Sensory Nerve Conduction Velocity Predicts Improvement of Hand Function with Nerve Gliding Exercise Following Carpal Tunnel Release Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
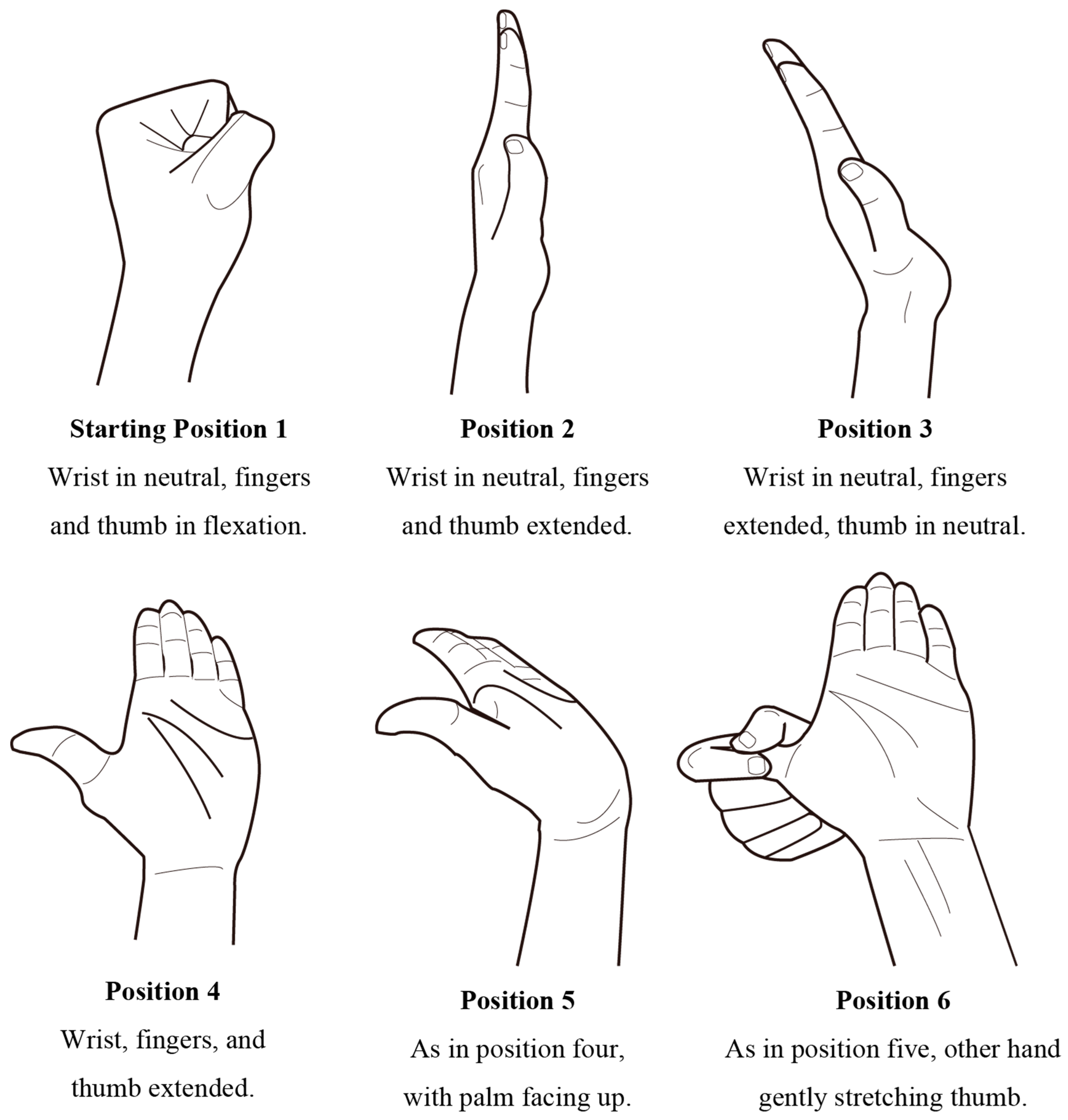
2.2. NGE
2.3. Outcome Measures
2.3.1. Grip-s and Pinch-s
2.3.2. Semmes-Weinstein Monofilament Test (SWMT)
2.3.3. Two-Point Discrimination (2PD)
2.3.4. Numerical Rating Scale (NRS) for Numbness and Pain
2.4. Statistical Analysis
3. Results
3.1. Comparison between Preintervention and Final Assessments
3.2. Multiple Regression Analysis
4. Discussion
4.1. Effectiveness of Surgical Treatment Combined with NGE
4.2. Functions and Factors Improved by Surgery and NGE
4.3. Limitation
4.4. Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gerritsen, A.A.; de Krom, M.C.; Struijs, M.A.; Scholten, R.J.; de Vet, H.C.; Bouter, L.M. Conservative treatment options for carpal tunnel syndrome: A systematic review of randomised controlled trials. J. Neurol. 2002, 249, 272–280. [Google Scholar] [CrossRef]
- Atroshi, I.; Gummesson, C.; Johnsson, R.; Ornstein, E.; Ranstam, J.; Rosén, I. Prevalence of carpal tunnel syndrome in a general population. JAMA 1999, 282, 153–158. [Google Scholar] [CrossRef]
- Papanicolaou, G.D.; McCabe, S.J.; Firrell, J. The prevalence and characteristics of nerve compression symptoms in the general population. J. Hand Surg. 2001, 26, 460–466. [Google Scholar] [CrossRef]
- Bakhtiary, A.H.; Rashidy-Pour, A. Ultrasound and laser therapy in the treatment of carpal tunnel syndrome. Aust. J. Physiother. 2004, 50, 147–151. [Google Scholar] [CrossRef]
- Vogt, T.; Scholz, J. Clinical outcome and predictive value of electrodiagnostics in endoscopic carpal tunnel surgery. Neurosurg. Rev. 2002, 25, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-Las-Peñas, C.; Pérez-de-Heredia-Torres, M.; Martínez-Piédrola, R.; de la Llave-Rincón, A.I.; Cleland, J.A. Bilateral deficits in fine motor control and pinch grip force in patients with unilateral carpal tunnel syndrome. Exp. Brain Res. 2009, 194, 29–37. [Google Scholar] [CrossRef]
- Li, K.; Evans, P.J.; Seitz, W.H., Jr.; Li, Z.M. Carpal tunnel syndrome impairs sustained precision pinch performance. Clin. Neurophysiol. 2015, 126, 194–201. [Google Scholar] [CrossRef]
- Huisstede, B.M.; Randsdorp, M.S.; Coert, J.H.; Glerum, S.; van Middelkoop, M.; Koes, B.W. Carpal tunnel syndrome. Part II: Effectiveness of surgical treatments—A systematic review. Arch. Phys. Med. Rehabil. 2010, 91, 1005–1024. [Google Scholar] [CrossRef] [PubMed]
- Burke, D.T.; Burke, M.M.; Stewart, G.W.; Cambré, A. Splinting for carpal tunnel syndrome: In search of the optimal angle. Arch. Phys. Med. Rehabil. 1994, 75, 1241–1244. [Google Scholar] [CrossRef]
- Burke, F.D.; Ellis, J.; McKenna, H.; Bradley, M.J. Primary care management of carpal tunnel syndrome. Postgrad. Med. J. 2003, 79, 433–437. [Google Scholar] [CrossRef]
- Michlovitz, S.L. Conservative interventions for carpal tunnel syndrome. J. Orthop. Sports Phys. Ther. 2004, 34, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Osterman, A.L.; Whitman, M.; Porta, L.D. Nonoperative carpal tunnel syndrome treatment. Hand Clin. 2002, 18, 279–289. [Google Scholar] [CrossRef]
- Ucan, H.; Yagci, I.; Yilmaz, L.; Yagmurlu, F.; Keskin, D.; Bodur, H. Comparison of splinting, splinting plus local steroid injection and open carpal tunnel release outcomes in idiopathic carpal tunnel syndrome. Rheumatol. Int. 2006, 27, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Verdugo, R.J.; Salinas, R.A.; Castillo, J.L.; Cea, J.G. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst. Rev. 2008, 2008, Cd001552. [Google Scholar] [CrossRef]
- Baysal, O.; Altay, Z.; Ozcan, C.; Ertem, K.; Yologlu, S.; Kayhan, A. Comparison of three conservative treatment protocols in carpal tunnel syndrome. Int. J. Clin. Pract. 2006, 60, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; O’Connor, D.; Pitt, V.; Massy-Westropp, N. Exercise and mobilisation interventions for carpal tunnel syndrome. Cochrane Database Syst. Rev. 2012, 2012, Cd009899. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; O’Connor, D.; Pitt, V.; Massy-Westropp, N. Therapeutic ultrasound for carpal tunnel syndrome. Cochrane Database Syst. Rev. 2013, 2013, Cd009601. [Google Scholar] [CrossRef] [PubMed]
- Hui, A.C.; Wong, S.; Leung, C.H.; Tong, P.; Mok, V.; Poon, D.; Li-Tsang, C.W.; Wong, L.K.; Boet, R. A randomized controlled trial of surgery vs steroid injection for carpal tunnel syndrome. Neurology 2005, 64, 2074–2078. [Google Scholar] [CrossRef]
- Ly-Pen, D.; Andréu, J.L.; de Blas, G.; Sánchez-Olaso, A.; Millán, I. Surgical decompression versus local steroid injection in carpal tunnel syndrome: A one-year, prospective, randomized, open, controlled clinical trial. Arthritis Rheum. 2005, 52, 612–619. [Google Scholar] [CrossRef]
- Marshall, S.; Tardif, G.; Ashworth, N. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst. Rev. 2007, 2007, Cd001554. [Google Scholar] [CrossRef]
- McKeon, J.M.M.; Yancosek, K.E. Neural gliding techniques for the treatment of carpal tunnel syndrome: A systematic review. J. Sport Rehabil. 2008, 17, 324–341. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lim, Y.H.; Chee, D.Y.; Girdler, S.; Lee, H.C. Median nerve mobilization techniques in the treatment of carpal tunnel syndrome: A systematic review. J. Hand Ther. 2017, 30, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Tsui, D.; Schnurr, R.; Biddulph-Deisroth, L.; Hard, J.; MacDermid, J.C. Effectiveness of hand therapy interventions in primary management of carpal tunnel syndrome: A systematic review. J. Hand Ther. 2004, 17, 210–228. [Google Scholar] [CrossRef]
- Akalin, E.; El, O.; Peker, O.; Senocak, O.; Tamci, S.; Gülbahar, S.; Cakmur, R.; Oncel, S. Treatment of carpal tunnel syndrome with nerve and tendon gliding exercises. Am. J. Phys. Med. Rehabil. 2002, 81, 108–113. [Google Scholar] [CrossRef]
- Pinar, L.; Enhos, A.; Ada, S.; Güngör, N. Can we use nerve gliding exercises in women with carpal tunnel syndrome? Adv. Ther. 2005, 22, 467–475. [Google Scholar] [CrossRef]
- Heebner, M.L.; Roddey, T.S. The effects of neural mobilization in addition to standard care in persons with carpal tunnel syndrome from a community hospital. J. Hand Ther. 2008, 21, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Coppieters, M.W.; Alshami, A.M. Longitudinal excursion and strain in the median nerve during novel nerve gliding exercises for carpal tunnel syndrome. J. Orthop. Res. 2007, 25, 972–980. [Google Scholar] [CrossRef]
- Tal-Akabi, A.; Rushton, A. An investigation to compare the effectiveness of carpal bone mobilisation and neurodynamic mobilisation as methods of treatment for carpal tunnel syndrome. Man. Ther. 2000, 5, 214–222. [Google Scholar] [CrossRef][Green Version]
- Horng, Y.S.; Hsieh, S.F.; Tu, Y.K.; Lin, M.C.; Horng, Y.S.; Wang, J.D. The comparative effectiveness of tendon and nerve gliding exercises in patients with carpal tunnel syndrome: A randomized trial. Am. J. Phys. Med. Rehabil. 2011, 90, 435–442. [Google Scholar] [CrossRef]
- Degnan, G.G. Postoperative management following carpal tunnel release surgery: Principles of rehabilitation. Neurosurg. Focus 1997, 3, e8. [Google Scholar] [CrossRef]
- Cook, A.C.; Szabo, R.M.; Birkholz, S.W.; King, E.F. Early mobilization following carpal tunnel release. A prospective randomized study. J. Hand Surg. 1995, 20, 228–230. [Google Scholar] [CrossRef]
- Steyers, C.M. Recurrent carpal tunnel syndrome. Hand Clin. 2002, 18, 339–345. [Google Scholar] [CrossRef]
- Seradge, H.; Jia, Y.C.; Owens, W. In Vivo measurement of carpal tunnel pressure in the functioning hand. J. Hand Surg. 1995, 20, 855–859. [Google Scholar] [CrossRef]
- Nazarieh, M.; Hakakzadeh, A.; Ghannadi, S.; Maleklou, F.; Tavakol, Z.; Alizadeh, Z. Non-Surgical Management and Post-Surgical Rehabilitation of Carpal Tunnel Syndrome: An Algorithmic Approach and Practical Guideline. Asian J. Sports Med. 2020, 11, e102631. [Google Scholar] [CrossRef]
- Rozmaryn, L.M.; Dovelle, S.; Rothman, E.R.; Gorman, K.; Olvey, K.M.; Bartko, J.J. Nerve and tendon gliding exercises and the conservative management of carpal tunnel syndrome. J. Hand Ther. 1998, 11, 171–179. [Google Scholar] [CrossRef]
- Wolny, T.; Linek, P. Reliability of two-point discrimination test in carpal tunnel syndrome patients. Physiother. Theory Pract. 2019, 35, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Simovic, D.; Weinberg, D.H. Carpal tunnel syndrome. Arch. Neurol. 2000, 57, 754–755. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Schlösser, F.J.; Sumpio, B.E. The Semmes Weinstein monofilament examination as a screening tool for diabetic peripheral neuropathy. J. Vasc. Surg. 2009, 50, 675–682.e671. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Andary, M. Carpal tunnel syndrome: Pathophysiology and clinical neurophysiology. Clin. Neurophysiol. 2002, 113, 1373–1381. [Google Scholar] [CrossRef]
- Phan, N.Q.; Blome, C.; Fritz, F.; Gerss, J.; Reich, A.; Ebata, T.; Augustin, M.; Szepietowski, J.C.; Ständer, S. Assessment of pruritus intensity: Prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm.-Venereol. 2012, 92, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Andary, M. Electrodiagnostic evaluation of carpal tunnel syndrome. Muscle Nerve 2011, 44, 597–607. [Google Scholar] [CrossRef]
- Lew, H.L.; Date, E.S.; Pan, S.S.; Wu, P.; Ware, P.F.; Kingery, W.S. Sensitivity, specificity, and variability of nerve conduction velocity measurements in carpal tunnel syndrome. Arch. Phys. Med. Rehabil. 2005, 86, 12–16. [Google Scholar] [CrossRef]
- Gelberman, R.H.; Hergenroeder, P.T.; Hargens, A.R.; Lundborg, G.N.; Akeson, W.H. The carpal tunnel syndrome. A study of carpal canal pressures. J. Bone Jt. Surgery. Am. Vol. 1981, 63, 380–383. [Google Scholar] [CrossRef]
- Choi, S.J.; Ahn, D.S. Correlation of clinical history and electrodiagnostic abnormalities with outcome after surgery for carpal tunnel syndrome. Plast. Reconstr. Surg. 1998, 102, 2374–2380. [Google Scholar] [CrossRef] [PubMed]
- Townshend, D.N.; Taylor, P.K.; Gwynne-Jones, D.P. The outcome of carpal tunnel decompression in elderly patients. J. Hand Surg. 2005, 30, 500–505. [Google Scholar] [CrossRef]
- Serrano Afonso, A.; Carnaval, T.; Videla Ces, S. Combination Therapy for Neuropathic Pain: A Review of Recent Evidence. J. Clin. Med. 2021, 10, 3533. [Google Scholar] [CrossRef] [PubMed]
- Hulkkonen, S.; Auvinen, J.; Miettunen, J.; Karppinen, J.; Ryhanen, J. Smoking as risk factor for carpal tunnel syndrome: A birth cohort study. Muscle Nerve 2019, 60, 299–304. [Google Scholar] [CrossRef]
- Nathan, P.A.; Keniston, R.C.; Lockwood, R.S.; Meadows, K.D. Tobacco, caffeine, alcohol, and carpal tunnel syndrome in American industry. A cross-sectional study of 1464 workers. J. Occup. Environ. Med. 1996, 38, 290–298. [Google Scholar] [CrossRef]
- Meirelles, L.M.; Fernandes, C.H.; Ejnisman, B.; Cohen, M.; dos Santos, J.B.G.; Albertoni, W.M. The prevalence of carpal tunnel syndrome in adapted Sports athletes based on clinical diagnostic. Orthop. Traumatol. Surg. Res. 2020, 106, 751–756. [Google Scholar] [CrossRef]
- Bartlett, O.; Farnsworth, J.L. The Influence of Kinesiophobia on Perceived Disability in Patients With an Upper-Extremity Injury: A Critically Appraised Topic. J. Sport Rehabil. 2021, 30, 818–823. [Google Scholar] [CrossRef]
- Tamaru, Y.; Yanagawa, A.; Matsugi, A. Dataset_CTS, Mendeley Data, Version 2. 2021. Available online: https://data.mendeley.com/research-data/?search=doi:10.17632/dpyg8dw49n.2 (accessed on 9 September 2021).
| n | |
|---|---|
| Subjects | 67 |
| Hands (Rt/Lt) | 86 (48/38) |
| Age (mean ± SD) | 66.8 ± 14.1 |
| Gender | M: 24; F: 43 |
| Dialysis | 4 |
| Trigger finger | 20 |
| TEA 1 (Rt/Lt) | 43 (26/17) |
| Difference between Pre vs. Post | 95% CL | p-Value | ||||
|---|---|---|---|---|---|---|
| Average | Standard Error | Lower | Upper | |||
| Grip-s (kgf) | 1.13 | 4.45 | 0.05 | 2.21 | 0.04 | ** |
| Pinch-s (kgf) | 0.45 | 1.33 | 0.13 | 0.77 | 0.007 | ** |
| SWMT | 0.96 | 1.26 | 0.66 | 1.26 | 0.001 | ** |
| 2PD (mm) | 1.71 | 4.99 | 0.52 | 2.91 | 0.005 | ** |
| Numb (score) | 4.18 | 2.38 | 3.60 | 4.75 | 0.001 | ** |
| Pain (score) | 0.56 | 3.19 | −0.20 | 1.33 | 0.14 | |
| Phalen (angle) | 16.01 | 19.49 | 11.37 | 20.66 | 0.001 | ** |
| B | Standard Error | β | B (95% CI) | F | t | p-Value | VIF | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||||
| Age | 0.03 | 0.09 | 0.05 | −0.14 | 0.20 | 0.12 | 0.35 | 0.727 | 1.43 | |
| Gender | −6.03 | 2.53 | −0.33 | −11.10 | −0.96 | 5.70 | −2.39 | 0.021 | * | 1.20 |
| Fault side | 2.42 | 2.36 | 0.14 | −2.33 | 7.16 | 1.05 | 1.02 | 0.311 | 1.21 | |
| Bilateral | 0.55 | 2.57 | 0.03 | −4.61 | 5.70 | 0.05 | 0.21 | 0.832 | 1.28 | |
| Trigger finger | −2.19 | 1.98 | −0.14 | −6.17 | 1.78 | 1.22 | −1.11 | 0.274 | 1.09 | |
| Dialysis | −4.64 | 5.54 | −0.12 | −15.76 | 6.47 | 0.70 | −0.84 | 0.406 | 1.23 | |
| TEA | −0.34 | 2.43 | −0.02 | −5.23 | 4.54 | 0.02 | −0.14 | 0.888 | 1.33 | |
| MCV | −0.08 | 0.14 | −0.08 | −0.36 | 0.20 | 0.34 | −0.58 | 0.562 | 1.24 | |
| SCV | 0.24 | 0.12 | 0.30 | 0.00 | 0.48 | 4.11 | 2.03 | 0.048 | * | 1.38 |
| Start-treat | 0.04 | 0.03 | 0.18 | −0.02 | 0.09 | 1.83 | 1.35 | 0.182 | 1.15 | |
| OT-inter | −0.01 | 0.10 | −0.02 | −0.21 | 0.19 | 0.01 | −0.11 | 0.914 | 1.23 | |
| Constant | −0.05 | 13.03 | −26.20 | 26.10 | 0.00 | 0.00 | 0.997 | |||
| B | Standard Error | β | B (95% CI) | F | t | p-Value | VIF | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||||
| Age | 0.00 | 0.02 | −0.03 | −0.04 | 0.03 | 0.05 | −0.23 | 0.822 | 1.43 | |
| Gender | −1.40 | 0.48 | −0.39 | −2.36 | −0.44 | 8.54 | −2.92 | 0.005 | ** | 1.20 |
| Fault side | 0.25 | 0.45 | 0.07 | −0.65 | 1.14 | 0.30 | 0.55 | 0.587 | 1.21 | |
| Bilateral | 0.24 | 0.49 | 0.07 | −0.74 | 1.21 | 0.24 | 0.49 | 0.629 | 1.28 | |
| Trigger finger | −0.20 | 0.38 | −0.07 | −0.96 | 0.55 | 0.29 | −0.54 | 0.594 | 1.09 | |
| Dialysis | −0.77 | 1.05 | −0.10 | −2.87 | 1.34 | 0.53 | −0.73 | 0.468 | 1.23 | |
| TEA | −0.44 | 0.46 | −0.13 | −1.37 | 0.48 | 0.92 | −0.96 | 0.343 | 1.33 | |
| MCV | −0.02 | 0.03 | −0.11 | −0.07 | 0.03 | 0.64 | −0.80 | 0.428 | 1.24 | |
| SCV | 0.05 | 0.02 | 0.29 | 0.00 | 0.09 | 4.18 | 2.04 | 0.046 | * | 1.38 |
| Start-treat | 0.01 | 0.01 | 0.21 | 0.00 | 0.02 | 2.65 | 1.63 | 0.109 | 1.15 | |
| OT-inter | −0.02 | 0.02 | −0.15 | −0.06 | 0.02 | 1.22 | −1.10 | 0.275 | 1.23 | |
| Constant | 2.18 | 2.47 | −2.78 | 7.13 | 0.78 | 0.88 | 0.383 | |||
| B | Standard Error | β | B (95% CI) | F | t | p-Value | VIF | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||||
| Age | −0.01 | 0.02 | −0.06 | −0.04 | 0.02 | 0.22 | −0.47 | 0.640 | 1.43 | |
| Gender | −0.62 | 0.44 | −0.18 | −1.50 | 0.26 | 1.99 | −1.41 | 0.164 | 1.20 | |
| Fault side | 0.45 | 0.41 | 0.14 | −0.38 | 1.27 | 1.18 | 1.08 | 0.283 | 1.21 | |
| Bilateral | −0.25 | 0.45 | −0.07 | −1.15 | 0.64 | 0.32 | −0.56 | 0.576 | 1.28 | |
| Trigger finger | 0.07 | 0.34 | 0.03 | −0.62 | 0.76 | 0.05 | 0.21 | 0.831 | 1.09 | |
| Dialysis | −0.19 | 0.96 | −0.03 | −2.12 | 1.73 | 0.04 | −0.20 | 0.840 | 1.23 | |
| TEA | 0.20 | 0.42 | 0.06 | −0.65 | 1.05 | 0.22 | 0.47 | 0.639 | 1.33 | |
| MCV | 0.03 | 0.02 | 0.14 | −0.02 | 0.08 | 1.28 | 1.13 | 0.263 | 1.24 | |
| SCV | 0.03 | 0.02 | 0.19 | −0.01 | 0.07 | 2.03 | 1.42 | 0.161 | 1.38 | |
| Start-treat | 0.01 | 0.00 | 0.30 | 0.00 | 0.02 | 5.81 | 2.41 | 0.019 | * | 1.15 |
| OT-inter | −0.05 | 0.02 | −0.33 | −0.08 | −0.01 | 6.82 | −2.61 | 0.012 | * | 1.23 |
| Constant | −1.46 | 2.26 | −6.00 | 3.09 | 0.41 | −0.64 | 0.523 | |||
| B | Standard Error | β | B (95% CI) | F | t | p-Value | VIF | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||||
| Age | 0.02 | 0.05 | 0.08 | −0.07 | 0.12 | 0.26 | 0.51 | 0.611 | 1.43 | |
| Gender | −0.92 | 1.38 | −0.09 | −3.69 | 1.86 | 0.44 | −0.66 | 0.509 | 1.20 | |
| Fault side | 1.55 | 1.29 | 0.16 | −1.04 | 4.15 | 1.44 | 1.20 | 0.236 | 1.21 | |
| Bilateral | 1.82 | 1.41 | 0.18 | −1.00 | 4.65 | 1.68 | 1.30 | 0.201 | 1.28 | |
| Trigger finger | 0.55 | 1.09 | 0.07 | −1.63 | 2.73 | 0.26 | 0.51 | 0.616 | 1.09 | |
| Dialysis | −1.54 | 3.03 | −0.07 | −7.62 | 4.55 | 0.26 | −0.51 | 0.614 | 1.23 | |
| TEA | −0.75 | 1.33 | −0.08 | −3.43 | 1.92 | 0.32 | −0.57 | 0.574 | 1.33 | |
| MCV | 0.10 | 0.08 | 0.18 | −0.05 | 0.25 | 1.68 | 1.30 | 0.201 | 1.24 | |
| SCV | 0.08 | 0.06 | 0.18 | −0.05 | 0.21 | 1.52 | 1.23 | 0.224 | 1.38 | |
| Start-treat | 0.03 | 0.01 | 0.27 | 0.00 | 0.06 | 4.23 | 2.06 | 0.045 | * | 1.15 |
| OT-intere | 0.03 | 0.05 | 0.07 | −0.08 | 0.14 | 0.25 | 0.50 | 0.618 | 1.23 | |
| Constant | −16.28 | 7.14 | −30.60 | −1.96 | 5.20 | −2.28 | 0.027 | * | ||
| B | Standard Error | β | B (95% CI) | F | t | p-Value | VIF | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||||
| Age | 0.01 | 0.03 | 0.05 | −0.05 | 0.07 | 0.21 | 0.35 | 0.73 | 1.43 | |
| Gender | −1.80 | 0.89 | −0.26 | −3.59 | −0.01 | 4.07 | −2.02 | 0.07 | 1.20 | |
| Fault side | 1.31 | 0.84 | 0.20 | −0.36 | 2.99 | 2.47 | 1.57 | 0.12 | 1.21 | |
| Bilateral | −0.24 | 0.91 | −0.04 | −2.07 | 1.58 | 0.07 | −0.27 | 0.79 | 1.28 | |
| Trigger finger | −0.02 | 0.70 | 0.00 | −1.43 | 1.39 | 0.00 | −0.03 | 0.98 | 1.09 | |
| Dialysis | −0.81 | 1.96 | −0.05 | −4.74 | 3.12 | 0.17 | −0.42 | 0.68 | 1.23 | |
| TEA | 1.62 | 0.86 | 0.26 | −0.11 | 3.35 | 3.55 | 1.88 | 0.07 | 1.33 | |
| MCV | −0.02 | 0.05 | −0.05 | −0.12 | 0.08 | 0.16 | −0.40 | 0.69 | 1.24 | |
| SCV | 0.07 | 0.04 | 0.24 | −0.01 | 0.16 | 3.14 | 1.77 | 0.08 | 1.38 | |
| Start-treat | 0.02 | 0.01 | 0.22 | 0.00 | 0.04 | 3.07 | 1.75 | 0.09 | 1.15 | |
| OT-intere | −0.06 | 0.04 | −0.21 | −0.13 | 0.01 | 2.51 | −1.58 | 0.12 | 1.23 | |
| Constant | −1.76 | 4.61 | −11.01 | 7.49 | 0.15 | −0.38 | 0.70 | |||
| B | Standard Error | β | B (95% CI) | F | t | p-Value | VIF | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||||
| Age | −0.03 | 0.03 | −0.12 | −0.09 | 0.04 | 0.71 | −0.84 | 0.404 | 1.43 | |
| Gender | 0.21 | 0.90 | 0.03 | −1.59 | 2.02 | 0.06 | 0.24 | 0.812 | 1.20 | |
| Fault side | 0.77 | 0.84 | 0.12 | −0.92 | 2.46 | 0.84 | 0.92 | 0.363 | 1.21 | |
| Bilateral | −0.87 | 0.91 | −0.13 | −2.71 | 0.96 | 0.91 | −0.96 | 0.343 | 1.28 | |
| Trigger finger | 0.85 | 0.70 | 0.15 | −0.56 | 2.27 | 1.47 | 1.21 | 0.232 | 1.09 | |
| Dialysis | −0.07 | 1.97 | 0.00 | −4.02 | 3.88 | 0.00 | −0.03 | 0.973 | 1.23 | |
| TEA | −0.08 | 0.87 | −0.01 | −1.82 | 1.65 | 0.01 | −0.09 | 0.926 | 1.33 | |
| MCV | 0.08 | 0.05 | 0.21 | −0.02 | 0.18 | 2.47 | 1.57 | 0.122 | 1.24 | |
| SCV | 0.08 | 0.04 | 0.27 | 0.00 | 0.17 | 3.83 | 1.96 | 0.056 | 1.38 | |
| Start-treat | 0.01 | 0.01 | 0.18 | −0.01 | 0.03 | 2.12 | 1.45 | 0.152 | 1.15 | |
| OT-intere | −0.03 | 0.04 | −0.11 | −0.10 | 0.04 | 0.72 | −0.85 | 0.399 | 1.23 | |
| Constant | −9.51 | 4.63 | −18.81 | −0.22 | 4.22 | −2.05 | 0.045 | * | ||
| B | Standard Error | β | B (95% CI) | F | t | p-Value | VIF | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||||
| Age | 0.39 | 0.24 | 0.24 | −0.09 | 0.88 | 2.64 | 1.63 | 0.110 | 1.43 | |
| Gender | −3.52 | 7.07 | −0.07 | −17.71 | 10.66 | 0.25 | −0.50 | 0.620 | 1.20 | |
| Fault side | 5.10 | 6.62 | 0.10 | −8.18 | 18.38 | 0.59 | 0.77 | 0.444 | 1.21 | |
| Bilateral | 7.56 | 7.19 | 0.15 | −6.87 | 21.99 | 1.11 | 1.05 | 0.298 | 1.28 | |
| Trigger finger | −7.77 | 5.55 | −0.18 | −18.90 | 3.37 | 1.96 | −1.40 | 0.168 | 1.09 | |
| Dialysis | 2.62 | 15.50 | 0.02 | −28.49 | 33.72 | 0.03 | 0.17 | 0.867 | 1.23 | |
| TEA | −7.04 | 6.81 | −0.15 | −20.72 | 6.63 | 1.07 | −1.03 | 0.306 | 1.33 | |
| MCV | 0.11 | 0.39 | 0.04 | −0.68 | 0.90 | 0.08 | 0.28 | 0.781 | 1.24 | |
| SCV | 0.42 | 0.33 | 0.18 | −0.24 | 1.09 | 1.63 | 1.28 | 0.207 | 1.38 | |
| Start-treat | 0.13 | 0.08 | 0.22 | −0.03 | 0.28 | 2.76 | 1.66 | 0.102 | 1.15 | |
| OT-intere | −0.33 | 0.28 | −0.16 | −0.88 | 0.23 | 1.37 | −1.17 | 0.247 | 1.23 | |
| Constant | −36.69 | 36.48 | −109.88 | 36.51 | 1.01 | −1.01 | 0.319 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamaru, Y.; Yanagawa, A.; Matsugi, A. Sensory Nerve Conduction Velocity Predicts Improvement of Hand Function with Nerve Gliding Exercise Following Carpal Tunnel Release Surgery. J. Clin. Med. 2021, 10, 4121. https://doi.org/10.3390/jcm10184121
Tamaru Y, Yanagawa A, Matsugi A. Sensory Nerve Conduction Velocity Predicts Improvement of Hand Function with Nerve Gliding Exercise Following Carpal Tunnel Release Surgery. Journal of Clinical Medicine. 2021; 10(18):4121. https://doi.org/10.3390/jcm10184121
Chicago/Turabian StyleTamaru, Yoshiki, Akiyoshi Yanagawa, and Akiyoshi Matsugi. 2021. "Sensory Nerve Conduction Velocity Predicts Improvement of Hand Function with Nerve Gliding Exercise Following Carpal Tunnel Release Surgery" Journal of Clinical Medicine 10, no. 18: 4121. https://doi.org/10.3390/jcm10184121
APA StyleTamaru, Y., Yanagawa, A., & Matsugi, A. (2021). Sensory Nerve Conduction Velocity Predicts Improvement of Hand Function with Nerve Gliding Exercise Following Carpal Tunnel Release Surgery. Journal of Clinical Medicine, 10(18), 4121. https://doi.org/10.3390/jcm10184121







