Differences in Immune Checkpoints Expression (TIM-3 and PD-1) on T Cells in Women with Recurrent Miscarriages—Preliminary Studies
Abstract
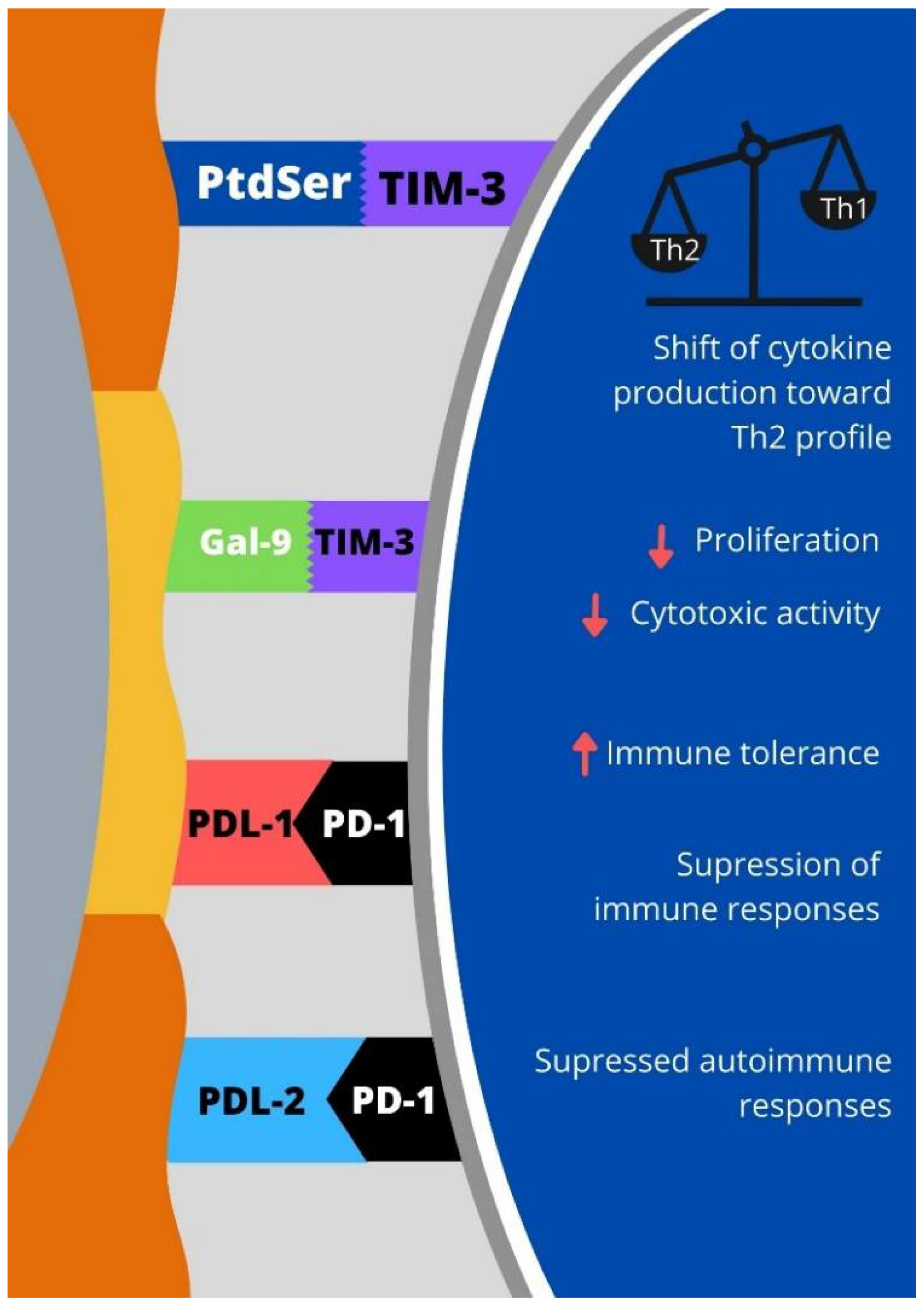
:1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Study Group
2.3. Control Group
2.4. Cell Preparation
2.5. Flow Cytometry Staining
2.6. Statistical Analyses
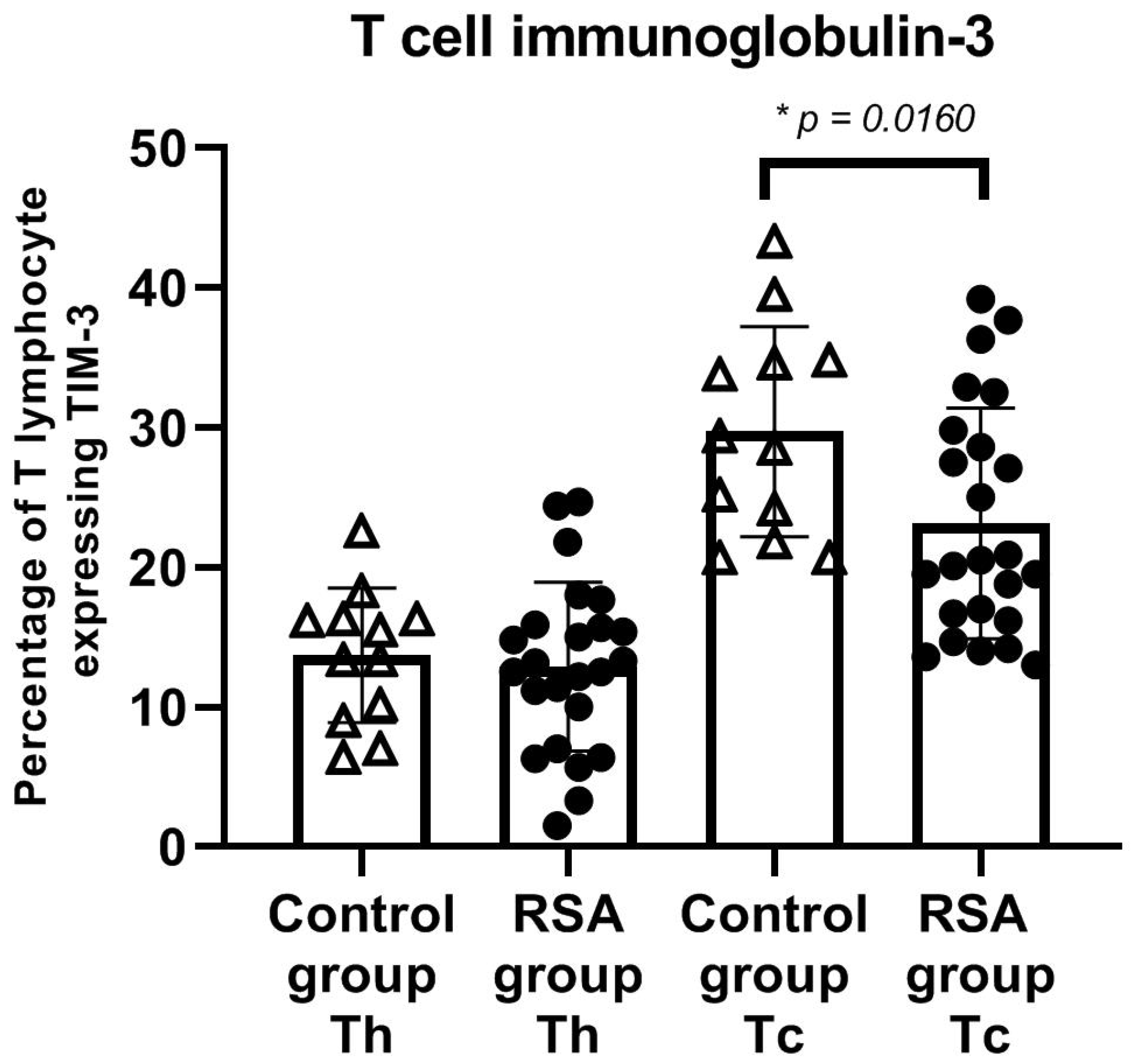
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marhelava, K.; Pilch, Z.; Bajor, M.; Graczyk-Jarzynka, A.; Zagozdzon, R. Targeting Negative and Positive Immune Checkpoints with Monoclonal Antibodies in Therapy of Cancer. Cancers 2019, 11, 1756. [Google Scholar] [CrossRef] [Green Version]
- Joller, N.; Kuchroo, V.K. Tim-3, Lag-3, and TIGIT. Curr. Top. Microbiol. Immunol. 2017, 410, 127–156. [Google Scholar] [CrossRef] [Green Version]
- Schildberg, F.A.; Klein, S.R.; Freeman, G.J.; Sharpe, A.H. Coinhibitory Pathways in the B7-CD28 Ligand-Receptor Family. Immunity 2016, 44, 955–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wing, K.; Onishi, Y.; Prieto-Martin, P.; Yamaguchi, T.; Miyara, M.; Fehervari, Z.; Nomura, T.; Sakaguchi, S. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008, 322, 271–275. [Google Scholar] [CrossRef]
- Gleason, M.K.; Lenvik, T.R.; McCullar, V.; Felices, M.; O’Brien, M.S.; Cooley, S.A.; Verneris, M.R.; Cichocki, F.; Holman, C.J.; Panoskaltsis-Mortari, A.; et al. Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood 2012, 119, 3064–3072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Sun, H.X. Immune checkpoint molecules in pregnancy: Focus on regulatory T cells. Eur. J. Immunol. 2020, 50, 160–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.Y.; Wang, S.C.; Li, D.J.; Du, M.R. Co-Signaling Molecules in Maternal-Fetal Immunity. Trends Mol. Med. 2017, 23, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Zhu, C.; Kondo, Y.; Anderson, A.C.; Gandhi, A.; Russell, A.; Dougan, S.K.; Petersen, B.S.; Melum, E.; Pertel, T.; et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature 2015, 517, 386–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.; Anderson, A.C.; Schubart, A.; Xiong, H.; Imitola, J.; Khoury, S.J.; Zheng, X.X.; Strom, T.B.; Kuchroo, V.K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005, 6, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.W.; Dutta, A.; Chang, L.Y.; Mahalingam, J.; Lin, Y.C.; Chiang, J.M.; Hsu, C.Y.; Huang, C.T.; Su, W.T.; Chu, Y.Y.; et al. Apoptosis of tumor infiltrating effector TIM-3+CD8+ T cells in colon cancer. Sci. Rep. 2015, 5, 15659. [Google Scholar] [CrossRef] [Green Version]
- Santiago, C.; Ballesteros, A.; Martinez-Munoz, L.; Mellado, M.; Kaplan, G.G.; Freeman, G.J.; Casasnovas, J.M. Structures of T cell immunoglobulin mucin protein 4 show a metal-Ion-dependent ligand binding site where phosphatidylserine binds. Immunity 2007, 27, 941–951. [Google Scholar] [CrossRef] [Green Version]
- Cao, E.; Zang, X.; Ramagopal, U.A.; Mukhopadhaya, A.; Fedorov, A.; Fedorov, E.; Zencheck, W.D.; Lary, J.W.; Cole, J.L.; Deng, H.; et al. T Cell Immunoglobulin Mucin-3 Crystal Structure Reveals a Galectin-9-Independent Ligand-Binding Surface. Immunity 2007, 26, 311–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiba, S.; Baghdadi, M.; Akiba, H.; Yoshiyama, H.; Kinoshita, I.; Dosaka-Akita, H.; Fujioka, Y.; Ohba, Y.; Gorman, J.V.; Colgan, J.D.; et al. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat. Immunol. 2012, 13, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Miko, E.; Meggyes, M.; Doba, K.; Barakonyi, A.; Szereday, L. Immune Checkpoint Molecules in Reproductive Immunology. Front. Immunol. 2019, 10, 846. [Google Scholar] [CrossRef] [PubMed]
- Meggyes, M.; Miko, E.; Polgar, B.; Bogar, B.; Farkas, B.; Illes, Z.; Szereday, L. Peripheral blood TIM-3 positive NK and CD8+ T cells throughout pregnancy: TIM-3/galectin-9 interaction and its possible role during pregnancy. PLoS ONE 2014, 9, e92371. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Fueyo, A.; Tian, J.; Picarella, D.; Domenig, C.; Zheng, X.X.; Sabatos, C.A.; Manlongat, N.; Bender, O.; Kamradt, T.; Kuchroo, V.K.; et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat. Immunol. 2003, 4, 1093–1101. [Google Scholar] [CrossRef]
- Hu, X.H.; Tang, M.X.; Mor, G.; Liao, A.H. Tim-3: Expression on immune cells and roles at the maternal-fetal interface. J. Reprod. Immunol. 2016, 118, 92–99. [Google Scholar] [CrossRef]
- Shimizu, Y.; Kabir-Salmani, M.; Azadbakht, M.; Sugihara, K.; Sakai, K.; Iwashita, M. Expression and localization of galectin-9 in the human uterodome. Endocr. J. 2008, 55, 879–887. [Google Scholar] [CrossRef] [Green Version]
- Chabtini, L.; Mfarrej, B.; Mounayar, M.; Zhu, B.; Batal, I.; Dakle, P.J.; Smith, B.D.; Boenisch, O.; Najafian, N.; Akiba, H.; et al. TIM-3 regulates innate immune cells to induce fetomaternal tolerance. J. Immunol. 2013, 190, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Miko, E.; Meggyes, M.; Bogar, B.; Schmitz, N.; Barakonyi, A.; Varnagy, A.; Farkas, B.; Tamas, P.; Bodis, J.; Szekeres-Bartho, J.; et al. Involvement of Galectin-9/TIM-3 pathway in the systemic inflammatory response in early-onset preeclampsia. PLoS ONE 2013, 8, e71811. [Google Scholar] [CrossRef]
- Li, J.; Li, F.F.; Zuo, W.; Zhou, Y.; Hao, H.Y.; Dang, J.; Jiang, M.; He, M.Z.; Deng, D.R. Up-regulated expression of Tim-3/Gal-9 at maternal-fetal interface in pregnant woman with recurrent spontaneous abortion. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2014, 34, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; He, M.; Li, J.; Zhou, Y.; Dang, J.; Li, F.; Yang, M.; Deng, D. Upregulation of the Tim-3/Gal-9 pathway and correlation with the development of preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 194, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhu, Y.; Zhao, J.; Ai, H.; Gong, Q.; Zhang, J.; Zhao, J.; Wang, Q.; La, X.; Ding, J. Soluble costimulatory molecule sTim3 regulates the differentiation of Th1 and Th2 in patients with unexplained recurrent spontaneous abortion. Int. J. Clin. Exp. Med. 2015, 8, 8812–8819. [Google Scholar]
- Meggyes, M.; Miko, E.; Szigeti, B.; Farkas, N.; Szereday, L. The importance of the PD-1/PD-L1 pathway at the maternal-fetal interface. BMC Pregnancy Childbirth 2019, 19, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef] [Green Version]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef] [Green Version]
- Sledzinska, A.; Menger, L.; Bergerhoff, K.; Peggs, K.S.; Quezada, S.A. Negative immune checkpoints on T lymphocytes and their relevance to cancer immunotherapy. Mol. Oncol. 2015, 9, 1936–1965. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhu, X.; Xu, Y.; Zhang, D.; Li, Y.; Tao, Y.; Piao, H.; Li, D.; Du, M. Programmed cell death-1 (PD-1) and T-cell immunoglobulin mucin-3 (Tim-3) regulate CD4+ T cells to induce Type 2 helper T cell (Th2) bias at the maternal-fetal interface. Hum. Reprod. 2016, 31, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Trowsdale, J.; Betz, A.G. Mother’s little helpers: Mechanisms of maternal-fetal tolerance. Nat. Immunol. 2006, 7, 241–246. [Google Scholar] [CrossRef]
- Rai, R.; Regan, L. Recurrent miscarriage. Lancet 2006, 368, 601–611. [Google Scholar] [CrossRef]
- Williams, Z. Inducing tolerance to pregnancy. N. Engl. J. Med. 2012, 367, 1159–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mekinian, A.; Cohen, J.; Alijotas-Reig, J.; Carbillon, L.; Nicaise-Roland, P.; Kayem, G.; Darai, E.; Fain, O.; Bornes, M. Unexplained Recurrent Miscarriage and Recurrent Implantation Failure: Is There a Place for Immunomodulation? Am. J. Reprod. Immunol. 2016, 76, 8–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Salazar Garcia, M.D.; Deutsch, G.; Sung, N.; Yang, X.; He, Q.; Jubiz, G.; Bilal, M.; Dambaeva, S.; Gilman-Sachs, A.; et al. PD-1 and PD-L1 expression on T-cell subsets in women with unexplained recurrent pregnancy losses. Am. J. Reprod. Immunol. 2020, 83, e13230. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, S.D.; Shin, H.; Haining, W.N.; Zou, T.; Workman, C.J.; Polley, A.; Betts, M.R.; Freeman, G.J.; Vignali, D.A.; Wherry, E.J. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2009, 10, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Hofmeyer, K.A.; Jeon, H.; Zang, X. The PD-1/PD-L1 (B7-H1) pathway in chronic infection-induced cytotoxic T lymphocyte exhaustion. J. Biomed. Biotechnol. 2011, 2011, 451694. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Thornley, T.B.; Gao, W.; Larocca, R.; Turka, L.A.; Kuchroo, V.K.; Strom, T.B. Allograft rejection is restrained by short-lived TIM-3+PD-1+Foxp3+ Tregs. J. Clin. Investig. 2012, 122, 2395–2404. [Google Scholar] [CrossRef] [Green Version]
- Lamprianidou, E.; Daniilidis, M.; Kordella, C.; Zoulia, E.; Nakou, E.; Gerofotis, A.; Vasilaki, A.; Pantos, G.; Kotsianidis, I. The STAT signaling profile at the single cell level reveals novel insights in the association of FOXP3+ T regulatory cells with recurrent spontaneous abortions before and after lymphocyte immunotherapy. Clin. Immunol. 2020, 210, 108261. [Google Scholar] [CrossRef]
- Kniotek, M.; Zych, M.; Roszczyk, A.; Szafarowska, M.; Jerzak, M.M. Decreased Production of TNF-α and IL-6 Inflammatory Cytokines in Non-Pregnant Idiopathic RPL Women Immunomodulatory Effect of Sildenafil Citrate on the Cellular Response of Idiopathic RPL Women. J. Clin. Med. 2021, 10, 3115. [Google Scholar] [CrossRef]
- Enninga, E.A.L.; Harrington, S.M.; Creedon, D.J.; Ruano, R.; Markovic, S.N.; Dong, H.; Dronca, R.S. Immune checkpoint molecules soluble program death ligand 1 and galectin-9 are increased in pregnancy. Am. J. Reprod. Immunol. 2018, 79, e12795. [Google Scholar] [CrossRef] [Green Version]
- Meggyes, M.; Nagy, D.U.; Szereday, L. Investigation of the PD-1 and PD-L1 Immune Checkpoint Molecules Throughout Healthy Human Pregnancy and in Nonpregnant Women. J. Clin. Med. 2020, 9, 2536. [Google Scholar] [CrossRef]
- Wang, S.; Cao, C.; Piao, H.; Li, Y.; Tao, Y.; Zhang, X.; Zhang, D.; Sun, C.; Zhu, R.; Wang, Y.; et al. Tim-3 protects decidual stromal cells from toll-like receptor-mediated apoptosis and inflammatory reactions and promotes Th2 bias at the maternal-fetal interface. Sci. Rep. 2015, 5, 9013. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.C.; Li, Y.H.; Piao, H.L.; Hong, X.W.; Zhang, D.; Xu, Y.Y.; Tao, Y.; Wang, Y.; Yuan, M.M.; Li, D.J.; et al. PD-1 and Tim-3 pathways are associated with regulatory CD8+ T-cell function in decidua and maintenance of normal pregnancy. Cell Death Dis. 2015, 6, e1738. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, X.; Xia, X.; Liu, L.; Zhang, Y.; Zhang, X.; Wang, C. Expression of Tim-3 in peripheral blood mononuclear cells and placental tissue in unexplained recurrent spontaneous abortion. Medicine 2018, 97, e12099. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yang, M.; Ban, Y.; Gao, W.; Song, B.; Wang, Y.; Zhang, Y.; Shao, Q.; Kong, B.; Qu, X. Tim-3 Is Upregulated in NK Cells during Early Pregnancy and Inhibits NK Cytotoxicity toward Trophoblast in Galectin-9 Dependent Pathway. PLoS ONE 2016, 11, e0147186. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Zhang, D.; Hong, X.; Tao, Y.; Wang, S.; Xu, Y.; Piao, H.; Yin, W.; Yu, M.; et al. Tim-3 signaling in peripheral NK cells promotes maternal-fetal immune tolerance and alleviates pregnancy loss. Sci. Signal. 2017, 10, eaah4323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.H.; Zhou, W.H.; Tao, Y.; Wang, S.C.; Jiang, Y.L.; Zhang, D.; Piao, H.L.; Fu, Q.; Li, D.J.; Du, M.R. The Galectin-9/Tim-3 pathway is involved in the regulation of NK cell function at the maternal-fetal interface in early pregnancy. Cell. Mol. Immunol. 2016, 13, 73–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zych, M.; Roszczyk, A.; Kniotek, M.; Dąbrowski, F.; Zagożdżon, R. Differences in Immune Checkpoints Expression (TIM-3 and PD-1) on T Cells in Women with Recurrent Miscarriages—Preliminary Studies. J. Clin. Med. 2021, 10, 4182. https://doi.org/10.3390/jcm10184182
Zych M, Roszczyk A, Kniotek M, Dąbrowski F, Zagożdżon R. Differences in Immune Checkpoints Expression (TIM-3 and PD-1) on T Cells in Women with Recurrent Miscarriages—Preliminary Studies. Journal of Clinical Medicine. 2021; 10(18):4182. https://doi.org/10.3390/jcm10184182
Chicago/Turabian StyleZych, Michał, Aleksander Roszczyk, Monika Kniotek, Filip Dąbrowski, and Radosław Zagożdżon. 2021. "Differences in Immune Checkpoints Expression (TIM-3 and PD-1) on T Cells in Women with Recurrent Miscarriages—Preliminary Studies" Journal of Clinical Medicine 10, no. 18: 4182. https://doi.org/10.3390/jcm10184182
APA StyleZych, M., Roszczyk, A., Kniotek, M., Dąbrowski, F., & Zagożdżon, R. (2021). Differences in Immune Checkpoints Expression (TIM-3 and PD-1) on T Cells in Women with Recurrent Miscarriages—Preliminary Studies. Journal of Clinical Medicine, 10(18), 4182. https://doi.org/10.3390/jcm10184182





