Treatment Guidelines for PTSD: A Systematic Review
Abstract
:1. Introduction
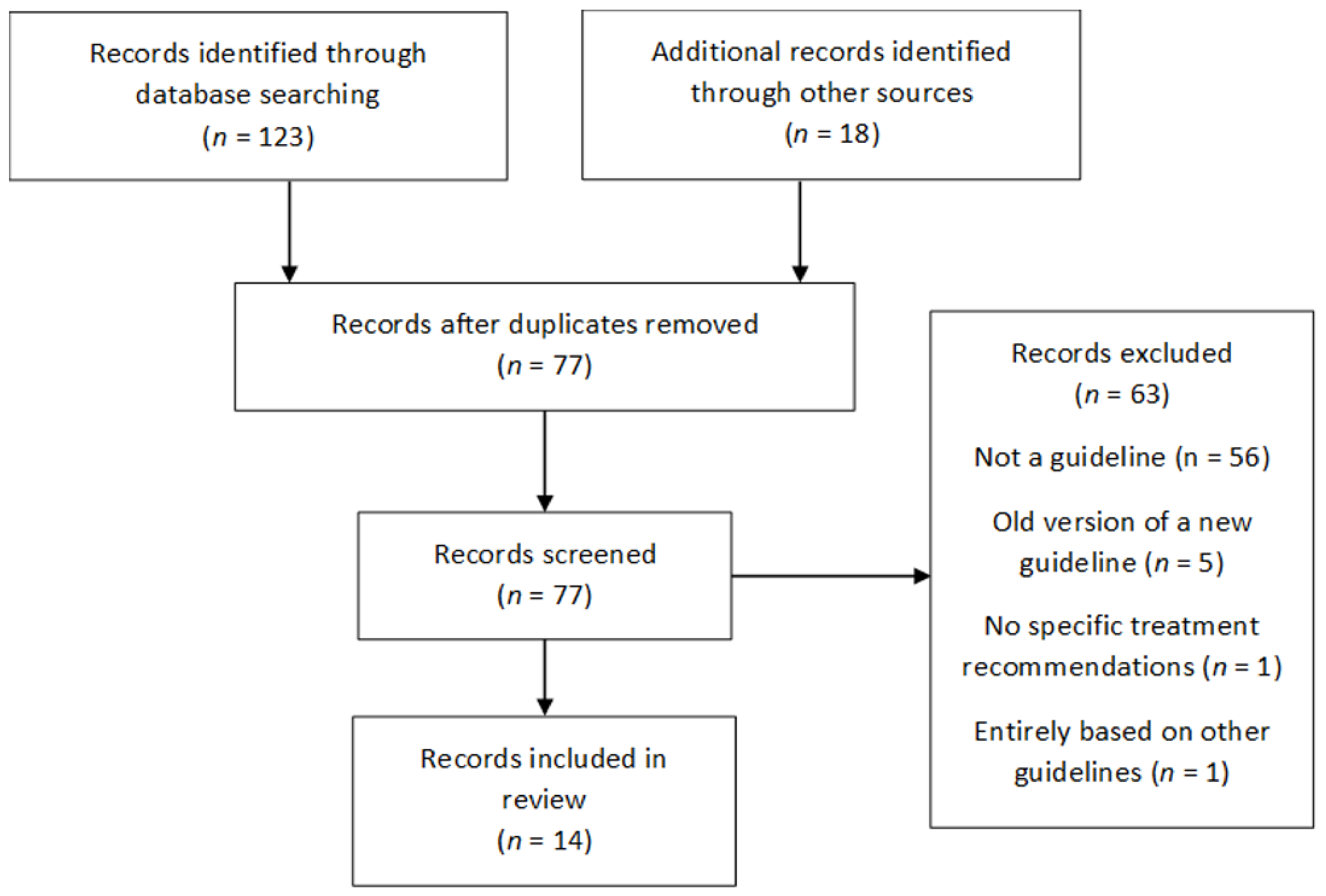
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Collection and Analysis
2.4. Evaluation of Guideline Quality
3. Results
3.1. Guideline Characteristics
3.2. Assessment of Guideline Quality
3.3. Recommendations in Guidelines
4. Discussion
4.1. Quality of Guidelines
4.2. Recommendations in Guidelines
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schnurr, P.; Lunney, C.; Bovin, M.; Marx, B. Posttraumatic stress disorder and quality of life: Extension of findings to veterans of the wars in Iraq and Afghanistan. Clin. Psychol. Rev. 2009, 29, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Giacco, D.; Matanov, A.; Priebe, S. Symptoms and subjective quality of life in post-traumatic stress disorder: A longitudinal study. PLoS ONE 2013, 8, e60991. [Google Scholar] [CrossRef] [Green Version]
- George, K.; Kebejian, L.; Ruth, L.; Miller, C.; Himelhoch, S. Meta-analysis of the efficacy and safety of prazosin versus placebo for the treatment of nightmares and sleep disturbances in adults with posttraumatic stress disorder. J. Trauma Dissociation Off. J. Int. Soc. Study Dissociation (ISSD) 2016, 17, 494–510. [Google Scholar] [CrossRef] [PubMed]
- Rapaport, M.; Clary, C.; Fayyad, R.; Endicott, J. Quality-of-life impairment in depressive and anxiety disorders. Am. J. Psychiatry 2005, 162, 1171–1178. [Google Scholar] [CrossRef]
- Kessler, R. Posttraumatic stress disorder: The burden to the individual and to society. J. Clin. Psychiatry 2000, 61 (Suppl. S5), 4–14. [Google Scholar] [PubMed]
- Stein, M.; McQuaid, J.; Pedrelli, P.; Lenox, R.; McCahill, M. Posttraumatic stress disorder in the primary care medical setting. Gen. Hosp. Psychiatry 2000, 22, 261–269. [Google Scholar] [CrossRef]
- LeBouthillier, D.; McMillan, K.; Thibodeau, M.; Asmundson, G. Types and Number of Traumas Associated With Suicidal Ideation and Suicide Attempts in PTSD: Findings From a U.S. Nationally Representative Sample. J. Trauma. Stress 2015, 28, 183–190. [Google Scholar] [CrossRef] [Green Version]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Sjostrom, N.; Waern, M.; Hetta, J. Nightmares and sleep disturbances in relation to suicidality in suicide attempters. Sleep 2007, 30, 91–95. [Google Scholar] [CrossRef]
- Khachatryan, D.; Groll, D.; Booij, L.; Sepehry, A.; Schütz, C. Prazosin for treating sleep disturbances in adults with posttraumatic stress disorder: A systematic review and meta-analysis of randomized controlled trials. Gen. Hosp. Psychiatry 2016, 39, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Spoormaker, V.; Montgomery, P. Disturbed sleep in post-traumatic stress disorder: Secondary symptom or core feature? Sleep Med. Rev. 2008, 12, 169–184. [Google Scholar] [CrossRef]
- Katz, C.; Stein, M.; Richardson, J.; Seedat, S.; Sareen, J. A Review of Interventions for Treatment-Resistant Posttraumatic Stress Disorder. Different Views of Anxiety Disorders; IntechOpen: London, UK, 2011. [Google Scholar]
- Detweiler, M.B.; Pagadala, B.; Candelario, J.; Boyle, J.S.; Detweiler, J.G.; Lutgens, B.W. Treatment of Post-Traumatic Stress Disorder Nightmares at a Veterans Affairs Medical Center. J. Clin. Med. 2016, 5, 117. [Google Scholar] [CrossRef] [Green Version]
- Seda, G.; Sanchez-Ortuno, M.; Welsh, C.; Halbower, A.; Edinger, J. Comparative meta-analysis of prazosin and imagery rehearsal therapy for nightmare frequency, sleep quality, and posttraumatic stress. J. Clin. Sleep Med. 2015, 11, 11–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Therapeutic Guidelines Limited. Posttraumatic Mental Health Disorders; Therapeutic Guidelines Limited: Melbourne, Australia, 2013. [Google Scholar]
- Phoenix Australia Centre for Posttraumatic Mental Health. Australian Guidelines for the Prevention and Treatment of Acute Stress Disorder, Posttraumatic Stress Disorder, and Complex Posttraumatic Stress Disorder; National Health and Medical Research Council: Canberra, Australia, 2020. [Google Scholar]
- Woolf, S.; Grol, R.; Hutchinson, A.; Eccles, M.; Grimshaw, J. Clinical guidelines: Potential benefits, limitations, and harms of clinical guidelines. BMJ 1999, 318, 527–530. [Google Scholar] [CrossRef]
- Kredo, T.; Bernhardsson, S.; Machingaidze, S.; Young, T.; Louw, Q.; Ochodo, E.; Grimmer, K. Guide to clinical practice guidelines: The current state of play. Int. J. Qual. Health Care 2016, 28, 122–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casey, D. Why don’t physicians (and patients) consistently follow clinical practice guidelines? JAMA Intern. Med. 2013, 173, 1581–1583. [Google Scholar] [CrossRef]
- Treweek, S.; Oxman, A.D.; Alderson, P.; Bossuyt, P.M.; Brandt, L.; Brożek, J.; Davoli, M.; Flottorp, S.; Harbour, R.; Hill, S.; et al. Developing and evaluating communication strategies to support informed decisions and practice based on evidence (DECIDE): Protocol and preliminary results. Implement. Sci. 2013, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- McIntyre, J. Usefulness and limitations of treatment guidelines in psychiatry. World Psychiatry 2002, 1, 186–189. [Google Scholar]
- Forsner, T.; Hansson, J.; Brommels, M.; Wistedt, A.-Å.; Forsell, Y. Implementing clinical guidelines in psychiatry: A qualitative study of perceived facilitators and barriers. BMC Psychiatry 2010, 10, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbui, C.; Girlanda, F.; Ay, E.; Cipriani, A.; Becker, T.; Koesters, M. Implementation of treatment guidelines for specialist mental health care. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, A.; Naunton, M.; Kosari, S.; Peterson, G.; Thomas, J.; Christiensen, J. Systematic Review of International Treatment Guidelines for PTSD. PROSPERO 2017 CRD42017084122. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42017084122 (accessed on 17 July 2021).
- McFetridge, M.; Swan, A.H.; Heke, S.; Karatzias, T.; Greenberg, N.; Kitchiner, N.; Morley, R. Guideline for the Treatment and Planning of Services for Complex Post-Traumatic Stress Disorder in Adults; UK Psychological Trauma Society: Edinburgh, UK, 2017; Available online: https://ray.yorksj.ac.uk/id/eprint/4862/ (accessed on 17 July 2021).
- Brouwers, M.C.; Kho, M.E.; Browman, G.P.; Burgers, J.S.; Cluzeau, F.; Feder, G.; Fervers, B.; Graham, I.D.; Grimshaw, J.; Hanna, S.E.; et al. AGREE II: Advancing guideline development, reporting and evaluation in health care. CMAJ 2010, 182, E839–E842. [Google Scholar] [CrossRef] [Green Version]
- Ursano, R.J.; Bell, C.; Eth, S.; Friedman, M.; Norwood, A.; Pfefferbaum, B.; Pynoos, J.D.; Zatzick, D.F.; Benedek, D.M.; McIntyre, J.S.; et al. Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Am. J. Psychiatry 2004, 161 (Suppl. S11), 3–31. [Google Scholar]
- Canadian Psychiatric Association. Clinical practice guidelines. Management of anxiety disorders. Can. J. Psychiatry Rev. Can. De Psychiatr. 2006, 51 (Suppl. S2), 9S–91S. [Google Scholar]
- Bandelow, B.; Zohar, J.; Hollander, E.; Kasper, S.; Möller, H.J.; WFSBP Task Force on Treatment Guidelines for Anxiety, Obsessive-Compulsive and Post-Traumatic Stress Disoders; Allgulander, C.; Ayuso-Gutierrez, J.; Baldwin, D.S.; Buenvicius, R.; et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and post-traumatic stress disorders—first revision. World J. Biol. Psychiatry 2008, 9, 248–312. [Google Scholar] [CrossRef] [Green Version]
- Standards of Practice Committee; Aurora, R.N.; Zak, R.S.; Auerbach, S.H.; Casey, K.R.; Chowdhuri, S.; Karippot, A.; Maganti, R.K.; Ramar, K.; Kristo, D.A.; et al. Best practice guide for the treatment of nightmare disorder in adults. J. Clin. Sleep Med. 2010, 6, 389–401. [Google Scholar]
- World Health Organisation. Guidelines for the Management of Conditions Specifically Related to Stress; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Emsley, R.; Flisher, A.J.; Grobler, G.; Seedat, S.; Szabo, C.P. The South African Society of Psychiatrists (SASOP) Treatment Guidlelines for Psychiatric Disorders. S. Afr. J. Psychiatry 2013, 19, 134–135. [Google Scholar] [CrossRef]
- Katzman, M.; Bleau, P.; Blier, P.; Chokka, P.; Kjernisted, K.; Van Ameringen, M. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry 2014, 14, S1. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, D.S.; Anderson, I.M.; Nutt, D.J.; Allgulander, C.; Bandelow, B.; den Boer, J.A.; Christmas, D.M.; Davies, S.; Fineberg, N.; Lidbetter, N.; et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: A revision of the 2005 guidelines from the British Association for Psychopharmacology. J. Psychopharmacol. Oxf. Engl. 2014, 28, 403–439. [Google Scholar] [CrossRef] [PubMed]
- Department of Veterans Affairs, Department of Defense. VA/DOD Clinical Practice Guideline for the Management of Posttraumatic Stress Disorder and Acute Stress Disorder; Department of Veterans Affairs, Department of Defense: Washington DC, USA, 2017.
- Guideline Development Panel for the Treatment of PTSD in Adults; Courtois, C.; Sonis, J.; Brown, L.S.; Cook, J.; Fairbank, J.A.; Friedman, M.; Gone, J.P.; Jones, R.; La Greca, A. Clinical Practice Guideline for the Treatment of Posttraumatic Stress Disorder (PTSD) in Adults; American Pyschological Association: Washington, DC, USA, 2017. [Google Scholar]
- National Institute for Health and Care Excellence (NICE). Post-Traumatic Stress Disorder; National Institute for Health and Care Excellence (NICE): London, UK, 2018. [Google Scholar]
- Forbes, D.; Bisson, J.; Monson, C.; Berliner, L. Effective Treatments for PTSD, Third Edition: International Society for Traumatic Stress Studies; Guilford Press: Chicago, IL, USA, 2020. [Google Scholar]
- Shekelle, P.G.; Ortiz, E.; Rhodes, S.; Morton, S.C.; Eccles, M.P.; Grimshaw, J.M.; Woolf, S.H. Validity of the agency for healthcare research and quality clinical practice guidelines: How quickly do guidelines become outdated? JAMA 2001, 286, 1461–1467. [Google Scholar] [CrossRef]
- Richard, G. Updating Clinical Practice Guidelines: How Do We Stay Current? Otolaryngol.-Head Neck Surg. 2015, 153, 488–490. [Google Scholar]
- Shekelle, P.; Eccles, M.; Grimshaw, J.; Woolf, S. When should clinical guidelines be updated? BMJ 2001, 323, 155–157. [Google Scholar] [CrossRef] [Green Version]
- Hoskins, M.; Pearce, J.; Bethell, A.; Dankova, L.; Barbui, C.; Tol, W.A.; van Ommeren, M.; de Jong, J.; Seedat, S.; Chen, H.; et al. Pharmacotherapy for post-traumatic stress disorder: Systematic review and meta-analysis. Br. J. Psychiatry 2015, 206, 93–100. [Google Scholar] [CrossRef]
- Puetz, T.; Youngstedt, S.; Herring, M. Effects of Pharmacotherapy on Combat-Related PTSD, Anxiety, and Depression: A Systematic Review and Meta-Regression Analysis. PLoS ONE 2015, 10, e0126529. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Schnitzlein, C.; Wolf, J.; Vythilingam, M.; Rasmusson, A.; Hoge, C. Psychotherapy versus pharmacotherapy for posttraumatic stress disorder: Systematic review and meta-analyses to determine first-line treatments. Depress. Anxiety 2016, 33, 792–806. [Google Scholar] [CrossRef] [Green Version]
- Mendes, D.; Marcelo, M.; Paula, V.; Cristiane-De-Medeiros, P.; Jair-De-Jesus, M. A Systematic Review on the Effectiveness of Cognitive Behavioral Therapy for Posttraumatic Stress Disorder. Int. J. Psychiatry Med. 2008, 38, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, G.; Hu, M.; Liang, X. Eye movement desensitization and reprocessing versus cognitive-behavioral therapy for adult posttraumatic stress disorder: Systematic review and meta-analysis. J. Nerv. Ment. Dis. 2015, 203, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Berardis, D.D.; Marini, S.; Serroni, N.; Iasevoli, F.; Tomasetti, C.; de Bartolomeis, A.; Mazza, M.; Tempesta, D.; Valchera, A.; Fornaro, M.; et al. Targeting the noradrenergic system in posttraumatic stress disorder: A systematic review and meta-analysis of prazosin trials. Curr. Drug Targets 2015, 16, 1094–1106. [Google Scholar] [CrossRef] [PubMed]
- Khazaie, H.; Nasouri, M.; Ghadami, M. Prazosin for Trauma Nightmares and Sleep Disturbances in Combat Veterans with Post-Traumatic Stress Disorder. Iran. J. Psychiatry Behav. Sci. 2016, 10, e2603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raskind, M.A.; Peskind, E.R.; Chow, B.; Harris, C.; Davis-Karim, A.; Holmes, H.A.; Hart, K.L.; McFall, M.; Reist, C.; Romesser, J.; et al. Trial of Prazosin for Post-Traumatic Stress Disorder in Military Veterans. N. Engl. J. Med. 2018, 378, 508–518. [Google Scholar] [CrossRef]
- Ressler, K. Alpha-Adrenergic Receptors in PTSD—Failure or Time for Precision Medicine? N. Engl. J. Med. 2018, 378, 575–576. [Google Scholar] [CrossRef] [PubMed]
- Keating, D.; McWilliams, S.; Schneider, I.; Hynes, C.; Cousins, G.; Strawbridge, J.; Clarke, M. Pharmacological guidelines for schizophrenia: A systematic review and comparison of recommendations for the first episode. BMJ Open 2017, 7, e013881. [Google Scholar] [CrossRef]
- Gaebel, W.; Weinmann, S.; Sartorius, N.; Rutz, W.; McIntyre, J. Schizophrenia practice guidelines: International survey and comparison. Br. J. Psychiatry 2005, 187, 248–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellani, A.; Girlanda, F.; Barbui, C. Rigour of development of clinical practice guidelines for the pharmacological treatment of bipolar disorder: Systematic review. J. Affect. Disord. 2015, 174, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.; Chadda, S.; Zhao, Z.; Barber, B.; Sykes, D. A systematic review of treatment guidelines for metastatic colorectal cancer. Colorectal Dis. 2012, 14, e31–e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| PTSD Terms | PTSD, Posttraumatic Stress Disorder, Post Traumatic Stress Disorder, Post-Traumatic Stress Disorder, Stress Disorders |
|---|---|
| Nightmares terms | Nightmares, sleep disturbance, sleep disruption |
| Guideline terms | Treatment guideline, practice guideline, evidence-based practice, best practice, consensus, clinical practice guideline, protocol |
| Three most populous English-speaking countries in each continent | Tanzania, Kenya, South Africa, India, Pakistan, Philippines, Australia, New Zealand, Papua New Guinea, England, Ireland, Scotland, The US, Canada, Jamaica |
| Guideline (Abbreviation) | Author/Institution | Country | Publication Date | First-Line Psychological Recommendation | First-Line Pharmacological Recommendation | Recommendation for Targeted Treatment of Nightmares (Y/N) | Recommendation for Use of Prazosin |
|---|---|---|---|---|---|---|---|
| Practice Guideline for the Treatment of Patients with Acute Stress Disorder and Posttraumatic Stress Disorder (APiA) [28] | American Psychiatric Association | United States | 2004 + minor update 2009 | CBT or EMDR | SSRIs | Y—IRT and prazosin suggested | “Low dose could be tried and increased if response is inadequate.” |
| Clinical Practice Guidelines for the Management of Anxiety Disorders (CPA) * [29] | Canadian Psychiatric Association | Canada | 2006 | CBT | Fluoxetine, paroxetine, sertraline, venlafaxine XR | N—But prazosin is included as a third-line adjunct option | Third-line as adjunct |
| World Federation of Societies of Biological Psychiatry Guidelines for the Pharmacological Treatment of Anxiety, Obsessive-Compulsive and Post-Traumatic Stress Disorders—First Revision (WFSBP) [30] | World Federation of Societies of Biological Psychiatry | International | 2009 | CBT | SSRIs (fluoxetine, sertraline, paroxetine), venlafaxine. | N—But prazosin is mentioned based on Category C evidence | Third-line |
| Best Practice Guide for the Treatment of Nightmare Disorder (AASM) [31] | American Academy of Sleep Medicine | United States | 2010 | CBT (IRT form specifically) | Prazosin | Y Prazosin & IRT = First-line, Level A | First-line—Level A |
| Guidelines for the Management of Conditions Specifically Related to Stress (WHO) [32] | World Health Organisation | International | 2013 | CBT or EMDR | Second-line to psychological therapies SSRIs, TCAs | N | No recommendation |
| eTG complete—Posttraumatic Mental Health Disorders (eTG) [15] | Therapeutic Guidelines Ltd. | Australia | 2013 | CBT or EMDR | Second-line to psychological therapies SSRIs | N | No recommendation |
| The South African Society of Psychiatrists (SASOP) Treatment Guidelines for Psychiatric Disorders (SASOP) [33] | The South African Society of Psychiatrists | South Africa | 2013 | CBT | SSRIs and SNRIs | Y | “Prazosin has shown promise in treating nightmares” |
| Canadian Clinical Practice Guidelines for the Management of Anxiety, Posttraumatic Stress and Obsessive-compulsive Disorders (ADAC) [34] | Anxiety Disorders Association of Canada | Canada | 2014 | Trauma-focused CBT or EMDR | Paroxetine, venlafaxine | Y | Level 1 for nightmares |
| Evidence-based Pharmacological Treatment of Anxiety Disorders, Post-traumatic Stress Disorder and Obsessive-compulsive Disorder: A Revision of the 2005 Guidelines from the British Association for Psychopharmacology (BAP) [35] | British Association for Psychopharmacology | United Kingdom | 2014 | Trauma-focused CBT or EMDR | Paroxetine, sertraline, venlafaxine | Y | As adjunct if initial treatment fails |
| VA/DOD Clinical Practice Guideline for the Management of Posttraumatic Stress Disorder and Acute Stress Disorder (VA) [36] | Department of Veterans Affairs and Department of Defense | United States | 2017 | Manualised trauma-focused CBT (including EMDR) | Second-line to psychological therapies SSRIs (fluoxetine, sertraline, paroxetine), venlafaxine. | Y | Insufficient evidence for or against its use |
| Clinical Practice Guidelines for the Treatment of PTSD (APoA) [37] | American Psychological Association | United States | 2017 | CPT, CT, Trauma-focused CBT, and PE | SSRIs (fluoxetine, sertraline, paroxetine), venlafaxine. | N | No recommendation |
| Post Traumatic Stress Disorder NICE Guidance (NICE) [38] | National Institute for Clinical Excellence | United Kingdom | 2018 | Trauma-focused CBT | Venlafaxine or SSRIs only if person has preference for drug treatment | N | No recommendation |
| Effective Treatments for PTSD: Third Edition (ISTSS) [39] | International Society for Traumatic Stress Studies | International | 2020 | CPT, CT, Trauma-focused CBT, EMDR and PE | SSRIs (fluoxetine, sertraline, paroxetine), venlafaxine. | N | No recommendation |
| Australian Guidelines for the Prevention and Treatment of Acute Stress Disorder, Posttraumatic Stress Disorder, and Complex Posttraumatic Stress Disorder (Phoenix) [16] | Phoenix Australia—Centre for Posttraumatic Mental Health | Australia | 2020 | CPT, CT, Trauma-focused CBT, EMDR and PE | Second-line to psychological therapies SSRIs (sertraline, fluoxetine, paroxetine), venlafaxine | N | No recommendation |
| Guideline (Abbreviation) | Domain 1 Scope and Purpose | Domain 2 Stakeholder Involvement | Domain 3 Rigour of development | Domain 4 Clarity of presentation | Domain 5 Applicability | Domain 6 Editorial independence | Recommended for Use? |
|---|---|---|---|---|---|---|---|
| Practice Guideline for the Treatment of Patients with Acute Stress Disorder and Posttraumatic Stress Disorder (APiA) [28] | 68% | 52% | 66% | 71% | 42% | 79% | Y/M |
| Clinical Practice Guidelines for the Management of Anxiety Disorders (CPA) [29] | 81% | 56% | 57% | 86% | 42% | 43% | Y/M |
| World Federation of Societies of Biological Psychiatry Guidelines for the Pharmacological Treatment of Anxiety, Obsessive-Compulsive and Post-Traumatic Stress Disorders—First Revision (WFSBP) [30] | 76% | 44% | 60% | 71% | 37% | 50% | N |
| Best Practice Guide for the Treatment of Nightmare Disorder (AASM) [31] | 89% | 30% | 85% | 90% | 30% | 93% | Y/M |
| Guidelines for the Management of Conditions Specifically Related to Stress (WHO) [32] | 98% | 73% | 92% | 100% | 67% | 86% | Y |
| eTG complete—Posttraumatic Mental Health Disorders (eTG) [15] | 68% | 68% | 55% | 83% | 37% | 93% | Y/M |
| The South African Society of Psychiatrists (SASOP) Treatment Guidelines for Psychiatric Disorders (SASOP) [33] | 79% | 67% | 44% | 89% | 49% | 43% | Y |
| Canadian Clinical Practice Guidelines for the Management of Anxiety, Posttraumatic Stress and Obsessive-compulsive Disorders (ADAC) [34] | 71% | 57% | 54% | 71% | 31% | 71% | N |
| Evidence-based Pharmacological Treatment of Anxiety Disorders, Post-traumatic Stress Disorder and Obsessive-compulsive Disorder: A Revision of the 2005 Guidelines from the British Association for Psychopharmacology (BAP) [35] | 87% | 76% | 60% | 95% | 48% | 76% | Y/M |
| VA/DOD Clinical Practice Guideline for the Management of Posttraumatic Stress Disorder and Acute Stress Disorder (VA) [36] | 100% | 100% | 84% | 100% | 67% | 52% | Y |
| Clinical Practice Guidelines for the Treatment of PTSD (APoA) [37] | 98% | 100% | 92% | 97% | 60% | 100% | Y |
| Post Traumatic Stress Disorder NICE Guidance (NICE) [38] | 95% | 100% | 86% | 98% | 61% | 64% | Y |
| Effective Treatments for PTSD: Third Edition (ISTSS) ** [39] | 86% | 70% | 64% | 78% | 57% | 26% | Y/M |
| Australian Guidelines for the Treatment of Acute Stress Disorder & Posttraumatic Stress Disorder (Phoenix) [16] | 100% | 93% | 89% | 92% | 74% | 95% | Y |
| Domain median (range) | 87% (68–100%) | 69% (30–100%) | 65% (44–92%) | 90% (71–100%) | 49% (30–74%) | 74% (26–100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, A.; Naunton, M.; Kosari, S.; Peterson, G.; Thomas, J.; Christenson, J.K. Treatment Guidelines for PTSD: A Systematic Review. J. Clin. Med. 2021, 10, 4175. https://doi.org/10.3390/jcm10184175
Martin A, Naunton M, Kosari S, Peterson G, Thomas J, Christenson JK. Treatment Guidelines for PTSD: A Systematic Review. Journal of Clinical Medicine. 2021; 10(18):4175. https://doi.org/10.3390/jcm10184175
Chicago/Turabian StyleMartin, Alicia, Mark Naunton, Sam Kosari, Gregory Peterson, Jackson Thomas, and Julia K. Christenson. 2021. "Treatment Guidelines for PTSD: A Systematic Review" Journal of Clinical Medicine 10, no. 18: 4175. https://doi.org/10.3390/jcm10184175
APA StyleMartin, A., Naunton, M., Kosari, S., Peterson, G., Thomas, J., & Christenson, J. K. (2021). Treatment Guidelines for PTSD: A Systematic Review. Journal of Clinical Medicine, 10(18), 4175. https://doi.org/10.3390/jcm10184175









