Rhythm Control in Patients with Heart Failure with Preserved Ejection Fraction: A Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Literature Review and Search Strategy
2.2. Selection Criteria
2.3. Data Abstraction
2.4. Statistical Analysis
3. Results
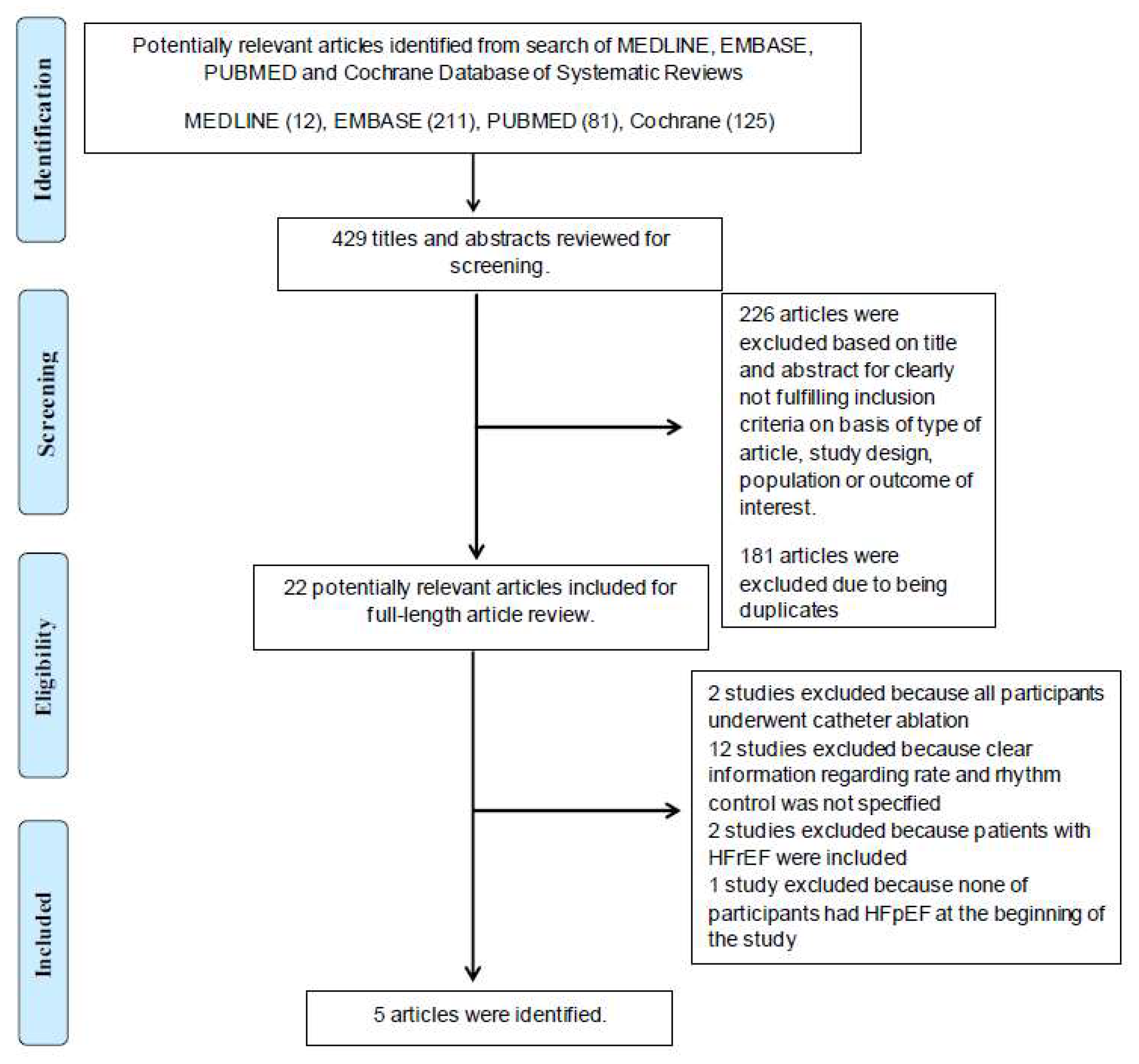
3.1. Study Characteristics and Quality Assessment
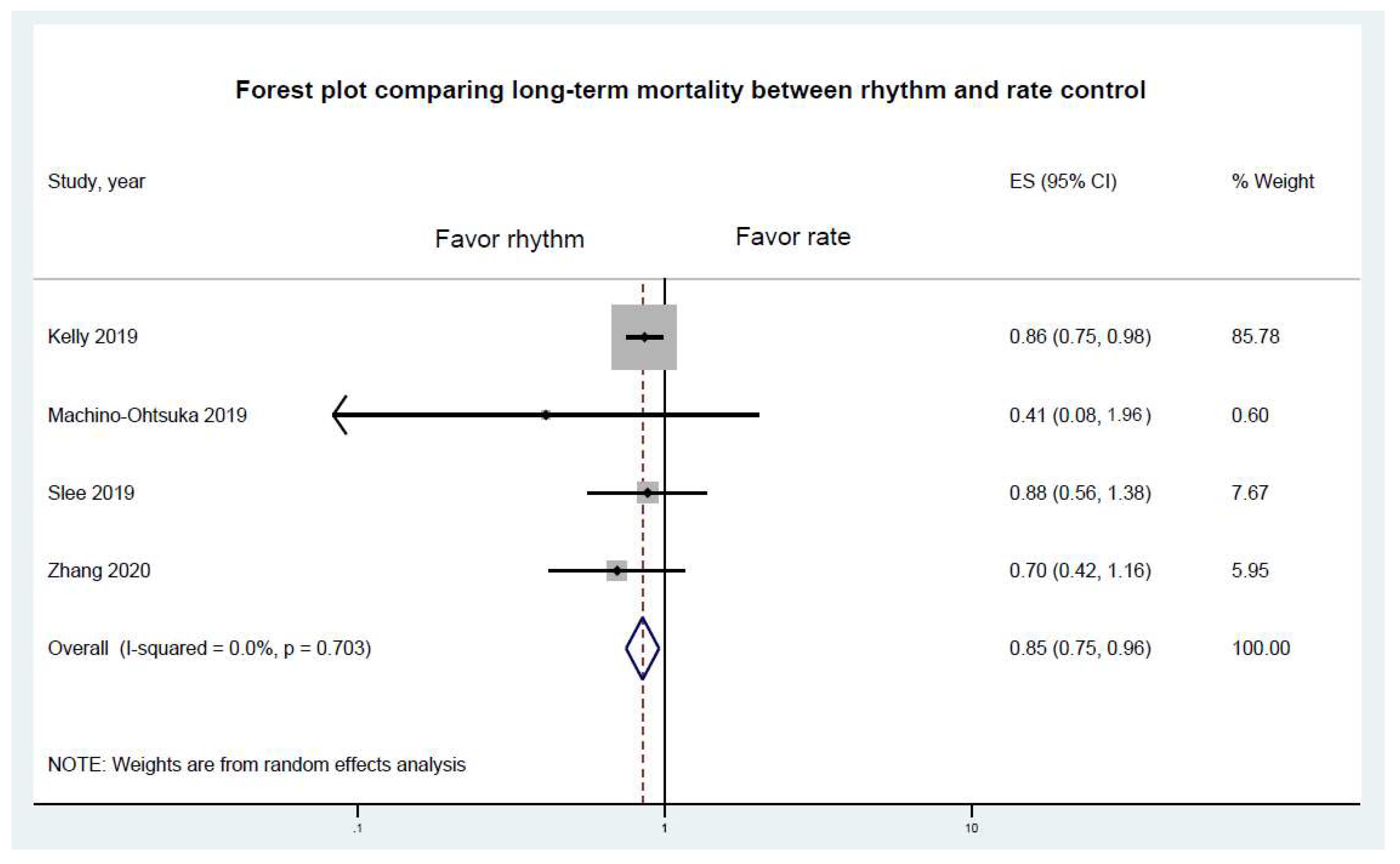
3.2. Primary Endpoints Results
3.3. Exploratory Analysis
3.4. Sensitivity Analysis and Publication Bias
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dunlay, S.M.; Roger, V.L.; Redfield, M.M. Epidemiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2017, 14, 591–602. [Google Scholar] [CrossRef]
- Shah, K.S.; Xu, H.; Matsouaka, R.A.; Bhatt, D.L.; Heidenreich, P.A.; Hernandez, A.F.; Devore, A.D.; Yancy, C.W.; Fonarow, G.C. Heart Failure With Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. J. Am. Coll. Cardiol. 2017, 70, 2476–2486. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.P.; Gamble, G.D.; Ling, L.H.; Sim, D.; Leong, K.T.G.; Yeo, P.S.D.; Ong, H.Y.; Jaufeerally, F.; Ng, T.P.; Cameron, A.V.; et al. Mortality associated with heart failure with preserved vs. reduced ejection fraction in a prospective international multi-ethnic cohort study. Eur. Heart J. 2018, 39, 1770–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: ThE Task Fource for the diagnosis and treatment of acute and chronic. Heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure:A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J. Am. Coll. Cardiol. 2017, 70, 776–803. [Google Scholar] [CrossRef] [PubMed]
- Sartipy, U.; Dahlström, U.; Fu, M.; Lund, L.H. Atrial Fibrillation in Heart Failure With Preserved, Mid-Range, and Reduced Ejection Fraction. JACC Heart Fail. 2017, 5, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; Van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.D.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.F.; Devereaux, P.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. Ann. Intern. Med. 2009, 151, W-65–W-94. [Google Scholar] [CrossRef] [Green Version]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [Green Version]
- Prasitlumkum, N.; Cheungpasitporn, W.; Sato, R.; Thangjui, S.; Thongprayoon, C.; Kewcharoen, J.; Bathini, T.; Vallabhajosyula, S.; Ratanapo, S.; Chokesuwattanaskul, R. Comparison of coronary artery bypass graft versus drug-eluting stents in dialysis patients: An updated systemic review and meta-analysis. J. Cardiovasc. Med. 2021, 22, 285–296. [Google Scholar] [CrossRef]
- Prasitlumkum, N.; Kewcharoen, J.; Kanitsoraphan, C.; Rattanawong, P.; Mekritthikrai, R.; Gillaspie, E.A.; Mao, M.A.; Cheungpasitporn, W. Previous coronary artery bypass graft is not associated with higher mortality in transcatheter aortic valve replacement: Systemic review and meta-analysis. Acta Cardiol. 2020, 75, 26–34. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.A.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kelly, J.P.; DeVore, A.D.; Wu, J.; Hammill, B.G.; Sharma, A.; Cooper, L.B.; Felker, G.M.; Piccini, J.P.; Allen, L.A.; Heidenreich, P.A.; et al. Rhythm Control Versus Rate Control in Patients With Atrial Fibrillation and Heart Failure With Preserved Ejection Fraction: Insights From Get With The Guidelines—Heart Failure. J. Am. Heart Assoc. 2019, 8, e011560. [Google Scholar] [CrossRef]
- Machino-Ohtsuka, T.; Seo, Y.; Ishizu, T.; Yamamoto, M.; Hamada-Harimura, Y.; Machino, T.; Yamasaki, H.; Sekiguchi, Y.; Nogami, A.; Aonuma, K.; et al. Relationships between maintenance of sinus rhythm and clinical outcomes in patients with heart failure with preserved ejection fraction and atrial fibrillation. J. Cardiol. 2019, 74, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Slee, A.; Saad, M.; Saksena, S. Heart failure progression and mortality in atrial fibrillation patients with preserved or reduced left ventricular ejection fraction. J. Interv. Card. Electrophysiol. 2019, 55, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chamberlain, A.M.; Hodge, D.O.; Cai, C.; Xiao, P.L.; Han, J.; Jiang, C.; Redfield, M.M.; Roger, V.L.; Cha, Y. Outcomes of incident atrial fibrillation in heart failure with preserved or reduced ejection fraction: A community-based study. J. Cardiovasc. Electrophysiol. 2020, 31, 2275–2283. [Google Scholar] [CrossRef] [PubMed]
- Zhirov, I.; Safronova, N.; Osmolovskaya, Y.; Alshevskaya, A.; Moskalev, A.; Tereshchenko, S. Predictors of Unfavorable Outcomes in Patients with Atrial Fibrillation and Concomitant Heart Failure with Different Ejection Fractions: RIF-CHF Register One-Year Follow-Up. Cardiol. Res. Pract. 2019, 2019, 1692104–1692114. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Hagens, V.E.; Bosker, H.A.; Kingma, J.H.; Kamp, O.; Kingma, T.; Said, S.A.; Darmanata, J.I.; Timmermans, A.J.M.; Tijssen, J.G.P.; et al. A Comparison of Rate Control and Rhythm Control in Patients with Recurrent Persistent Atrial Fibrillation. N. Engl. J. Med. 2002, 347, 1834–1840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyse, D.G.; Waldo, A.L.; DiMarco, J.P.; Domanski, M.J.; Rosenberg, Y.; Schron, E.B.; Kellen, J.C.; Greene, H.L.; Mickel, M.C.; E Dalquist, J.; et al. A Comparison of Rate Control and Rhythm Control in Patients with Atrial Fibrillation. N. Engl. J. Med. 2002, 347, 1825–1833. [Google Scholar] [CrossRef]
- Camm, A.J.; Breithardt, G.; Crijns, H.; Dorian, P.; Kowey, P.; Le Heuzey, J.-Y.; Merioua, I.; Pedrazzini, L.; Prystowsky, E.N.; Schwartz, P.J.; et al. Real-Life Observations of Clinical Outcomes With Rhythm- and Rate-Control Therapies for Atrial Fibrillation: RECORDAF (Registry on Cardiac Rhythm Disorders Assessing the Control of Atrial Fibrillation). J. Am. Coll. Cardiol. 2011, 58, 493–501. [Google Scholar] [CrossRef] [Green Version]
- McManus, D.D.; Hsu, G.; Sung, S.H.; Saczynski, J.S.; Smith, D.H.; Magid, D.J.; Gurwitz, J.H.; Goldberg, R.J.; Go, A.S.; for the Cardiovascular Research Network PRESERVE Study. Atrial Fibrillation and Outcomes in Heart Failure With Preserved Versus Reduced Left Ventricular Ejection Fraction. J. Am. Heart Assoc. 2013, 2, e005694. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, A.; Taillandier, S.; Olesen, J.B.; Lane, D.A.; Lallemand, B.; Lip, G.Y.; Fauchier, L. Ejection fraction and outcomes in patients with atrial fibrillation and heart failure: The Loire Valley Atrial Fibrillation Project. Eur. J. Heart Fail. 2012, 14, 295–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakeri, R.; Chamberlain, A.M.; Roger, V.L.; Redfield, M.M. Temporal Relationship and Prognostic Significance of Atrial Fibrillation in Heart Failure Patients With Preserved Ejection Fraction: A community-based study. Circulation 2013, 128, 1085–1093. [Google Scholar] [CrossRef] [Green Version]
- Kotecha, D.; Chudasama, R.; Lane, D.A.; Kirchhof, P.; Lip, G.Y. Atrial fibrillation and heart failure due to reduced versus preserved ejection fraction: A systematic review and meta-analysis of death and adverse outcomes. Int. J. Cardiol. 2016, 203, 660–666. [Google Scholar] [CrossRef]
- Cheng, M.; Lu, X.; Huang, J.; Zhang, J.; Zhang, S.; Gu, D. The prognostic significance of atrial fibrillation in heart failure with a preserved and reduced left ventricular function: Insights from a meta-analysis. Eur. J. Heart Fail. 2014, 16, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Mamas, M.A.; Caldwell, J.C.; Chacko, S.; Garratt, C.J.; Fath-Ordoubadi, F.; Neyses, L. A meta-analysis of the prognostic significance of atrial fibrillation in chronic heart failure. Eur. J. Heart Fail. 2009, 11, 676–683. [Google Scholar] [CrossRef] [Green Version]
- Marrouche, N.F.; Brachmann, J.; Andresen, D.; Siebels, J.; Boersma, L.; Jordaens, L.; Merkely, B.; Pokushalov, E.; Sanders, P.; Proff, J.; et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. N. Engl. J. Med. 2018, 378, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Taylor, A.; Costello, B.T.; Kaye, D.M.; McLellan, A.J.; Voskoboinik, A.; Sugumar, H.; Lockwood, S.M.; Stokes, M.B.; Pathik, B.; et al. Catheter Ablation Versus Medical Rate Control in Atrial Fibrillation and Systolic Dysfunction: The CAMERA-MRI Study. J. Am. Coll. Cardiol. 2017, 70, 1949–1961. [Google Scholar] [CrossRef]
- Jones, D.G.; Haldar, S.K.; Hussain, W.; Sharma, R.; Francis, D.P.; Rahman-Haley, S.L.; McDonagh, T.A.; Underwood, S.R.; Markides, V.; Wong, T. A Randomized Trial to Assess Catheter Ablation Versus Rate Control in the Management of Persistent Atrial Fibrillation in Heart Failure. J. Am. Coll. Cardiol. 2013, 61, 1894–1903. [Google Scholar] [CrossRef] [Green Version]
- Maisel, W.H.; Stevenson, L.W. Atrial fibrillation in heart failure: Epidemiology, pathophysiology, and rationale for therapy. Am. J. Cardiol. 2003, 91, 2D–8D. [Google Scholar] [CrossRef]
- Lam, C.S.; Rienstra, M.; Tay, W.T.; Liu, L.C.; Hummel, Y.M.; van der Meer, P.; de Boer, R.A.; Van Gelder, I.C.; van Veldhuisen, D.J.; Voors, A.A.; et al. Atrial Fibrillation in Heart Failure With Preserved Ejection Fraction: Associ-ation With Exercise Capacity, Left Ventricular Filling Pressures, Natriuretic Peptides, and Left Atrial Volume. JACC: Heart Fail. 2017, 5, 92–98. [Google Scholar] [CrossRef]
- Odutayo, A.; Wong, C.; Hsiao, A.J.; Hopewell, S.; Altman, D.G.; Emdin, A.C. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: Systematic review and meta-analysis. BMJ 2016, 354, i4482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.; Sung, J.; Jang, E.; Yu, H.T.; Kim, T.; Uhm, J.; Kim, J.; Pak, H.; Lee, M.; Joung, B. Catheter Ablation Improves Mortality and Other Outcomes in Real-World Patients With Atrial Fibrillation. J. Am. Heart Assoc. 2020, 9, e015740. [Google Scholar] [CrossRef] [PubMed]
- Turagam, M.K.; Garg, J.; Whang, W.; Sartori, S.; Koruth, J.; Miller, M.A.; Langan, N.; Sofi, A.; Gomes, A.; Choudry, S.; et al. Catheter Ablation of Atrial Fibrillation in Patients With Heart Failure: A Meta-analysis of Randomized Controlled Trials. Ann. Intern. Med. 2019, 170, 41–50. [Google Scholar] [CrossRef]
- Packer, D.L.; Mark, D.B.; Robb, R.A.; Monahan, K.H.; Bahnson, T.D.; Poole, J.E.; Noseworthy, P.A.; Rosenberg, Y.D.; Jeffries, N.; Mitchell, L.B.; et al. Effect of Catheter Ablation vs Antiarrhythmic Drug Therapy on Mortality, Stroke, Bleeding, and Cardiac Arrest Among Patients with Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA 2019, 321, 1261–1274. [Google Scholar] [CrossRef]
- Wożakowska-Kapłon, B.; Opolski, G. Effects of sinus rhythm restoration in patients with persistent atrial fibrillation: A clinical, echocardiographic and hormonal study. Int. J. Cardiol. 2004, 96, 171–176. [Google Scholar] [CrossRef]
- Machino-Ohtsuka, T.; Seo, Y.; Ishizu, T.; Sugano, A.; Atsumi, A.; Yamamoto, M.; Kawamura, R.; Machino, T.; Kuroki, K.; Yamasaki, H.; et al. Efficacy, Safety, and Outcomes of Catheter Ablation of Atrial Fibrillation in Patients With Heart Failure With Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2013, 62, 1857–1865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, G.; Lopez-Jimenez, F.; Boyd, J.; D’Amico, F.; Durant, N.H.; Hlatky, M.; Howard, G.; Kirley, K.; Masi, C.; Powell-Wiley, T.M.; et al. Methodological Standards for Meta-Analyses and Qualitative Systematic Reviews of Cardiac Prevention and Treatment Studies: A Scientific Statement From the American Heart Association. Circulation 2017, 136, e172–e194. [Google Scholar] [CrossRef] [PubMed]
| Study | Kelly 2019 [16] | Machino–Ohtsuka 2019 [17] | Slee 2019 [18] | Zhang 2020 [19] | Zhirov 2019 [20] |
|---|---|---|---|---|---|
| Types | Retro | Retro | Retro | Retro | Pros |
| Country | USA | Japan | USA | USA | Russia |
| Participants | HF patients >65 years old with concurrent AF who were discharged alive | HFpEF patients >20 years old with AF | >65 years old HF patients with prior AF who were at high risk of stroke | HF patients >18 years old with prior or current AF | HF patients >18 years old with prior or current non-valvular AF |
| Database | Medicare data from 2008 to 2014 | Multicenter from 2012 to 2015 | AFFIRM registry | REP from 2000 to 2014 | Multicenter from 2015 to 2016 |
| Exclusion | Patients who did not receive either rhythm or rate control strategies, patients who were not admitted | Younger than 20 years old, prior MI, valvular disease requiring intervention, history of pacemaker implantation, severe lung and liver disease | N/A | Patients without documented EF, patients with AF who died within 1 year | Recent stroke/TIA, recent MI, valvular AF, BiV implantation, severe life-limiting comorbidities, recent VTE |
| HFpEF criteria | HF with EF > 50% | HF with EF > 50% | HF with EF > 40% | HF with EF > 50% | HF with EF > 50% |
| Mean EF (%) | 58.0 | 63.8 ± 8 | N/A | 61.2 ± 6.7 | 60.0 ± 5.0 |
| Mean age (years) | 83.0 | 71 ± 8 | 70.9 ± 8.7 | 79.2 ± 11.1 | 72.0 |
| Sex (Female%) | 65.8 | 39.9 | 40.1 | 60.2 | 65.4 |
| CHA2DS2-VASc | N/A | Rate: 4.2 ± 1.4, Rhythm: 3.9 ± 1.3 | N/A | Rate: 5.2 ± 1.7, Rhythm: 4.7 ± 2 | N/A |
| Total participants | 15,682 | 283 | 349 | 447 | 387 |
| Hypertension | Rate: 81.9%, Rhythm: 83.8% | Rate: 75.0%, Rhythm: 80.4% | Rate: 80.1%, Rhythm: 76.4% | Rate: 85.4%, Rhythm: 75.0% | 68% |
| Diabetes | Rate: 36.2%, Rhythm: 36.0% | Rate: 31.3%, Rhythm: 34.6% | Rate: 33.3%, Rhythm: 20.8% | Rate: 36.8%, Rhythm: 30.0% | 23% |
| Coronary artery disease | Rate: 45.8%, Rhythm: 48.7% | Rate: 17.0%, Rhythm: 25.2% | Rate: 17.0%, Rhythm: 10.7% | Rate: 16.5%, Rhythm: 15.0% | 70% |
| CVA/TIA | Rate: 19.3%, Rhythm: 17.5% | Rate: 9.1%, Rhythm: 12.1% | Rate: 11.7%, Rhythm: 16.9% | Rate: 22.6%, Rhythm: 2.5% | 15% |
| Proportion of paroxysmal AF | N/A | 37.4% | N/A | N/A | 37.2% |
| AF duration (years) | N/A | 5.8 ± 6.7 | N/A | 4.2 (IQR 2–9) | N/A |
| Mean follow up (months) | 12 | 24 | 48 | 49.2 | 12 |
| Proportion of rhythm control | 11.8% | 37.8% | 51.0% | 15.9% | 40.6% |
| Proportion of rate control | 78.2% | 62.2% | 49.0% | 74.1% | 59.4% |
| Adjusted variables | Age, sex, race, prior MI, hypertension, hyperlipidemia, smoking history, prior CVA/TIA, DM, CKD, anemia, PVD, prior HF, COPD | Age, sex, body mass index, vital signs, prior MI, hypertension, hyperlipidemia, CKD, DM, medications, laboratory data, LVEF, LA volume, E/E’, TRPG, GLS, LV mass | Age, sex, failed antiarrhythmic drugs, hypertension, MI, stroke, DM, hypertension, cardiomyopathy, valvular heart disease | Age, sex, time interval from HF to AF, body mass index, hypertension, COPD, prior MI, prior stroke | N/A |
| Study | Rhythm Control | Rate Control |
|---|---|---|
| Kelly 2019 [16] | Class III 80.9%, Cardioversion 13.6%, AF ablation 1%, Unspecified 11.4% | Beta blocker 89.4%, CCB 25.3%, Digoxin 17.1% |
| Machino–Ohtsuka 2019 [17] | Class IA 6.5%, Class IC 37.4%, Class III 52.3%, Unspecified 10.3% | Beta blocker 54.5%, CCB 42.6% |
| Slee 2019 [18] | Class IC 3.9%, Class III 78.7%, Unspecified 8.4% | Beta blocker 36.8%, CCB 39.2%, Digoxin 51.9% |
| Zhang 2020 [19] | N/A | Beta blocker 80%, CCB 30%, Digoxin 20% |
| Zhirov 2019 [20] | N/A | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasitlumkum, N.; Chokesuwattanaskul, R.; Cheungpasitporn, W.; Kewcharoen, J.; Thongprayoon, C.; Bathini, T.; Vallabhajosyula, S.; Jongnarangsin, K. Rhythm Control in Patients with Heart Failure with Preserved Ejection Fraction: A Meta-Analysis. J. Clin. Med. 2021, 10, 4038. https://doi.org/10.3390/jcm10184038
Prasitlumkum N, Chokesuwattanaskul R, Cheungpasitporn W, Kewcharoen J, Thongprayoon C, Bathini T, Vallabhajosyula S, Jongnarangsin K. Rhythm Control in Patients with Heart Failure with Preserved Ejection Fraction: A Meta-Analysis. Journal of Clinical Medicine. 2021; 10(18):4038. https://doi.org/10.3390/jcm10184038
Chicago/Turabian StylePrasitlumkum, Narut, Ronpichai Chokesuwattanaskul, Wisit Cheungpasitporn, Jakrin Kewcharoen, Charat Thongprayoon, Tarun Bathini, Saraschandra Vallabhajosyula, and Krit Jongnarangsin. 2021. "Rhythm Control in Patients with Heart Failure with Preserved Ejection Fraction: A Meta-Analysis" Journal of Clinical Medicine 10, no. 18: 4038. https://doi.org/10.3390/jcm10184038
APA StylePrasitlumkum, N., Chokesuwattanaskul, R., Cheungpasitporn, W., Kewcharoen, J., Thongprayoon, C., Bathini, T., Vallabhajosyula, S., & Jongnarangsin, K. (2021). Rhythm Control in Patients with Heart Failure with Preserved Ejection Fraction: A Meta-Analysis. Journal of Clinical Medicine, 10(18), 4038. https://doi.org/10.3390/jcm10184038








