Effectiveness of a New 3D-Printed Dynamic Hand–Wrist Splint on Hand Motor Function and Spasticity in Chronic Stroke Patients
Abstract
1. Introduction
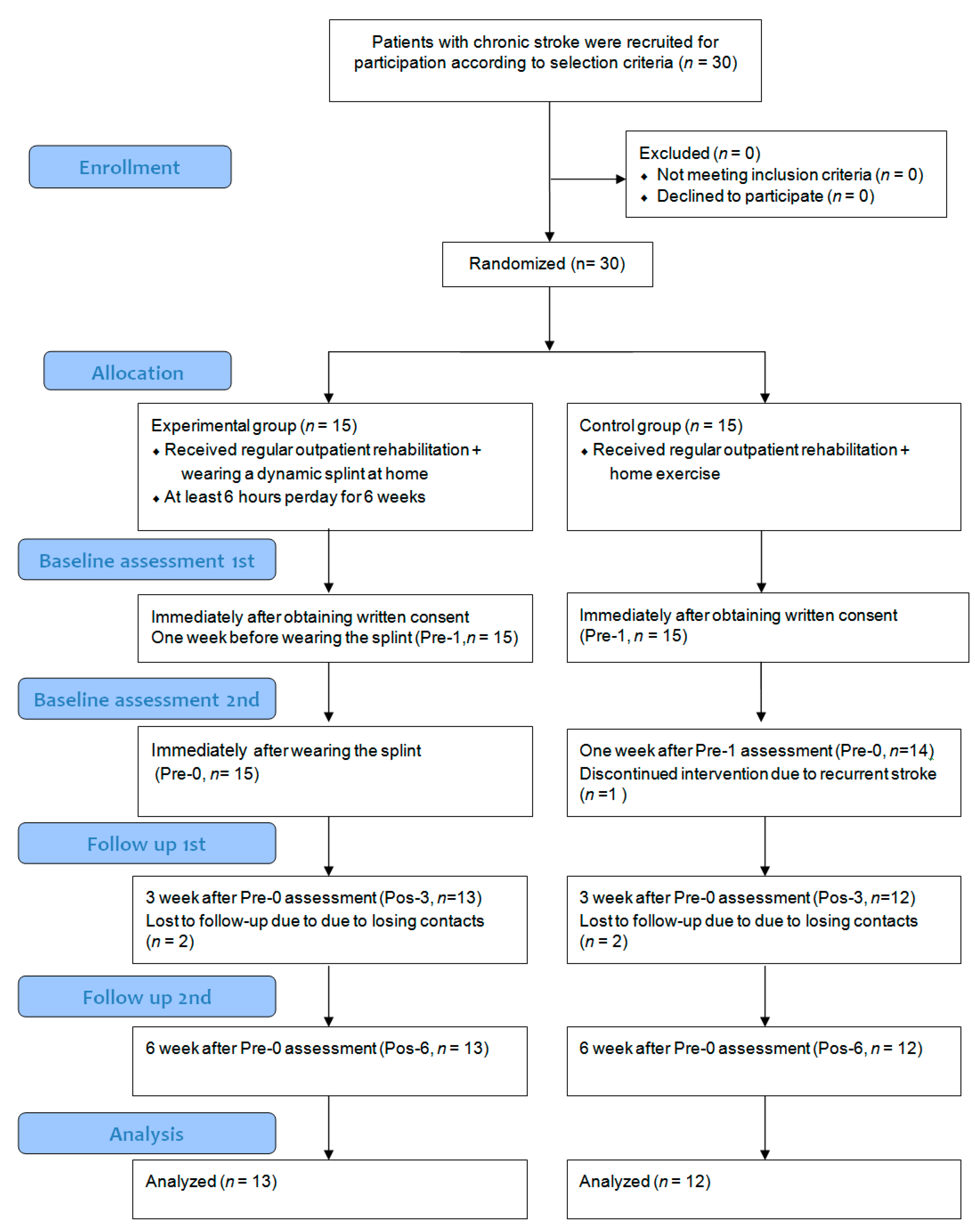
2. Materials and Methods
2.1. Participants
2.2. Intervention
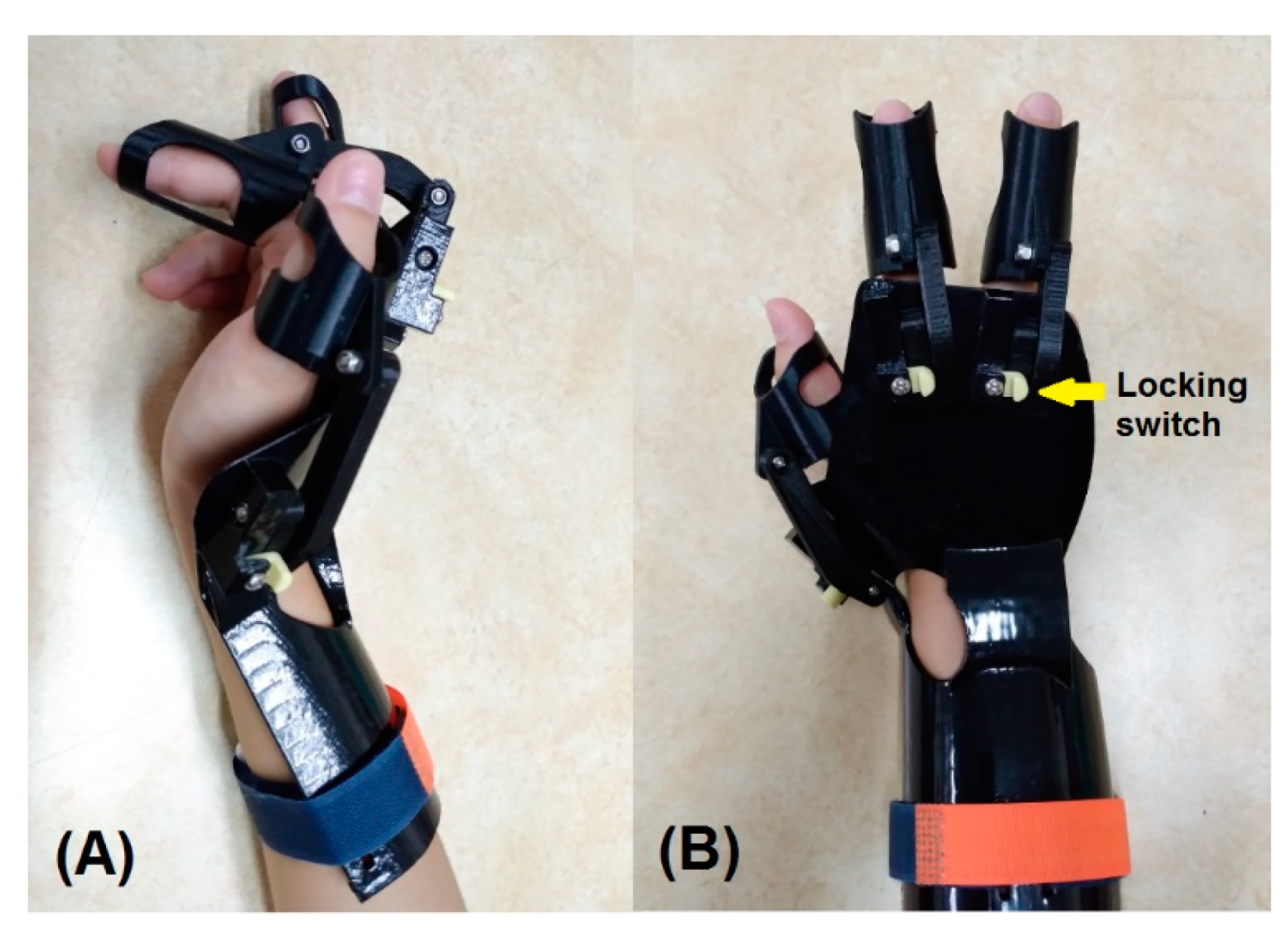
2.3. D-Printed Dynamic Splint
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Clinical Assessments
3.3. Subjective Self-Reported Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kong, K.H.; Chua, K.S.; Lee, J. Symptomatic upper limb spasticity in patients with chronic stroke attending a rehabilitation clinic: Frequency, clinical correlates and predictors. J. Rehabil. Med. 2010, 42, 453–457. [Google Scholar] [CrossRef]
- Urban, P.P.; Wolf, T.; Uebele, M.; Marx, J.J.; Vogt, T.; Stoeter, P.; Bauermann, T.; Weibrich, C.; Vucurevic, G.D.; Schneider, A.; et al. Occurence and clinical predictors of spasticity after ischemic stroke. Stroke 2010, 41, 2016–2020. [Google Scholar] [CrossRef] [PubMed]
- Pandyan, A.; Cameron, M.; Powell, J.; Stott, D.; Granat, M. Contractures in the post-stroke wrist: A pilot study of its time course of development and its association with upper limb recovery. Clin. Rehabil. 2003, 17, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.; Pandyan, A.; Rosewilliam, S.; Roffe, C.; Hermens, H. Spasticity and contractures at the wrist after stroke: Time course of development and their association with functional recovery of the upper limb. Clin. Rehabil. 2011, 25, 184–191. [Google Scholar] [CrossRef]
- Salazar, A.P.; Pinto, C.; Mossi, J.V.R.; Figueiro, B.; Lukrafka, J.L.; Pagnussat, A.S. Effectiveness of static stretching positioning on post-stroke upper-limb spasticity and mobility: Systematic review with meta-analysis. Ann. Phys. Rehabil. Med. 2019, 62, 274–282. [Google Scholar] [CrossRef] [PubMed]
- O’dwyer, N.; Ada, L.; Neilson, P. Spasticity and muscle contracture following stroke. Brain 1996, 119, 1737–1749. [Google Scholar] [CrossRef] [PubMed]
- Gracies, J.M. Pathophysiology of spastic paresis. I: Paresis and soft tissue changes. Muscle Nerve 2005, 31, 535–551. [Google Scholar] [CrossRef]
- Franceschini, M.; La Porta, F.; Agosti, M.; Massucci, M. Is health-related-quality of life of stroke patients influenced by neurological impairments at one year after stroke? Eur. J. Phys. Rehabil. Med. 2010, 46, 389–399. [Google Scholar]
- Schinwelski, M.; Sławek, J. Prevalence of spasticity following stroke and its impact on quality of life with emphasis on disability in activities of daily living. Systematic review. Neurol. Neurochir. Pol. 2010, 44, 404–411. [Google Scholar] [CrossRef][Green Version]
- Sonmez, I.; Karasel, S. Poor sleep quality i related to impaired functional status following stroke. J. Stroke Cerebrovasc. Dis. 2019, 28, 104349. [Google Scholar] [CrossRef]
- Heijnen, I.; Franken, R.; Bevaart, B.; Meijer, J. Long-term outcome of superficialis-to-profundus tendon transfer in patients with clenched fist due to spastic hemiplegia. Disabil. Rehabil. 2008, 30, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Lieber, R.L.; Fridén, J. Spasticity causes a fundamental rearrangement of muscle–joint interaction. Muscle Nerve 2002, 25, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Boven’Eerdt, T.J.; Newman, M.; Barker, K.; Dawes, H.; Minelli, C.; Wade, D.T. The effects of stretching in spasticity: A systematic review. Arch. Phys. Med. Rehabil. 2008, 89, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Harvey, L.A.; Katalinic, O.M.; Herbert, R.D.; Moseley, A.M.; Lannin, N.A.; Schurr, K. Stretch for the treatment and prevention of contracture: An abridged republication of a cochrane systematic review. J. Physiother. 2017, 63, 67–75. [Google Scholar] [CrossRef]
- Jung, Y.J.; Hong, J.H.; Kwon, H.G.; Song, J.C.; Kim, C.; Park, S.; Kim, Y.K.; Ahn, S.H.; Jang, S.H. The effect of a stretching device on hand spasticity in chronic hemiparetic stroke patients. NeuroRehabilitation 2011, 29, 53–59. [Google Scholar] [CrossRef]
- Lannin, N.A.; Cusick, A.; Hills, C.; Kinnear, B.; Vogel, K.; Matthews, K.; Bowring, G. Upper limb motor training using a saebo™ orthosis is feasible for increasing task-specific practice in hospital after stroke. Aust. Occup. Ther. J. 2016, 63, 364–372. [Google Scholar] [CrossRef]
- Andringa, A.S.; Van de Port, I.G.; Meijer, J.W. Tolerance and effectiveness of a new dynamic hand-wrist orthosis in chronic stroke patients. NeuroRehabilitation 2013, 33, 225–231. [Google Scholar] [CrossRef]
- Kim, E.H.; Chang, M.C.; Seo, J.P.; Jang, S.H.; Song, J.C.; Jo, H.M. The effect of a hand-stretching device during the management of spasticity in chronic hemiparetic stroke patients. Ann. Rehabil. Med. 2013, 37, 235–240. [Google Scholar] [CrossRef]
- Greg Pitts, D.; Peganoff O’Brien, S. Splinting the hand to enhance motor control and brain plasticity. Top. Stroke. Rehabil. 2008, 15, 456–467. [Google Scholar] [CrossRef]
- Lannin, N.A.; Horsley, S.A.; Herbert, R.; McCluskey, A.; Cusick, A. Splinting the hand in the functional position after brain impairment: A randomized, controlled trial. Arch. Phys. Med. Rehabil. 2003, 84, 297–302. [Google Scholar] [CrossRef]
- Lannin, N.A.; Cusick, A.; McCluskey, A.; Herbert, R.D. Effects of splinting on wrist contracture after stroke: A randomized controlled trial. Stroke 2007, 38, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Lannin, N.A.; Herbert, R.D. Is hand splinting effective for adults following stroke? A systematic review and methodologic critique of published research. Clin. Rehabil. 2003, 17, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Bianca, C.; Machuki, J.; Chen, W. A dynamic splint for the treatment of spasticity of the hand after stroke-recognition of its design, functionality and limitations: A narrative review article. J. Neurol. Neurorehabil. Res. 2018, 3, 17–19. [Google Scholar]
- Andringa, A.; van de Port, I.; Meijer, J.-W. Long-term use of a static hand-wrist orthosis in chronic stroke patients: A pilot study. Stroke. Res. Treat. 2013, 2013, 546093. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Shi, L.; Wang, D. A rapid and intelligent designing technique for patient-specific and 3d-printed orthopedic cast. 3D Print. Med. 2016, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Lunsford, C.; Grindle, G.; Salatin, B.; Dicianno, B.E. Innovations with 3-dimensional printing in physical medicine and rehabilitation: A review of the literature. PM&R 2016, 8, 1201–1212. [Google Scholar]
- Barry, J.G.; Ross, S.A.; Woehrle, J. Therapy incorporating a dynamic wrist-hand orthosis versus manual assistance in chronic stroke: A pilot study. J. Neurol. Phys. Ther. 2012, 36, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.Y.; Pan, L.H.; Yang, W.W.; Huang, L.Y.; Sun, P.C.; Chen, C.S. Biomechanical evaluation of three-dimensional printed dynamic hand device for patients with chronic stroke. IEEE Trans. Neural. Syst. Rehabil. Eng. 2019, 27, 1246–1252. [Google Scholar] [CrossRef]
- Dudley, D.R.; Knarr, B.A.; Siu, K.C.; Peck, J.; Ricks, B.; Zuniga, J.M. Testing of a 3d printed hand exoskeleton for an individual with stroke: A case study. Disabil. Rehabil. Assist. Technol. 2021, 16, 209–213. [Google Scholar] [CrossRef]
- Sarlegna, F.R.; Sainburg, R.L. The roles of vision and proprioception in the planning of reaching movements. Adv. Exp. Med. Biol. 2009, 629, 317–335. [Google Scholar]
- Scheidt, R.A.; Conditt, M.A.; Secco, E.L.; Mussa-Ivaldi, F.A. Interaction of visual and proprioceptive feedback during adaptation of human reaching movements. J. Neurophysiol. 2005, 93, 3200–3213. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Emzain, Z.F.; Huang, S.C. Biomechanical evaluation of dynamic splint based on pulley rotation design for management of hand spasticity. IEEE Trans. Neural. Syst. Rehabil. Eng. 2021, 29, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Sands, D.; Pérez Gracia, A.; McCormack, J.; Wolbrecht, E.T. Design method for a reconfigurable mechanism for finger rehabilitation. In Proceedings of the 15th IASTED International Conference on Robotics and Applications, Cambridge, MA, USA, 1–3 November 2010; pp. 1–8. [Google Scholar]
- Charalambous, C.P. Interrater reliability of a modified ashworth scale of muscle spasticity. In Classic Papers in Orthopaedics; Springer: New York, NY, USA, 2014; pp. 415–417. [Google Scholar]
- Craven, B.; Morris, A. Modified ashworth scale reliability for measurement of lower extremity spasticity among patients with SCI. SpinalCord 2010, 48, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Meseguer-Henarejos, A.-B.; Sanchez-Meca, J.; Lopez-Pina, J.-A.; Carles-Hernandez, R. Inter-and intra-rater reliability of the modified ashworth scale: A systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 2017, 54, 576–590. [Google Scholar] [PubMed]
- Baker, K.; Cano, S.J.; Playford, E.D. Outcome measurement in stroke: A scale selection strategy. Stroke 2011, 42, 1787–1794. [Google Scholar] [CrossRef]
- Kwakkel, G.; Lannin, N.A.; Borschmann, K.; English, C.; Ali, M.; Churilov, L.; Saposnik, G.; Winstein, C.; Van Wegen, E.E.; Wolf, S.L. Standardized measurement of sensorimotor recovery in stroke trials: Consensus-based core recommendations from the stroke recovery and rehabilitation roundtable. Neurorehabil. Neural. Repair 2017, 31, 784–792. [Google Scholar] [CrossRef]
- Page, S.J.; Levine, P.; Hade, E. Psychometric properties and administration of the wrist/hand subscales of the fugl-meyer assessment in minimally impaired upper extremity hemiparesis in stroke. Arch. Phys. Med. Rehabil. 2012, 93, 2373–2376. [Google Scholar] [CrossRef]
- Lundquist, C.B.; Maribo, T. The fugl–meyer assessment of the upper extremity: Reliability, responsiveness and validity of the danish version. Disabil. Rehabil. 2017, 39, 934–939. [Google Scholar] [CrossRef]
- Hernandez, E.D.; Galeano, C.P.; Barbosa, N.E.; Forero, S.M.; Nordin, Å.; Sunnerhagen, K.S.; Alt Murphy, M. Intra-and inter-rater reliability of fugl-meyer assessment of upper extremity in stroke. J. Rehabil. Med. 2019, 51, 652–659. [Google Scholar] [CrossRef]
- Chang, W.D.; Lai, P.T. New design of home-based dynamic hand splint for hemiplegic hands: A preliminary study. J. Phys. Ther. Sci. 2015, 27, 829–831. [Google Scholar] [CrossRef]
- Page, S.J.; Fulk, G.D.; Boyne, P. Clinically important differences for the upper-extremity fugl-meyer scale in people with minimal to moderate impairment due to chronic stroke. Phys. Ther. 2012, 92, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Vaughan-Graham, J.; Cott, C.; Wright, F.V. The bobath (NDT) concept in adult neurological rehabilitation: What is the state of the knowledge? A scoping review. Part I: Conceptual perspectives. Disabil. Rehabil. 2015, 37, 1793–1807. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.; Yang, G.; Park, B.K.; Labatia, I. Muscle weakness and cocontraction in upper limb hemiparesis: Relationship to motor impairment and physical disability. Neurorehabil. Neural. Repair 2002, 16, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Gracies, J.M. Pathophysiology of spastic paresis. II: Emergence of muscle overactivity. Muscle Nerve 2005, 31, 552–571. [Google Scholar] [CrossRef]
- Hu, X.L.; Tong, K.Y.; Song, R.; Zheng, X.J.; Leung, W.W. A comparison between electromyography-driven robot and passive motion device on wrist rehabilitation for chronic stroke. Neurorehabil. Neural. Repair 2009, 23, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.S.; Hsieh, Y.W.; Wu, C.Y.; Lin, K.C.; Lin, J.C.; Yeh, L.M.; Yin, H.P. Comparative assessment of two robot-assisted therapies for the upper extremity in people with chronic stroke. Am. J. Occup. Ther. 2019, 73, 7301205010p1–7301205010p9. [Google Scholar] [CrossRef]
- Lee, K.W.; Kim, S.B.; Lee, J.H.; Lee, S.J.; Yoo, S.W. Effect of upper extremity robot-assisted exercise on spasticity in stroke patients. Ann. Rehabil. Med. 2016, 40, 961. [Google Scholar] [CrossRef]
- Ansari, N.N.; Naghdi, S.; Moammeri, H.; Jalaie, S. Ashworth scales are unreliable for the assessment of muscle spasticity. Physiother. Theory Pract. 2006, 22, 119–125. [Google Scholar] [CrossRef]
- Pandyan, A.D.; Johnson, G.R.; Price, C.I.; Curless, R.H.; Barnes, M.P.; Rodgers, H. A review of the properties and limitations of the ashworth and modified ashworth scales as measures of spasticity. Clin. Rehabil. 1999, 13, 373–383. [Google Scholar] [CrossRef]
- Patrick, E.; Ada, L. The tardieu scale differentiates contracture from spasticity whereas the ashworth scale is confounded by it. Clin. Rehabil. 2006, 20, 173–182. [Google Scholar] [CrossRef]
- Haugh, A.B.; Pandyan, A.D.; Johnson, G.R. A systematic review of the tardieu scale for the measurement of spasticity. Disabil. Rehabil. 2006, 28, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wu, Y.; Li, X. Test-retest reliability and inter-rater reliability of the modified tardieu scale and the modified ashworth scale in hemiplegic patients with stroke. Eur. J. Phys. Rehabil. Med. 2014, 50, 9–15. [Google Scholar] [PubMed]

| Characteristic/Feature | Group | ||
|---|---|---|---|
| Experimental (n =13) | Control (n =12) | p-Value | |
| Mean age, years | 44.4 (10.2) | 47.1 (9.2) | 0.55 |
| Gender (male/female) | 11/2 | 10/2 | 0.93 |
| History of stroke, months | 22.5 (11.9) | 20.0 (10.4) | 0.48 |
| Affected side (right/left) | 7/6 | 6/6 | 0.86 |
| Group | Pre-1 | Pre-0 | p-Value a | Pos-3 | p-Value a | Pos-6 | p-Value a |
|---|---|---|---|---|---|---|---|
| E-wrist | 3.0 (0.6) | 2.8 (0.7) | 0.99 | 2.4 (0.8) | 0.57 | 1.8 (0.7) | <0.01 * |
| C-wrist | 2.5 (0.8) | 2.3 (0.8) | 0.99 | 2.4 (0.8) | 0.9 | 2.2 (0.9) | 0.99 |
| p-value b | 0.92 | 0.92 | - | 0.57 | - | <0.01 * | - |
| Partial EtaSquared | <0.01 | <0.01 | 0.02 | 0.28 | |||
| E-finger | 3.3 (0.6) | 3.2 (0.6) | 0.99 | 2.4 (0.9) | 0.03 * | 2.0 (0.6) | <0.01 * |
| C-finger | 2.8 (0.4) | 2.9 (0.3) | 0.99 | 2.8 (0.4) | 0.99 | 2.8 (0.5) | 0.99 |
| p-value b | 0.22 | 0.22 | - | 0.05 * | - | <0.01 * | - |
| Partial Eta Squared | 0.07 | 0.07 | 0.17 | 0.61 |
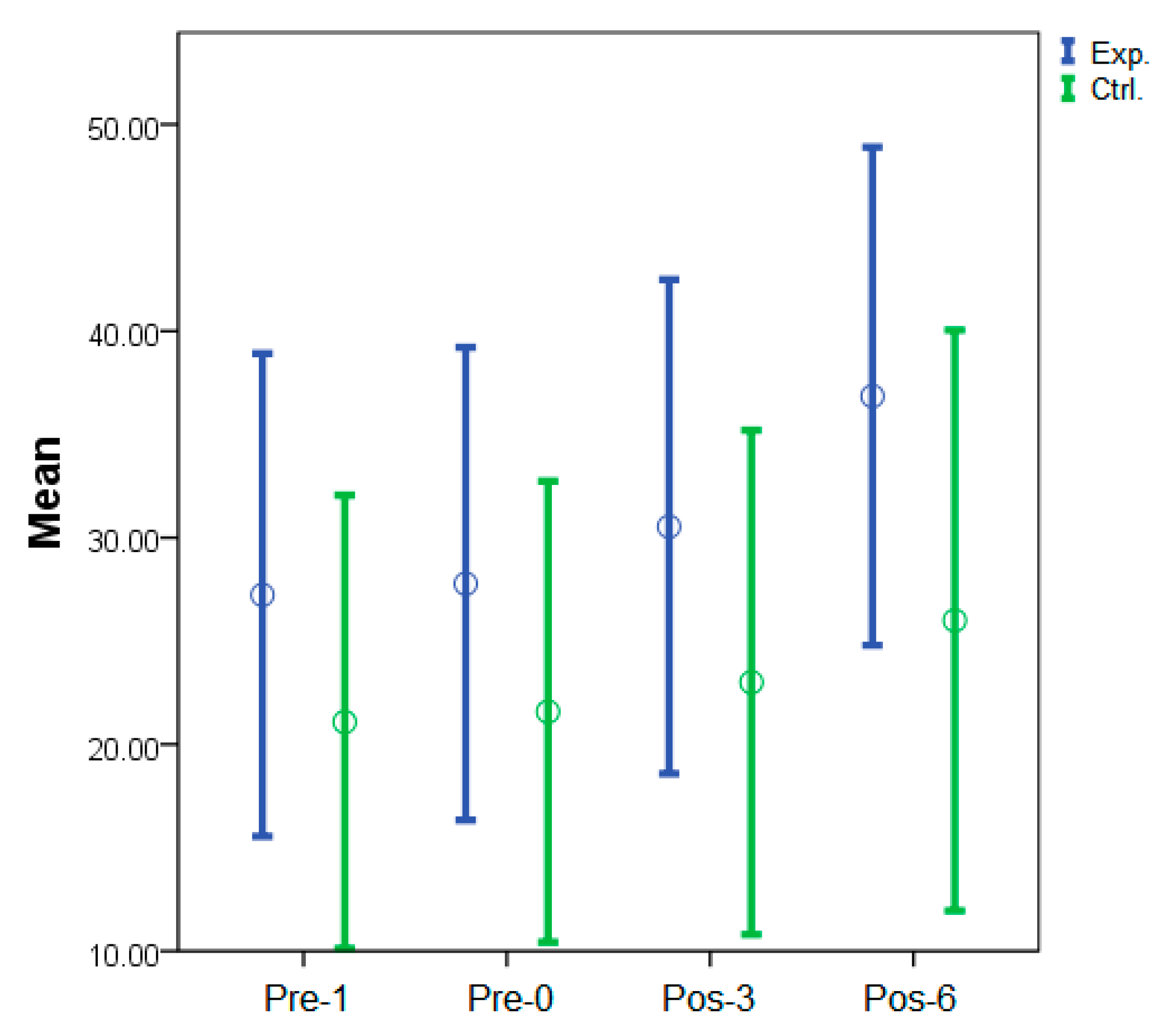
| Group | Pre-1 | Pre-0 | p-value a | Pos-3 | p-Value a | Pos-6 | p-Value a |
|---|---|---|---|---|---|---|---|
| Exp | 27.2 (11.4) | 27.8 (11.7) | 0.07 | 30.5 (12.0) | 0.02 * | 36.8 (11.2) | <0.01 * |
| Ctrl | 21.1 (11.6) | 21.6 (11.5) | 0.13 | 23.0 (12.7) | 0.06 | 26.0 (14.7) | 0.01 * |
| p-value b | 0.89 | 0.89 | 0.39 | - | 0.05 * | - | |
| Partial EtaSquared | <0.01 | <0.01 | 0.03 | 0.17 |
| Time (n =13) | Wearing Time (h) | Pain (0–10) | Spasticity (0–10) | Satisfaction (0–10) | Easy to Use (0–10) |
|---|---|---|---|---|---|
| Pos-3 | 7.2 | 2.6 | 7.4 | 7.7 | 8.0 |
| Pos-6 | 7.9 | 2.5 | 5.6 | 8.4 | 8.8 |
| p-value | <0.01 * | 0.89 | <0.01 * | <0.01 * | <0.01 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.-S.; Tseng, C.-H.; Fang, W.-C.; Han, I.-W.; Huang, S.-C. Effectiveness of a New 3D-Printed Dynamic Hand–Wrist Splint on Hand Motor Function and Spasticity in Chronic Stroke Patients. J. Clin. Med. 2021, 10, 4549. https://doi.org/10.3390/jcm10194549
Yang Y-S, Tseng C-H, Fang W-C, Han I-W, Huang S-C. Effectiveness of a New 3D-Printed Dynamic Hand–Wrist Splint on Hand Motor Function and Spasticity in Chronic Stroke Patients. Journal of Clinical Medicine. 2021; 10(19):4549. https://doi.org/10.3390/jcm10194549
Chicago/Turabian StyleYang, Yu-Sheng, Chi-Hsiang Tseng, Wei-Chien Fang, Ia-Wen Han, and Shyh-Chour Huang. 2021. "Effectiveness of a New 3D-Printed Dynamic Hand–Wrist Splint on Hand Motor Function and Spasticity in Chronic Stroke Patients" Journal of Clinical Medicine 10, no. 19: 4549. https://doi.org/10.3390/jcm10194549
APA StyleYang, Y.-S., Tseng, C.-H., Fang, W.-C., Han, I.-W., & Huang, S.-C. (2021). Effectiveness of a New 3D-Printed Dynamic Hand–Wrist Splint on Hand Motor Function and Spasticity in Chronic Stroke Patients. Journal of Clinical Medicine, 10(19), 4549. https://doi.org/10.3390/jcm10194549






