., & Murakami, A. (2021). Role of Immune Cell Diversity and Heterogeneity in Corneal Graft Survival: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 10(20), 4667. https://doi.org/10.3390/jcm10204667







