Management of Spontaneous Bleeding in COVID-19 Inpatients: Is Embolization Always Needed?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Setting
2.2. Definitions
2.3. CT Examination
2.4. PTAE
2.5. Outcomes
2.6. Statistical Analysis
3. Results
3.1. COVID-Related Clinical Characteristics
3.2. Radiological Characteristics and Interventional Outcomes
- 21/1075 (1.95%) COVID-19 patients with haemorrhagic events were observed.
- Eleven patients were treated conservatively, based on clinical judgement, resuscitated with intravenous fluid, transfusions of red blood cells and, in addiction, further standard supportive measures.
- Ten patients, in addition to the conservative treatment, underwent PTAE after the haemorrhagic event.
- Three patients treated with PTAE died after the event within 30 days (14% of lethality) whereas one patient died after more than 90 days of the procedure for independent reasons.
- Patients treated with a conservative manner only had a 30-day survival of 90.9%. A deceased patient in this group presented terminal complication of recent major hepatic surgery, and was not considered for PTAE.
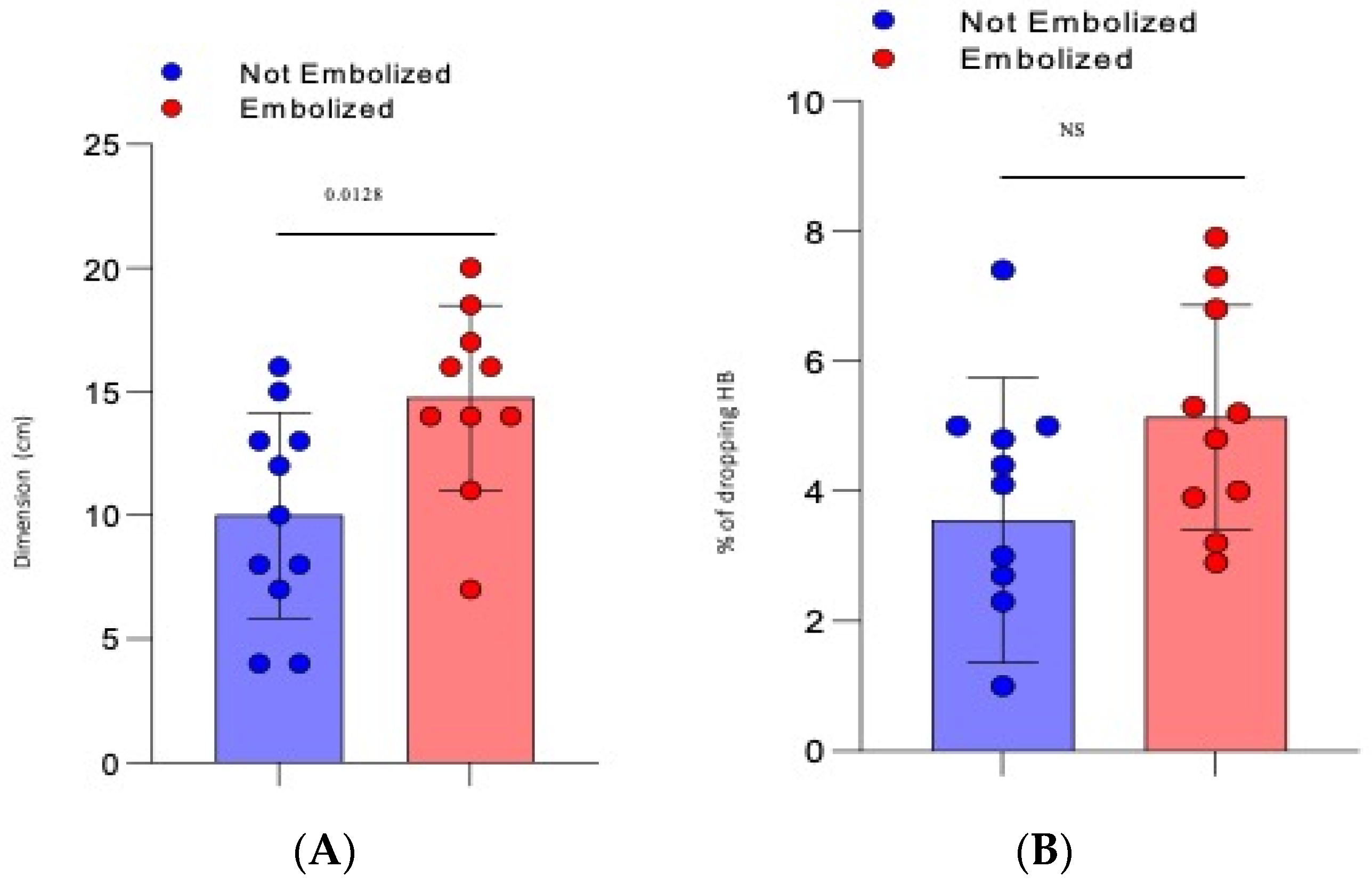
- The mean dimension of the hematoma was 10 cm (±1.25 SD) for the not-embolized group vs. 14.75 cm (±1.2 SD) for the embolized group (p = 0.0128), Figure 1A.
- There was a mean drop in haemoglobin of 3618 g/dL (±0.622 SD) for the not-embolized group vs. 5.13 g/dL (±0.55 SD) for the embolized group (p = 0.086), Figure 1B.
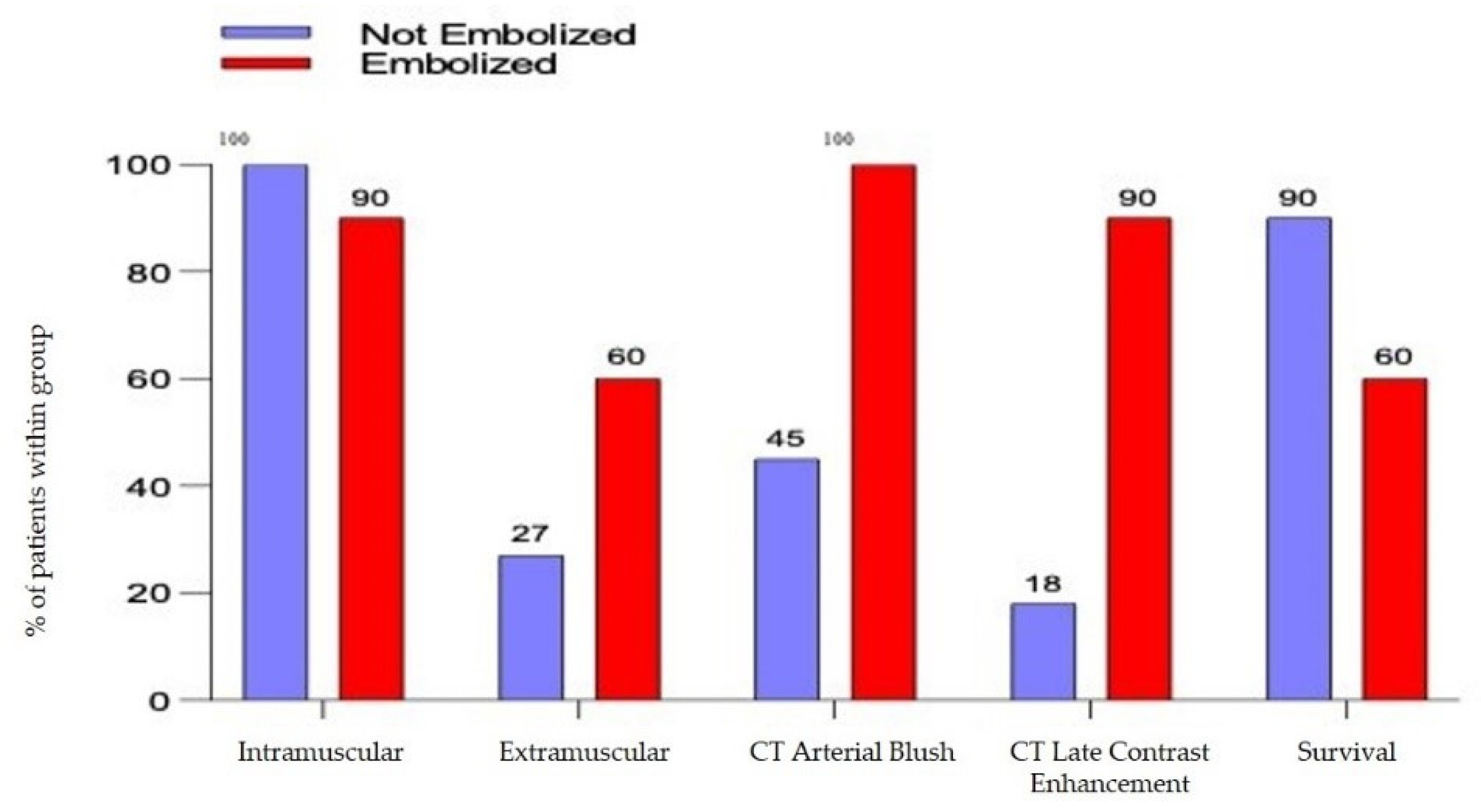
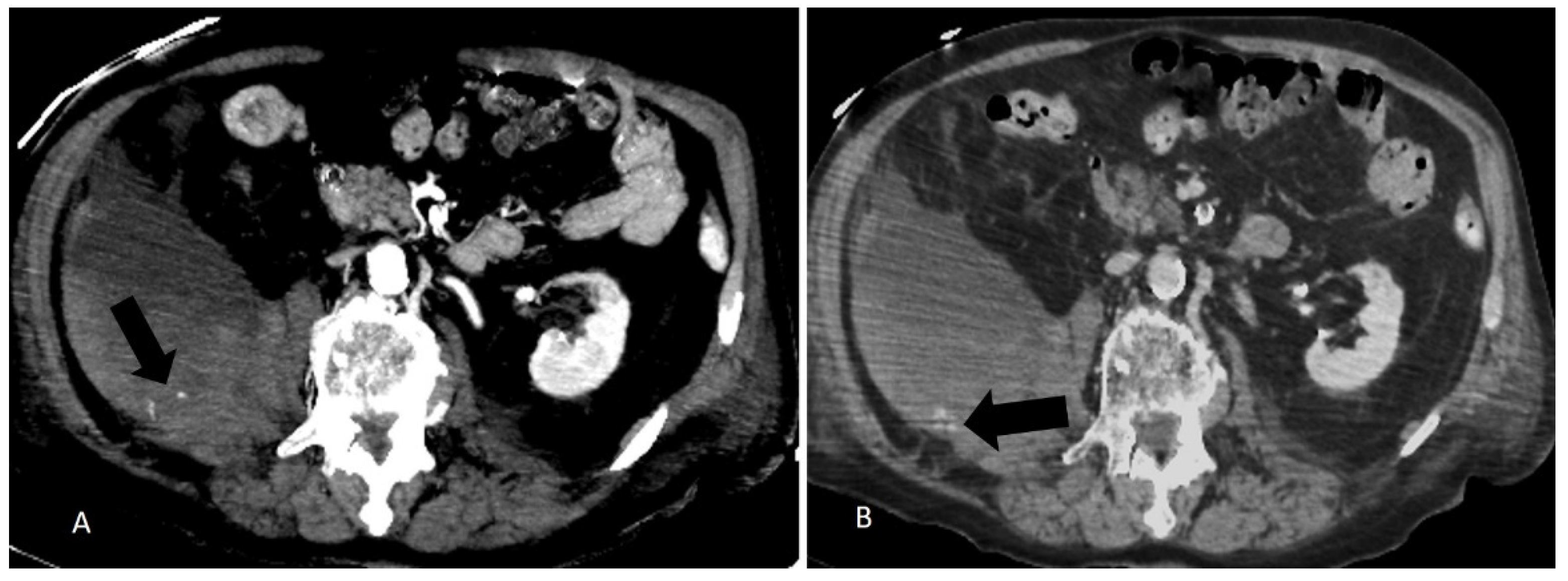
- The arterial blush at CT scan was presented in all the embolized patients but only in 5 patients not embolized (45%) with a significative difference (p= 0.0124). There was a significant difference also in the presence of a CT Late Contrast Enhancement between the 2 groups (90% vs. 18,1%, embolized vs. not embolized, p = 0.0019), Figure 2.
- The presence of an intra or extra-muscular hematoma was not statistically significant (Figure 2).
3.3. Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Coronavirus Disease 2019 (COVID-19) Situation Report—72. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200401-sitrep-72-covid-19.pdf?sfvrsn=3dd8971b_2 (accessed on 1 April 2020).
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef]
- Cevik, M.; Kuppalli, K.; Kindrachuk, J.; Peiris, M. Virology, Transmission, and Pathogenesis of SARS-CoV-2. BMJ 2020, 371, m3862. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Nigoghossian, C.D.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef]
- Agenzia Italiana del Farmaco. Uso delle Eparine nei Pazienti Adulti con COVID-19. Available online: https://www.aifa.gov.it/documents/20142/0/Eparine_Basso_Peso_Molecolare_13.05.2021.pdf (accessed on 13 May 2021).
- Thachil, J.; Tang, N.; Gando, S.; Falanga, A.; Cattaneo, M.; Levi, M.; Clark, C.; Iba, T. ISTH Interim Guidance on Recognition and Management of Coagulopathy in COVID-19. J. Thromb. Haemost. 2020, 18, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Musoke, N.; Lo, K.B.; Albano, J.; Peterson, E.; Bhargav, R.; Gul, F.; DeJoy, R.; Salacup, G.; Pelayo, J.; Tipparaju, P.; et al. Anticoagulation and Bleeding Risk in Patients with COVID-19. Thromb. Res. 2020, 196, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, G.N.; Lala, A.; Bagiella, E.; Chang, H.L.; Moreno, P.R.; Pujadas, E.; Arvind, V.; Bose, S.; Charney, A.W.; Chen, M.D.; et al. Anticoagulation, Bleeding, Mortality, and Pathology in Hospitalized Patients With COVID-19. J. Am. Coll. Cardiol. 2020, 76, 1815–1826. [Google Scholar] [CrossRef]
- Lucatelli, P.; Rocco, B.; Nardis, P.G.; Cannavale, A.; Bezzi, M.; Catalano, C.; Corona, M. Bleeding in COVID Patients: What We Have Understood So Far. CardioVasc. Interv. Radiol. 2021, 44, 666–668. [Google Scholar] [CrossRef]
- Touma, L.; Cohen, S.; Cassinotto, C.; Reinhold, C.; Barkun, A.; Tran, V.T.; Banon, O.; Valenti, D.; Gallix, B.; Dohan, A. Transcatheter Arterial Embolization of Spontaneous Soft Tissue Hematomas: A Systematic Review. CardioVasc. Interv. Radiol. 2019, 42, 335–343. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. COVID-19 Clinical Management: Living Guidance. 2021. Available online: https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-clinical-2021-1 (accessed on 9 August 2021).
- Reisi-Vanani, V.; Lorigooini, Z.; Dayani, M.A.; Mardani, M.; Rahmani, F. Massive Intraperitoneal Hemorrhage in Patients with COVID-19: A Case Series. J Thromb Thrombolysis 2021, 52, 338–344. [Google Scholar] [CrossRef]
- Al-Samkari, H.; Gupta, S.; Leaf, R.K.; Wang, W.; Rosovsky, R.P.; Brenner, S.K.; Hayek, S.S.; Berlin, H.; Kapoor, R.; Shaefi, S.; et al. Thrombosis, Bleeding, and the Observational Effect of Early Therapeutic Anticoagulation on Survival in Critically Ill Patients With COVID-19. Ann. Intern. Med. 2021, 174, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Llitjos, J.F.; Daviaud, F.; Grimaldi, D.; Legriel, S.; Georges, J.L.; Guerot, E.; Bedos, J.P.; Fagon, J.Y.; Charpentier, J.; Mira, J.P. Ilio-psoas hematoma in the intensive care unit: A multicentric study. Ann. Intensive Care 2016, 6, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estivill Pallejà, X.; Domingo, P.; Fontcuberta, J.; Félez, J. Spontaneous Retroperitoneal Hemorrhage during Oral Anticoagulant Therapy. Arch. Intern. Med. 1985, 145, 1531–1534. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial Cell Infection and Endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Palumbo, D.; Guazzarotti, G.; De Cobelli, F. Spontaneous Major Hemorrhage in COVID-19 Patients: Another Brick in the Wall of SARS-CoV-2-Associated Coagulation Disorders? J. Vasc. Interv. Radiol. 2020, 31, 1494–1496. [Google Scholar] [CrossRef] [PubMed]
- Artzner, T.; Clere-Jehl, R.; Schenck, M.; Greget, M.; Merdji, H.; De Marini, P.; Tuzin, N.; Helms, J.; Meziani, F. Spontaneous Ilio-Psoas Hematomas Complicating Intensive Care Unit Hospitalizations. PLoS ONE 2019, 14, e0211680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conti, C.B.; Henchi, S.; Coppeta, G.P.; Testa, S.; Grassia, R. Bleeding in COVID-19 Severe Pneumonia: The Other Side of Abnormal Coagulation Pattern? Eur. J. Intern. Med. 2020, 77, 147–149. [Google Scholar] [CrossRef]
- Bargellini, I.; Cervelli, R.; Lunardi, A.; Scandiffio, R.; Daviddi, F.; Giorgi, L.; Cicorelli, A.; Crocetti, L.; Cioni, R. Spontaneous Bleedings in COVID-19 Patients: An Emerging Complication. CardioVasc. Interv. Radiol. 2020, 43, 1095–1096. [Google Scholar] [CrossRef]
- Vergori, A.; Pianura, E.; Lorenzini, P.; D’Abramo, A.; Di Stefano, F.; Grisetti, S.; Vita, S.; Pinnetti, C.; Donno, D.R.; Marini, M.C.; et al. Spontaneous Ilio-Psoas Haematomas (IPHs): A Warning for COVID-19 Inpatients. Ann. Med. 2021, 53, 295–301. [Google Scholar] [CrossRef]
- Chan, Y.C.; Morales, J.P.; Reidy, J.F.; Taylor, P.R. Management of Spontaneous and Iatrogenic Retroperitoneal Haemorrhage: Conservative Management, Endovascular Intervention or Open Surgery? Int. J. Clin. Pr. 2008, 62, 1604–1613. [Google Scholar] [CrossRef]
- Farrelly, C.; Fidelman, N.; Durack, J.C.; Hagiwara, E.; Kerlan, R.K. Transcatheter Arterial Embolization of Spontaneous Life-Threatening Extraperitoneal Hemorrhage. J. Vasc. Interv. Radiol. 2011, 22, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Dohan, A.; Sapoval, M.; Chousterman, B.G.; di Primio, M.; Guerot, E.; Pellerin, O. Spontaneous Soft-Tissue Hemorrhage in Anticoagulated Patients: Safety and Efficacy of Embolization. AJR Am. J. Roentgenol. 2015, 204, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Pelage, J.P.; Le Dref, O.; Mateo, J.; Soyer, P.; Jacob, D.; Kardache, M.; Dahan, H.; Repiquet, D.; Payen, D.; Truc, J.B.; et al. Life-Threatening Primary Postpartum Hemorrhage: Treatment with Emergency Selective Arterial Embolization. Radiology 1998, 208, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Cullinane, D.C.; Schiller, H.J.; Zielinski, M.D.; Bilaniuk, J.W.; Collier, B.R.; Como, J.; Holevar, M.; Sabater, E.A.; Sems, S.A.; Vassy, W.M.; et al. Eastern Association for the Surgery of Trauma Practice Management Guidelines for Hemorrhage in Pelvic Fracture--Update and Systematic Review. Trauma 2011, 71, 1850–1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barral, M.; Pellerin, O.; Tran, V.T.; Gallix, B.; Boucher, L.-M.; Valenti, D.; Sapoval, M.; Soyer, P.; Dohan, A. Predictors of Mortality from Spontaneous Soft-Tissue Hematomas in a Large Multicenter Cohort Who Underwent Percutaneous Transarterial Embolization. Radiology 2019, 291, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, M.; Bertinato, E.M.; Birocchi, S.; Brizio, C.; Malavolta, D.; Manzoni, M.; Muscarella, G.; Orlandi, M. Pulmonary Embolism or Pulmonary Thrombosis in COVID-19? Is the Recommendation to Use High-Dose Heparin for Thromboprophylaxis Justified? Thromb. Haemost. 2020, 120, 1230–1232. [Google Scholar] [CrossRef]
- Kong, W.K.; Cho, K.-T.; Lee, H.J.; Choi, J.-S. Femoral Neuropathy Due to Iliacus Muscle Hematoma in a Patient on Warfarin Therapy. J. Korean Neurosurg. Soc. 2012, 51, 51–53. [Google Scholar] [CrossRef]
- Fraissé, M.; Logre, E.; Pajot, O.; Mentec, H.; Plantefève, G.; Contou, D. Thrombotic and Hemorrhagic Events in Critically Ill COVID-19 Patients: A French Monocenter Retrospective Study. Crit. Care 2020, 24, 275. [Google Scholar] [CrossRef]
- Palumbo, D.; Guazzarotti, G.; De Cobelli, F. Spontaneous Major Haemorrhage in COVID-19 Patients: A Proposal for a Pathophysiology-Based Angiographic Treatment. CardioVasc. Interv. Radiol. 2021, 44, 1289–1290. [Google Scholar] [CrossRef]

| N (%), Median (IQR) | Overall | Not Embolized N = 11 | Embolized N = 10 | p-Value |
|---|---|---|---|---|
| Gender | ||||
| Male | 13 (61.9%) | 5 (45.5%) | 8 (80%) | 0.104 |
| Female | 8 (38.1%) | 6 (54.5%) | 2 (20) | |
| Age, years | 66 (62–76) | 64 (56–74) | 75 (62–80) | 0.573 |
| BMI | 27 (25–29) | 28 (23–29) | 25 (25–29) | 0.906 |
| N. of co-morbidities | 0.086 | |||
| 0 | 2 (9.5%) | 2 (18.2%) | 0 | |
| 1 | 6 (28.6%) | 2 (18.2%) | 4 (40%) | |
| 2 | 6 (28.6%) | 5 (45.5%) | 1 (10%) | |
| 3+ | 7 (33.3%) | 2 (18.2%) | 5 (50%) | |
| CVD co-morbidity | 15 (71.4%) | 8 (72.7%) | 7 (70%) | 0.890 |
| DM co-morbidity | 4 (19.1%) | 3 (27.3%) | 1 (10%) | 0.314 |
| PaO2/FiO2 at admission < 300 mmHg | 200 (103–273) | 200 (103–251) | 215 (123–292) | 0.724 |
| Ferritin, pg/mL | 541 (297–1113) | 521 (336–831) | 707 (248–1412) | 0.790 |
| CRP, mg/dL | 6.6 (4.5–16.0) | 8.6 (3.5–16.2) | 6.2 (4.8–16.0) | 0.725 |
| LDH, U/L | 328 (277–404) | 325 (254–433) | 356 (309–404) | 0.291 |
| D-dimer, ng/mL | 740 (555–1473) | 879 (555–1496) | 664 (403–1473) | 0.481 |
| Lymphocytes, cells × 1000/mcL | 960 (640–2650) | 1200 (960–7500) | 605 (510–760) | 0.003 |
| D-dimer, ng/mL | 0.639 | |||
| <500 | 4 (19.1%) | 1 (9.1%) | 3 (30%) | |
| 501–1000 | 10 (47.5%) | 6 (54.5%) | 4 (40%) | |
| 1000–2500 | 4 (19.1%) | 2 (18.2%) | 2 (2.0%) | |
| >2500 | 3 (14.3%) | 2 (18.2%) | 1 (10%) | |
| Antiviral therapy | 0.620 | |||
| LPV/r | 2 (9.5%) | 1 (9.1%) | 1 (10%) | |
| HCQ | 0 | 0 | 0 | |
| LPV/r+HCQ | 1 (4.8%) | 1 (9.1%) | 0 | |
| Neither LPV nor HCQ | 18 (85.7%) | 9 (81.8%) | 9 (90%) | |
| Steroides | 18 (85.7%) | 9 (81.8%) | 9 (90%) | 0.228 |
| Remdesivir | 10 (52.6%) | 6 (54.6%) | 4 (50%) | 0.845 |
| Hospitalization, days | 34 (24–51) | 37 (29–50) | 26 (17–79) | 0.409 |
| Days from symptoms onset to Hematoma | 25 (14–35) | 25 (15–33) | 30 (13–37) | 0.790 |
| Intramuscular Hematoma | 20 (95.2%) | 11 (100.0%) | 9 (90%) | 0.283 |
| Deaths | 5 (23.8%) | 1 (9.1%) | 4 (40%) | 0.097 |
| Overall | Not Embolized N = 11 | Embolized N = 10 | p-Value | |
|---|---|---|---|---|
| Dropping HB | 4.3 | 3.55 | 5.13 | 0.084 |
| Bleeding localization | 0.598 | |||
| Left iliopsoas | 6 (28.5%) | 4 (36.3%) | 2 (20%) | |
| Right iliopsoas | 5 (23.8%) | 4 (36.3%) | 1 (10%) | |
| Abductor muscles | 1 (4.7%) | 1 (9.09%) | 0 (0%) | |
| Paravertebral muscles | 1 (4.7%) | 1 (9.09%) | 0 (0%) | |
| Thoracic muscles | 2 (9.5%) | 0 (0%) | 2 (20%) | |
| Rectus abdominis | 3 (14.2%) | 1 (9.09%) | 2 (20%) | |
| Gluteus | 1 (4.7%) | 0 (0%) | 1 (10%) | |
| Retropancreatic | 1 (4.7%) | 0 (0%) | 1 (10%) | |
| Left pectineus | 1 (4.7%) | 0 (0%) | 1 (10%) | |
| Intramuscular | 20 (95.2%) | 11 (100%) | 9 (90%) | NS |
| Extramuscular | 9 (42.8%) | 3 (27.3%) | 6 (60%) | NS |
| Dimension (cm) | 12.26 | 10 | 14.75 | 0.0128 * |
| CT Arterial Blush | 15 (71.4%) | 5 (45.4%) | 10 (100%) | 0.0124 * |
| CT Late CE | 11 (52.4%) | 2 (18.2%) | 9 (90%) | 0.0019 * |
| Outcome (survival) | 16 (76.2%) | 10 (90.9%) | 6 (60%) | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riu, P.; Albarello, F.; Di Stefano, F.; Vergori, A.; D’Abramo, A.; Cerini, C.; Nocioni, M.; Morucci, M.; Tetaj, N.; Cristofaro, M.; et al. Management of Spontaneous Bleeding in COVID-19 Inpatients: Is Embolization Always Needed? J. Clin. Med. 2021, 10, 4119. https://doi.org/10.3390/jcm10184119
Riu P, Albarello F, Di Stefano F, Vergori A, D’Abramo A, Cerini C, Nocioni M, Morucci M, Tetaj N, Cristofaro M, et al. Management of Spontaneous Bleeding in COVID-19 Inpatients: Is Embolization Always Needed? Journal of Clinical Medicine. 2021; 10(18):4119. https://doi.org/10.3390/jcm10184119
Chicago/Turabian StyleRiu, Pascale, Fabrizio Albarello, Federica Di Stefano, Alessandra Vergori, Alessandra D’Abramo, Carlo Cerini, Martina Nocioni, Maurizio Morucci, Nardi Tetaj, Massimo Cristofaro, and et al. 2021. "Management of Spontaneous Bleeding in COVID-19 Inpatients: Is Embolization Always Needed?" Journal of Clinical Medicine 10, no. 18: 4119. https://doi.org/10.3390/jcm10184119
APA StyleRiu, P., Albarello, F., Di Stefano, F., Vergori, A., D’Abramo, A., Cerini, C., Nocioni, M., Morucci, M., Tetaj, N., Cristofaro, M., Schininà, V., Campioni, P., Petrone, A., Fusco, N., Marchioni, L., Antinori, A., Nicastri, E., Cianni, R., & Ianniello, S. (2021). Management of Spontaneous Bleeding in COVID-19 Inpatients: Is Embolization Always Needed? Journal of Clinical Medicine, 10(18), 4119. https://doi.org/10.3390/jcm10184119






