Ribophorin II Overexpression Is Associated with Poor Response to Induction Chemotherapy with Docetaxel, Cisplatin, and Fluorouracil in P16-Negative Locally Advanced Head and Neck Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Treatment and Response Evaluation
2.3. Immunohistochemical (IHC) Staining
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Relationship between RPN2 Expression, the Response of Induction Chemotherapy with TPF, and Clinicopathologic Parameters
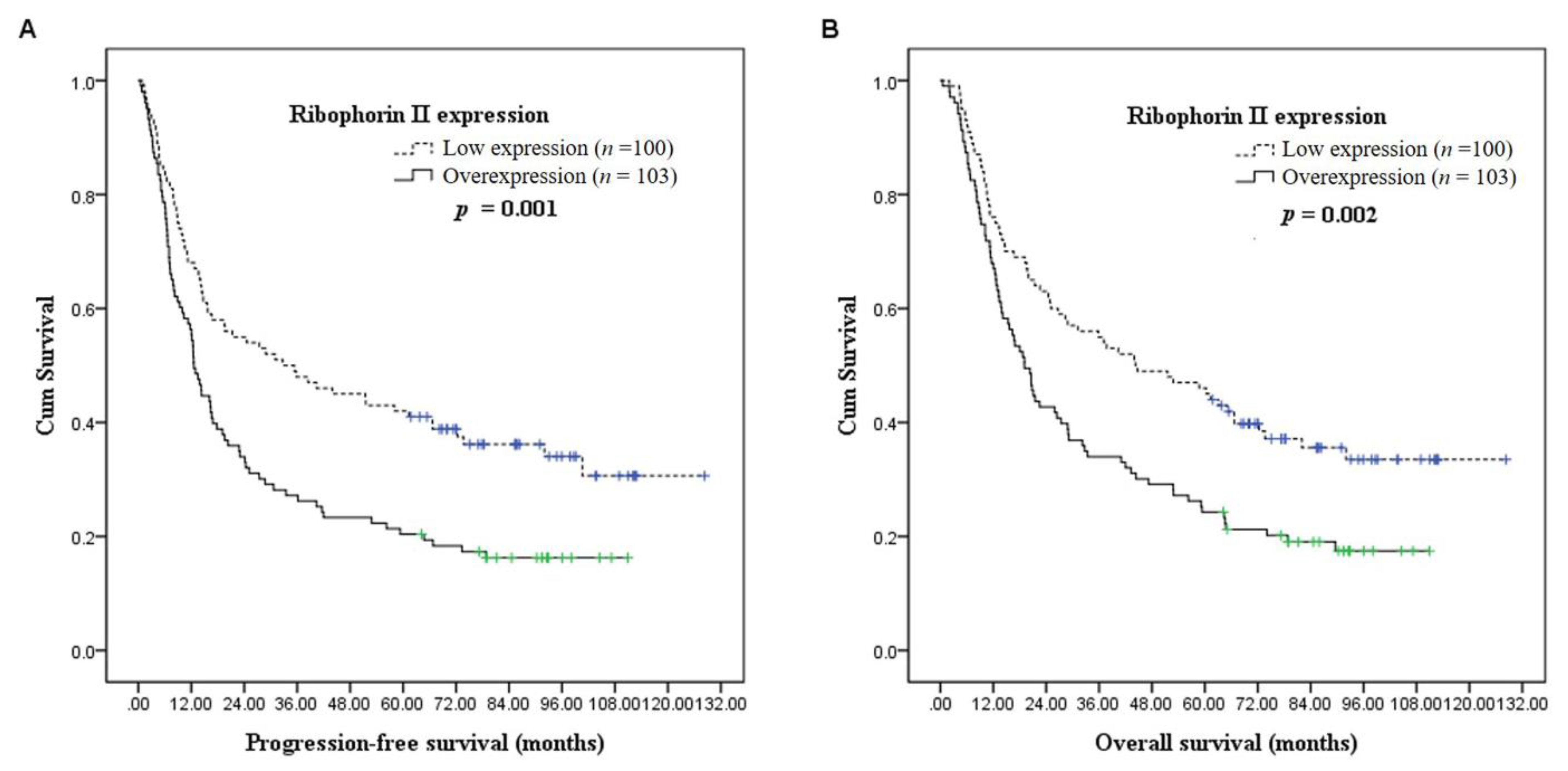
3.3. Survival Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cancer Registry Annual Report, 2017; Taiwan. Health Promotion Administration, Ministry of Health and Welfare: Taiwan, December 2019.
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haddad, R.I.; Shin, D.M. Recent advances in head and neck cancer. N. Engl. J. Med. 2008, 359, 1143–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermorken, J.B.; Remenar, E.; van Herpen, C.; Gorlia, T.; Mesia, R.; Degardin, M.; Stewart, J.S.; Jelic, S.; Betka, J.; Preiss, J.H.; et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N. Engl. J. Med. 2007, 357, 1695–1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorch, J.H.; Goloubeva, O.; Haddad, R.I.; Cullen, K.; Sarlis, N.; Tishler, R.; Tan, M.; Fasciano, J.; Sammartino, D.E.; Posner, M.R. Induction chemotherapy with cisplatin and fluorouracil alone or in combination with docetaxel in locally advanced squamous-cell cancer of the head and neck: Long-term results of the TAX 324 randomised phase 3 trial. Lancet Oncol. 2011, 12, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Honma, K.; Iwao-Koizumi, K.; Takeshita, F.; Yamamoto, Y.; Yoshida, T.; Nishio, K.; Nagahara, S.; Kato, K.; Ochiya, T. RPN2 gene confers docetaxel resistance in breast cancer. Nat. Med. 2008, 14, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Kurashige, J.; Watanabe, M.; Iwatsuki, M.; Kinoshita, K.; Saito, S.; Nagai, Y.; Ishimoto, T.; Baba, Y.; Mimori, K.; Baba, H. RPN2 expression predicts response to docetaxel in oesophageal squamous cell carcinoma. Br. J. Cancer 2012, 107, 1233–1238. [Google Scholar] [CrossRef] [Green Version]
- Fujita, Y.; Yagishita, S.; Takeshita, F.; Yamamoto, Y.; Kuwano, K.; Ochiya, T. Prognostic and therapeutic impact of RPN2-mediated tumor malignancy in non-small-cell lung cancer. Oncotarget 2015, 6, 3335–3345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouvier, C.; Macagno, N.; Nguyen, Q.; Loundou, A.; Jiguet-Jiglaire, C.; Gentet, J.C.; Jouve, J.L.; Rochwerger, A.; Mattei, J.C.; Bouvard, D.; et al. Prognostic value of the Hippo pathway transcriptional coactivators YAP/TAZ and beta1-integrin in conventional osteosarcoma. Oncotarget 2016, 7, 64702–64710. [Google Scholar] [CrossRef] [Green Version]
- Kuo, K.T.; Hsiao, C.H.; Lin, C.H.; Kuo, L.T.; Huang, S.H.; Lin, M.C. The biomarkers of human papillomavirus infection in tonsillar squamous cell carcinoma-molecular basis and predicting favorable outcome. Mod. Pathol. 2008, 21, 376–386. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.S., Jr. p16 Immunohistochemistry as a standalone test for risk stratification in oropharyngeal squamous cell carcinoma. Head Neck Pathol. 2012, 6 (Suppl. S1), S75–S82. [Google Scholar] [CrossRef]
- Zhu, J.; He, J.; Liu, Y.; Simeone, D.M.; Lubman, D.M. Identification of glycoprotein markers for pancreatic cancer CD24+CD44+ stem-like cells using nano-LC-MS/MS and tissue microarray. J. Proteome Res. 2012, 11, 2272–2281. [Google Scholar] [CrossRef] [Green Version]
- Bi, C.; Jiang, B. Downregulation of RPN2 induces apoptosis and inhibits migration and invasion in colon carcinoma. Oncol. Rep. 2018, 40, 283–293. [Google Scholar] [CrossRef] [Green Version]
- Hong, F.; Li, Y.; Ni, H.; Li, J. Downregulation of ribophorin II suppresses tumor growth, migration, and invasion of nasopharyngeal carcinoma. Onco Targets Ther. 2018, 11, 3485–3494. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Duan, B.; Shi, H.; Li, Y.; Ai, P.; Tian, J.; Chen, N. Comparison of GP and TPF induction chemotherapy for locally advanced nasopharyngeal carcinoma. Oral Oncol. 2019, 97, 37–43. [Google Scholar] [CrossRef]
- Brockstein, B.; Haraf, D.J.; Rademaker, A.W.; Kies, M.S.; Stenson, K.M.; Rosen, F.; Mittal, B.B.; Pelzer, H.; Fung, B.B.; Witt, M.E.; et al. Patterns of failure, prognostic factors and survival in locoregionally advanced head and neck cancer treated with concomitant chemoradiotherapy: A 9-year, 337-patient, multi-institutional experience. Ann. Oncol. 2004, 15, 1179–1186. [Google Scholar] [CrossRef]
- Fujimoto, D.; Goi, T.; Koneri, K.; Hirono, Y. RPN2 is effective biomarker to predict the outcome of combined chemotherapy docetaxel and cisplatin for advanced gastric cancer. Oncotarget 2018, 9, 15208–15218. [Google Scholar] [CrossRef] [Green Version]
- Zhong, L.P.; Zhang, C.P.; Ren, G.X.; Guo, W.; William, W.N., Jr.; Sun, J.; Zhu, H.G.; Tu, W.Y.; Li, J.; Cai, Y.L.; et al. Randomized phase III trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil followed by surgery versus up-front surgery in locally advanced resectable oral squamous cell carcinoma. J. Clin. Oncol. 2013, 31, 744–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vokes, E.E.; Athanasiadis, I. Chemotherapy of squamous cell carcinoma of head and neck: The future is now. Ann. Oncol. 1996, 7, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Kies, M.S.; Boatright, D.H.; Li, G.; Blumenschein, G.; El-Naggar, A.K.; Brandon Gunn, G.; Lewin, J.S.; Steinhaus, G.D.; Sturgis, E.M. Phase II trial of induction chemotherapy followed by surgery for squamous cell carcinoma of the oral tongue in young adults. Head Neck 2012, 34, 1255–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hitt, R.; Grau, J.J.; Lopez-Pousa, A.; Berrocal, A.; Garcia-Giron, C.; Irigoyen, A.; Sastre, J.; Martinez-Trufero, J.; Brandariz Castelo, J.A.; Verger, E.; et al. A randomized phase III trial comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as treatment of unresectable head and neck cancer. Ann. Oncol. 2014, 25, 216–225. [Google Scholar] [CrossRef] [PubMed]

| Parameters | n (%) | |
|---|---|---|
| Age | ||
| median | 52 | |
| mean | 52 | |
| range | 29–82 | |
| Sex | ||
| male | 192 (95%) | |
| female | 11 (5%) | |
| Primary tumor site | ||
| hypopharynx | 30 (15%) | |
| larynx | 22 (11%) | |
| oropharynx | 63 (31%) | |
| oral cavity | 88 (43%) | |
| Clinical T classification | ||
| T1 | 5 (2%) | |
| T2 | 23 (11%) | |
| T3 | 28 (14%) | |
| T4a | 68 (34%) | |
| T4b | 79 (39%) | |
| Clinical N classification | ||
| N0 | 42 (21%) | |
| N1 | 24 (12%) | |
| N2a | 5 (2%) | |
| N2b | 58 (28%) | |
| N2c | 52 (26%) | |
| N3 | 22 (11%) | |
| Clinical 7th AJCC stage | ||
| III | 16 (8%) | |
| IVA | 89 (44%) | |
| IVB | 98 (48%) | |
| Betel nut chewing | ||
| Absent | 44 (22%) | |
| Present | 159 (78%) | |
| Smoking | ||
| Absent | 16 (8%) | |
| Present | 187 (92%) | |
| Alcohol | ||
| Absent | 33 (16%) | |
| Present | 170 (84%) | |
| Ribophorin II expression | ||
| Low expression | 100 (49%) | |
| Overexpression | 103 (51%) | |
| Response to induction chemotherapy | ||
| Complete response | 12 (6%) | |
| Partial response | 114 (56%) | |
| Stable disease | 53 (26%) | |
| Progression disease | 24 (12%) |
| Parameters | Ribophorin II Expression | |||
|---|---|---|---|---|
| Low | Over | p Value | ||
| Age | <52 y/o | 45 | 55 | 0.23 |
| ≥52 y/o | 55 | 48 | ||
| Sex | Male | 96 | 96 | 0.38 |
| Female | 4 | 7 | ||
| Clinical T classification | T1~3 | 32 | 24 | 0.17 |
| T4 | 68 | 79 | ||
| Clinical T classification | T1~4a | 63 | 62 | 0.68 |
| T4b | 37 | 41 | ||
| Clinical N classification | N0 | 23 | 19 | 0.42 |
| N1~3 | 77 | 84 | ||
| Clinical N classification | N0~1 | 33 | 33 | 0.88 |
| N2~3 | 67 | 70 | ||
| Clinical 7th AJCC stage | III | 10 | 6 | 0.27 |
| IVA, IVB | 90 | 97 | ||
| Clinical 7th AJCC stage | III, IVA | 53 | 52 | 0.72 |
| IVB | 47 | 51 | ||
| Primary tumor site | Oral cavity | 40 | 48 | 0.34 |
| Others | 60 | 55 | ||
| Primary tumor site | Larynx/Hypopharynx | 29 | 23 | 0.28 |
| Others | 71 | 80 | ||
| Primary tumor site | Oropharynx | 31 | 32 | 0.99 |
| Others | 69 | 71 | ||
| Betel-nut chewing | Absent | 23 | 21 | 0.65 |
| Present | 77 | 82 | ||
| Smoking history | Absent | 11 | 5 | 0.11 |
| Present | 89 | 98 | ||
| Alcohol history | Absent | 20 | 13 | 0.15 |
| Present | 80 | 90 | ||
| Parameters | Response to Induction Chemotherapy | |||
|---|---|---|---|---|
| CR/PR 2 | SD/PD 3 | p Value | ||
| Age | <52 y/o | 64 | 36 | 0.58 |
| ≥52 y/o | 62 | 41 | ||
| Sex | Male | 119 | 73 | 1.00 |
| Female | 7 | 4 | ||
| Ribophorin II expression | Low | 71 | 29 | 0.01 * |
| Over | 55 | 48 | ||
| Clinical T classification | T1~3 | 41 | 15 | 0.043 * |
| T4 | 85 | 62 | ||
| Clinical T classification | T1~4a | 80 | 45 | 0.47 |
| T4b | 46 | 32 | ||
| Clinical N classification | N0 | 30 | 12 | 0.16 |
| N1~3 | 96 | 65 | ||
| Clinical N classification | N0~1 | 46 | 20 | 0.12 |
| N2~3 | 80 | 57 | ||
| Clinical 7th AJCC stage | III | 13 | 3 | 0.099 |
| IVA, IVB | 113 | 74 | ||
| Clinical 7th AJCC stage | III, IVA | 69 | 36 | 0.27 |
| IVB | 57 | 41 | ||
| Primary tumor site | Oral cavity | 48 | 40 | 0.053 |
| Others | 78 | 37 | ||
| Primary tumor site | Larynx/Hypopharynx | 34 | 18 | 0.57 |
| Others | 92 | 59 | ||
| Primary tumor site | Oropharynx | 44 | 19 | 0.13 |
| Others | 82 | 58 | ||
| Betel-nut chewing | Absent | 30 | 14 | 0.35 |
| Present | 96 | 63 | ||
| Smoking | Absent | 11 | 5 | 0.57 |
| Present | 115 | 72 | ||
| Alcohol | Absent | 22 | 11 | 0.55 |
| Present | 104 | 66 | ||
| Factors | No. of Patients | Progression-Free Survival (PFS) | Overall Survival (OS) | ||
|---|---|---|---|---|---|
| 5-Year PFS Rate (%) | p Value | 5-Year OS Rate (%) | p Value | ||
| Age | |||||
| <52 y/o | 100 | 25% | 0.14 | 30% | 0.20 |
| ≥52 y/o | 103 | 37% | 40% | ||
| Sex | |||||
| Male | 192 | 31% | 0.99 | 35% | 0.89 |
| Female | 11 | 27% | 36% | ||
| Ribophorin II expression | |||||
| Low expression | 100 | 42% | 0.001 * | 46% | 0.002 * |
| Overexpression | 103 | 20% | 24% | ||
| Clinical T classification | |||||
| T1~3 | 56 | 41% | 0.036 * | 43% | 0.03 * |
| T4 | 147 | 27% | 32% | ||
| Clinical T classification | |||||
| T1~4a | 125 | 34% | 0.12 | 37% | 0.14 |
| T4b | 78 | 27% | 32% | ||
| Clinical N classification | |||||
| N0 | 42 | 45% | 0.021 * | 52% | 0.006 * |
| N1~3 | 161 | 27% | 30% | ||
| Clinical N classification | |||||
| N0~1 | 66 | 42% | 0.012 * | 50% | 0.005 * |
| N2~3 | 137 | 26% | 28% | ||
| Clinical 7th AJCC stage | |||||
| III | 16 | 50% | 0.083 | 56% | 0.039 * |
| IVA, IVB | 187 | 29% | 33% | ||
| Clinical 7th AJCC stage | |||||
| III, IVA | 105 | 35% | 0.034 * | 38% | 0.043 * |
| IVB | 98 | 27% | 32% | ||
| Primary tumor site | |||||
| Oral cavity | 88 | 30% | 0.24 | 33% | 0.27 |
| Others | 115 | 32% | 37% | ||
| Primary tumor site | |||||
| Larynx/Hypopharynx | 52 | 40% | 0.085 | 46% | 0.082 |
| Others | 151 | 28% | 31% | ||
| Primary tumor site | |||||
| Oropharynx | 63 | 25% | 0.64 | 29% | 0.58 |
| Others | 140 | 34% | 38% | ||
| Betel-nut chewing | |||||
| Absent | 44 | 39% | 0.21 | 43% | 0.31 |
| Present | 159 | 29% | 33% | ||
| Smoking | |||||
| Absent | 16 | 38% | 0.33 | 44% | 0.31 |
| Present | 187 | 31% | 34% | ||
| Alcohol | |||||
| Absent | 33 | 46% | 0.079 | 49% | 0.093 |
| Present | 170 | 28% | 32% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.-S.; Luo, S.-D.; Chiu, T.-J.; Wang, Y.-M.; Chen, W.-C.; Chien, C.-Y.; Fang, F.-M.; Huang, T.-L.; Li, S.-H. Ribophorin II Overexpression Is Associated with Poor Response to Induction Chemotherapy with Docetaxel, Cisplatin, and Fluorouracil in P16-Negative Locally Advanced Head and Neck Squamous Cell Carcinoma. J. Clin. Med. 2021, 10, 4118. https://doi.org/10.3390/jcm10184118
Chen W-S, Luo S-D, Chiu T-J, Wang Y-M, Chen W-C, Chien C-Y, Fang F-M, Huang T-L, Li S-H. Ribophorin II Overexpression Is Associated with Poor Response to Induction Chemotherapy with Docetaxel, Cisplatin, and Fluorouracil in P16-Negative Locally Advanced Head and Neck Squamous Cell Carcinoma. Journal of Clinical Medicine. 2021; 10(18):4118. https://doi.org/10.3390/jcm10184118
Chicago/Turabian StyleChen, Wei-Shan, Sheng-Dean Luo, Tai-Jan Chiu, Yu-Ming Wang, Wei-Chih Chen, Chih-Yen Chien, Fu-Min Fang, Tai-Lin Huang, and Shau-Hsuan Li. 2021. "Ribophorin II Overexpression Is Associated with Poor Response to Induction Chemotherapy with Docetaxel, Cisplatin, and Fluorouracil in P16-Negative Locally Advanced Head and Neck Squamous Cell Carcinoma" Journal of Clinical Medicine 10, no. 18: 4118. https://doi.org/10.3390/jcm10184118
APA StyleChen, W.-S., Luo, S.-D., Chiu, T.-J., Wang, Y.-M., Chen, W.-C., Chien, C.-Y., Fang, F.-M., Huang, T.-L., & Li, S.-H. (2021). Ribophorin II Overexpression Is Associated with Poor Response to Induction Chemotherapy with Docetaxel, Cisplatin, and Fluorouracil in P16-Negative Locally Advanced Head and Neck Squamous Cell Carcinoma. Journal of Clinical Medicine, 10(18), 4118. https://doi.org/10.3390/jcm10184118








