Role of Nucleocapsid Protein Antigen Detection for Safe End of Isolation of SARS-CoV-2 Infected Patients with Long Persistence of Viral RNA in Respiratory Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Testing
2.3. Viral Culture
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, J.; Wang, M.; Zhang, G.; Lu, E. Seven discharged patients turning positive again for SARS-CoV-2 on quantitative RT-PCR. Am. J. Infect. Control. 2020, 48, 725–726. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Chen, Q.; Wang, T. Letter to the Editor: Three cases of re-detectable positive SARS-CoV-2 RNA in recovered COVID-19 patients with antibodies. J. Med. Virol. 2020, 92, 2298–2301. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, A. COVID-19 follow up testing. J. Infect. 2020, 81, 647–679. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.D.; Chang, S.Y.; Wang, J.T.; Tsai, M.J.; Hung, C.C.; Hsu, C.L.; Chang, S.C. Prolonged virus shedding even after seroconversion in a patient with COVID-19. J. Infect. 2020, 81, 318–356. [Google Scholar] [CrossRef] [PubMed]
- Cento, V.; Colagrossi, L.; Nava, A.; Lamberti, A.; Senatore, S.; Travi, G.; Rossotti, R.; Vecchi, M.; Casati, O.; Matarazzo, E.; et al. Persistent positivity and fluctuations of SARS-CoV-2 RNA in clinically-recovered COVID-19 patients. J. Infect. 2020, 81, e90–e92. [Google Scholar] [CrossRef] [PubMed]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef] [Green Version]
- Bullard, J.; Dust, K.; Funk, D.; Strong, J.E.; Alexander, D.; Garnett, L.; Boodman, C.; Bello, A.; Hedley, A.; Schiffman, Z.; et al. Predicting Infectious Severe Acute Respiratory Syndrome Coronavirus 2 From Diagnostic Samples. Clin. Infect. Dis. 2020, 71, 2663–2666. [Google Scholar] [CrossRef]
- Cevik, M.; Tate, M.; Lloyd, O.; Maraolo, A.E.; Schafers, J.; Ho, A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: A systematic review and meta-analysis. Lancet Microbe 2021, 2, e13–e22. [Google Scholar] [CrossRef]
- Badu, K.; Oyebola, K.; Zahouli, J.Z.B.; Fagbamigbe, A.F.; de Souza, D.K.; Dukhi, N.; Amankwaa, E.F.; Tolba, M.F.; Sylverken, A.A.; Mosi, L.; et al. SARS-CoV-2 Viral Shedding and Transmission Dynamics: Implications of WHO COVID-19 Discharge Guidelines. Front. Med. 2021, 8, 648660. [Google Scholar] [CrossRef]
- Aydillo, T.; Gonzalez-Reiche, A.S.; Aslam, S.; van de Guchte, A.; Khan, Z.; Obla, A.; Dutta, J.; van Bakel, H.; Aberg, J.; García-Sastre, A.; et al. Shedding of Viable SARS-CoV-2 after Immunosuppressive Therapy for Cancer. N. Engl. J. Med. 2020, 384, 1677–1678. [Google Scholar]
- Avanzato, V.A.; Matson, M.J.; Seifert, S.N.; Pryce, R.; Williamson, B.N.; Anzick, S.L.; Barbian, K.; Judson, S.D.; Fischer, E.R.; Martens, C.; et al. Case study: Prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell 2020, 183, 1901–1912.e9. [Google Scholar] [CrossRef] [PubMed]
- Baang, J.H.; Smith, C.; Mirabelli, C.; Valesano, A.L.; Manthei, D.M.; Bachman, M.A.; Wobus, C.E.; Adams, M.; Washer, L.; Martin, E.T.; et al. Prolonged Severe Acute Respiratory Syndrome Coronavirus 2 Replication in an Immunocompromised Patient. J. Infect. Dis. 2021, 223, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Choudhary, M.C.; Regan, J.; Sparks, J.A.; Padera, R.F.; Qiu, X.; Solomon, I.H.; Kuo, H.H.; Boucau, J.; Bowman, K.; et al. Persistence and Evolution of SARS-CoV-2 in an Immunocompromised Host. N. Engl. J. Med. 2020, 383, 2291–2293. [Google Scholar] [CrossRef]
- Tarhini, H.; Recoing, A.; Bridier-Nahmias, A.; Rahi, M.; Lambert, C.; Martres, P.; Lucet, J.C.; Rioux, C.; Bouzid, D.; Lebourgeois, S.; et al. Long term SARS-CoV-2 infectiousness among three immunocompromised patients: From prolonged viral shedding to SARS-CoV-2 superinfection. J. Infect. Dis. 2021, 223, 1522–1527. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Interim Guidance on Ending Isolation and Precautions for Adults with COVID-19. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html (accessed on 17 August 2021).
- European Center for Disease Prevention and Control. Guidance for Discharge and Ending of Isolation of People with COVID-19. Available online: https://www.ecdc.europa.eu/en/publications-data/covid-19-guidance-discharge-and-ending-isolation (accessed on 17 August 2021).
- Circolare del Ministero Della Salute. Aggiornamento Sulle Misure di Quarantena e di Isolamento Raccomandate Alla Luce Della Circolazione Delle Nuove Varianti SARS-CoV-2 in Italia ed in Particolare Della Diffusione Della Variante Delta (Lignaggio B.1.617.2). Available online: https://fimmg.bari.it/documenti/FDRBC_1.pdf (accessed on 17 August 2021).
- Rodríguez-Fernández, P.; González-Santos, J.; Santamaría-Peláez, M.; Soto-Cámara, R.; Sánchez-González, E.; González-Bernal, J.J. Psychological Effects of Home Confinement and Social Distancing Derived from COVID-19 in the General Population-A Systematic Review. Int. J. Environ. Res. Public. Health 2021, 18, 6528. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.F.; Ma, B.; Shahzad, L. A brief review of socio-economic and environmental impact of Covid-19. Air Qual. Atmos. Health 2020, 1, 1403–1409. [Google Scholar] [CrossRef]
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. 2020, 78, 185–193. [Google Scholar] [CrossRef]
- Brodeur, A.; Gray, D.; Islam, A.; Bhuiyan, S. A literature review of the economics of COVID-19. J. Econ. Surv. 2021, 35, 1007–1044. [Google Scholar] [CrossRef]
- Kohmer, N.; Rabenau, H.F.; Hoehl, S.; Kortenbusch, M.; Ciesek, S.; Berger, A. Comparative analysis of point-of-care, high-throughput and laboratory-developed SARS-CoV-2 nucleic acid amplification tests (NATs). J. Virol. Methods 2021, 291, 114102. [Google Scholar] [CrossRef]
- Hirotsu, Y.; Maejima, M.; Shibusawa, M.; Nagakubo, Y.; Hosaka, K.; Amemiya, K.; Sueki, H.; Hayakawa, M.; Mochizuki, H.; Tsutsui, T.; et al. Comparison of automated SARS-CoV-2 antigen test for COVID-19 infection with quantitative RT-PCR using 313 nasopharyngeal swabs, including from seven serially followed patients. Int. J. Infect. Dis. 2020, 99, 397–402. [Google Scholar] [CrossRef]
- Gili, A.; Paggi, R.; Russo, C.; Cenci, E.; Pietrella, D.; Graziani, A.; Stracci, F.; Mencacci, A. Evaluation of Lumipulse® G SARS-CoV-2 antigen assay automated test for detecting SARS-CoV-2 nucleocapsid protein (NP) in nasopharyngeal swabs for community and population screening. Int. J. Infect. Dis. 2021, 105, 391–396. [Google Scholar] [CrossRef]
- Hirotsu, Y.; Maejima, M.; Shibusawa, M.; Amemiya, K.; Nagakubo, Y.; Hosaka, K.; Sueki, H.; Hayakawa, M.; Mochizuki, H.; Tsutsui, T.; et al. Analysis of a persistent viral shedding patient infected with SARS-CoV-2 by RT-qPCR, FilmArray Respiratory Panel v2.1, and antigen detection. J. Infect. Chemother. 2021, 27, 406–409. [Google Scholar] [CrossRef]
- Hirotsu, Y.; Maejima, M.; Shibusawa, M.; Amemiya, K.; Nagakubo, Y.; Hosaka, K.; Sueki, H.; Hayakawa, M.; Mochizuki, H.; Tsutsui, T.; et al. Prospective study of 1308 nasopharyngeal swabs from 1033 patients using the LUMIPULSE SARS-CoV-2 antigen test: Comparison with RT-qPCR. Int. J. Infect. Dis. 2021, 105, 7–14. [Google Scholar] [CrossRef]
- Gidari, A.; Nofri, M.; Saccarelli, L.; Bastianelli, S.; Sabbatini, S.; Bozza, S.; Camilloni, B.; Fusco-Moffa, I.; Monari, C.; De Robertis, E.; et al. Is recurrence possible in coronavirus disease 2019 (COVID-19)? Case series and systematic review of literature. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Keyaerts, E.; Vijgen, L.; Maes, P.; Neyts, J.; Van Ranst, M. Growth kinetics of SARS-coronavirus in Vero E6 cells. Biochem. Biophys. Res. Commun. 2005, 329, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Kim, H.M.; Lee, E.J.; Jo, H.J.; Yoon, Y.; Lee, N.J.; Son, J.; Lee, Y.J.; Kim, M.S.; Lee, Y.P.; et al. Detection and Isolation of SARS-CoV-2 in Serum, Urine, and Stool Specimens of COVID-19 Patients from the Republic of Korea. Osong Public Health Res. Perspect. 2020, 11, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, W.S. Robust locally weighted regression and smoothing scatterplots. J. Am. Stat. Assoc. 1979, 74, 829–836. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis; Springer: New York, NY, USA, 2001. [Google Scholar]
- La Scola, B.; Le Bideau, M.; Andreani, J.; Hoang, V.T.; Grimaldier, C.; Colson, P.; Gautret, P.; Raoult, D. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1059–1061. [Google Scholar] [CrossRef]
- Million, M.; Lagier, J.C.; Gautret, P.; Colson, P.; Fournier, P.E.; Amrane, S.; Hocquart, M.; Mailhe, M.; Esteves-Vieira, V.; Doudier, B.; et al. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: A retrospective analysis of 1061 cases in Marseille, France. Travel Med. Infect. Dis. 2020, 35, 101738. [Google Scholar] [CrossRef]
- Perera, R.A.P.M.; Tso, E.; Tsang, O.T.Y.; Tsang, D.N.C.; Fung, K.; Leung, Y.W.Y.; Chin, A.W.H.; Chu, D.K.W.; Cheng, S.M.S.; Poon, L.L.M.; et al. SARS-CoV-2 virus culture and subgenomic RNA for respiratory specimens from patients with mild coronavirus disease. Emerg. Infect. Dis. 2020, 26, 2701–2704. [Google Scholar] [CrossRef]
- van Kampen, J.J.A.; van de Vijver, D.A.M.C.; Fraaij, P.L.A.; Haagmans, B.L.; Lamers, M.M.; Okba, N.; van den Akker, J.P.C.; Endeman, H.; Gommers, D.A.M.P.J.; Cornelissen, J.J.; et al. Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19). Nat. Commun. 2021, 12, 267. [Google Scholar] [CrossRef] [PubMed]
- Manzulli, V.; Scioscia, G.; Giganti, G.; Capobianchi, M.R.; Lacedonia, D.; Pace, L.; Cipolletta, D.; Tondo, P.; De Nittis, R.; Rondinone, V.; et al. Real Time PCR and Culture-Based Virus Isolation Test in Clinically Recovered Patients: Is the Subject Still Infectious for SARS-CoV2? J. Clin. Med. 2021, 10, 309. [Google Scholar] [CrossRef]
- Zhou, Y.; Ding, F.; Bao, W.; Xue, Y.; Han, L.; Zhang, X.; Zhang, P.; Ji, Y.; Yin, D.; Bao, A.; et al. Clinical features in coronavirus disease 2019 (COVID-19) patients with early clearance and prolonged shedding of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA. Ann. Transl. Med. 2021, 9, 665. [Google Scholar] [CrossRef]
- Kim, M.C.; Cui, C.; Shin, K.R.; Bae, J.Y.; Kweon, O.J.; Lee, M.K.; Choi, S.H.; Jung, S.Y.; Park, M.S.; Chung, J.W. Duration of Culturable SARS-CoV-2 in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Helleberg, M.; Niemann, C.U.; Moestrup, K.S.; Kirk, O.; Lebech, A.M.; Lane, C.; Lundgren, J. Persistent COVID-19 in an immunocompromised patient temporarily responsive to two courses of remdesivir therapy. J. Infect. Dis. 2020, 222, 1103–1107. [Google Scholar] [CrossRef]
- Manabe, Y.C.; Sharfstein, J.S.; Armstrong, K. The Need for More and Better Testing for COVID-19. JAMA 2020, 324, 2153–2154. [Google Scholar] [CrossRef] [PubMed]
- Arons, M.M.; Hatfield, K.M.; Reddy, S.C.; Kimball, A.; James, A.; Jacobs, J.R.; Taylor, J.; Spicer, K.; Bardossy, A.C.; Oakley, L.P.; et al. Public Health–Seattle and King County and CDC COVID-19 Investigation Team. Presymptomatic SARS-CoV-2 Infections and Transmission in a Skilled Nursing Facility. N. Engl. J. Med. 2020, 382, 2081–2090. [Google Scholar] [CrossRef]
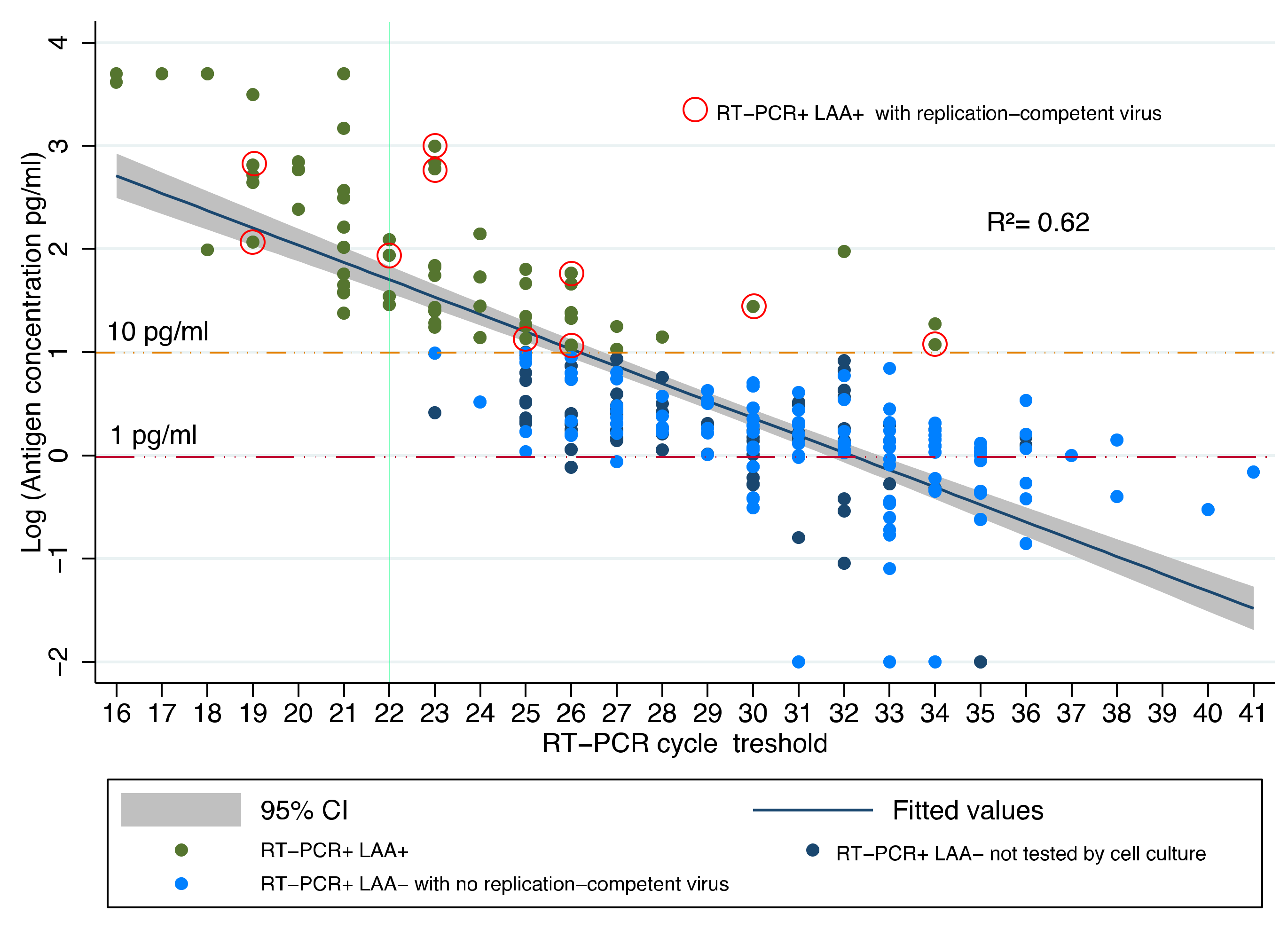
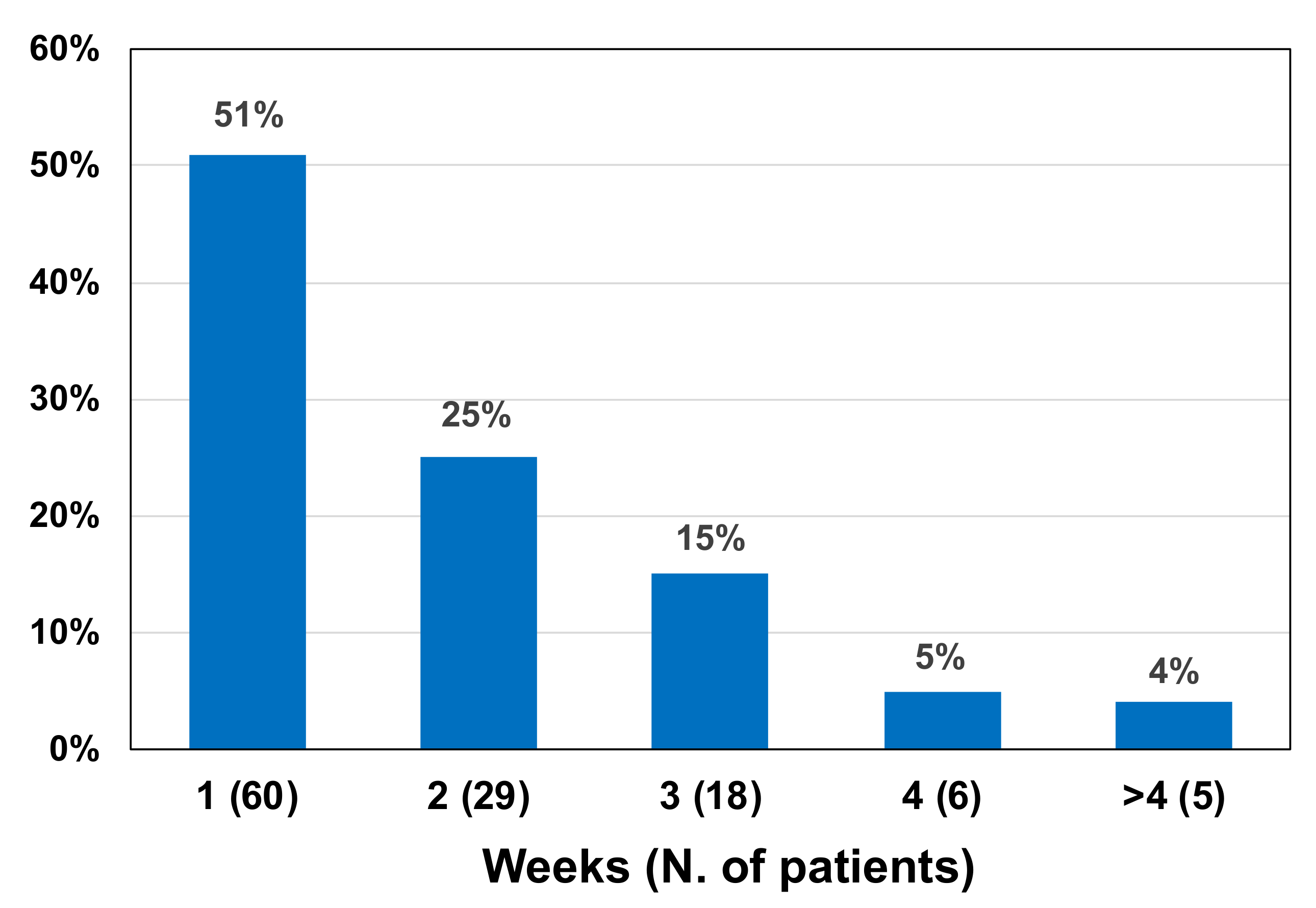
| LAA Positive | LAA Negative | Total | |
|---|---|---|---|
| RT-PCR positive | 68 (26.7%) | 187 (73.3%) | 255 (100.0%) |
| RT-PCR negative | 0 (0%) | 416 (100.0%) | 416 (100.0%) |
| Total | 68 (10.1%) | 603 (89.9%) | 671 (100%) |
| Samples Assessed by Culture | Samples with Viable Virus | |||||
|---|---|---|---|---|---|---|
| Sample Group | Number | Ct Mean (Range) | Antigen Mean (Range) | Number (%) | Ct Mean (Range) | Antigen Mean (Range) |
| RT-PCR+/LAA+ | 20 | 24.9 (19–34) | 146.6 * (13.6–990.1) | 10 (50%) | 24.7 (19–33) | 263.1 (13.6–990.1) |
| RT-PCR+/LAA– | 118 | 31 (22–41) | 1.38 (0.4–9.6) | 0 (0%) | NA | NA |
| Culture Result | Patient Age (Years) | Hospitalization | Days after Diagnosis | Ct Value | Antigen Level (pg/mL) |
|---|---|---|---|---|---|
| Positive | 73 | Yes | 22 | 30 | 17.5 |
| 33 | No | 23 | 26 | 19.8 | |
| 51 | Yes | 26 | 34 | 14.7 | |
| 45 | Yes | 22 | 22 | 93.2 | |
| 48 | Yes | 25 | 26 | 65.2 | |
| 76 | Yes | 23 | 19 | 732.2 | |
| 88 | Yes | 60 | 25 | 13.6 | |
| 47 | No | 24 | 23 | 990.1 | |
| 86 | Yes | 43 | 19 | 94.3 | |
| 46 | Yes | 48 | 23 | 590.7 | |
| Negative | 72 | No | 39 | 28 | 15.5 |
| 65 | No | 47 | 25 | 13.9 | |
| 73 | No | 37 | 34 | 18.8 | |
| 75 | No | 38 | 28 | 14.2 | |
| 76 | Yes | 25 | 23 | 14.4 | |
| 46 | No | 25 | 23 | 90.0 | |
| 81 | No | 27 | 22 | 31.2 | |
| 86 | No | 40 | 20 | 35.5 | |
| 90 | No | 35 | 22 | 52.3 | |
| 88 | No | 37 | 25 | 14.6 |
| vs. RT-PCR (95% C.I.) | vs. Culture (95% C.I.) | |
|---|---|---|
| Sensitivity | 26.7% (21.3–32.5) | 100% (69.0–100) |
| Specificity | 100.0% (99.1–100) | 92.2% (86.1–96.2) |
| NPV | 69.0% (65.1–72.7) | 100% (96.1–100) |
| PPV | 100.0% (94.7–100) | 50.0% (35.6–64.5) |
| LR+ | NA | 12.8 (7.1–23.2) |
| LR– | 0.73 (0.68–0.79) | NA |
| AUC | 63.2% (61.4–66.3) | 92.7% (87.1–96.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mencacci, A.; Gili, A.; Gidari, A.; Schiaroli, E.; Russo, C.; Cenci, E.; Camilloni, B.; Graziani, A.; Melelli-Roia, A.; Francisci, D.; et al. Role of Nucleocapsid Protein Antigen Detection for Safe End of Isolation of SARS-CoV-2 Infected Patients with Long Persistence of Viral RNA in Respiratory Samples. J. Clin. Med. 2021, 10, 4037. https://doi.org/10.3390/jcm10184037
Mencacci A, Gili A, Gidari A, Schiaroli E, Russo C, Cenci E, Camilloni B, Graziani A, Melelli-Roia A, Francisci D, et al. Role of Nucleocapsid Protein Antigen Detection for Safe End of Isolation of SARS-CoV-2 Infected Patients with Long Persistence of Viral RNA in Respiratory Samples. Journal of Clinical Medicine. 2021; 10(18):4037. https://doi.org/10.3390/jcm10184037
Chicago/Turabian StyleMencacci, Antonella, Alessio Gili, Anna Gidari, Elisabetta Schiaroli, Carla Russo, Elio Cenci, Barbara Camilloni, Alessandro Graziani, Arduino Melelli-Roia, Daniela Francisci, and et al. 2021. "Role of Nucleocapsid Protein Antigen Detection for Safe End of Isolation of SARS-CoV-2 Infected Patients with Long Persistence of Viral RNA in Respiratory Samples" Journal of Clinical Medicine 10, no. 18: 4037. https://doi.org/10.3390/jcm10184037
APA StyleMencacci, A., Gili, A., Gidari, A., Schiaroli, E., Russo, C., Cenci, E., Camilloni, B., Graziani, A., Melelli-Roia, A., Francisci, D., & Stracci, F. (2021). Role of Nucleocapsid Protein Antigen Detection for Safe End of Isolation of SARS-CoV-2 Infected Patients with Long Persistence of Viral RNA in Respiratory Samples. Journal of Clinical Medicine, 10(18), 4037. https://doi.org/10.3390/jcm10184037








