Changes in Physical Fitness and Body Composition Associated with Physical Exercise in Patients with Myasthenia Gravis: A Longitudinal Prospective Study
Abstract
1. Introduction
2. Subjects and Methods
2.1. Study Design and Ethical Considerations
2.2. Participants
2.3. Physical Exercise Regimen
2.4. Outcome Measures
2.5. Clinical Measures
2.6. Definition of Sarcopenia, Obesity, and Sarcopenic Obesity
2.7. Body Composition Assessment
2.8. Physical Fitness Measures
2.9. Statistical Analyses
3. Results
3.1. Clinical Features of the 35 MG Patients
3.2. Changes in Body Composition before and after Resistance Training
3.3. Subgroup Analysis According to the Time Spent Exercising per Week
3.4. Subgroup Analysis by the Presence or Absence of Sarcopenia and Obesity
3.5. Subgroup Analysis of the QMG Total Score
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilhus, N.E. Myasthenia Gravis. N. Engl. J. Med. 2016, 375, 2570–2581. [Google Scholar] [CrossRef]
- Krivickas, L.S. Exercise in neuromuscular disease. J. Clin. Neuromuscul. Dis. 2003, 5, 29–39. [Google Scholar] [CrossRef]
- Wolfe, G.I.; Kaminski, H.J.; Aban, I.B.; Minisman, G.; Kuo, H.-C.; Marx, A.; Ströbel, P.; Mazia, C.; Oger, J.; Cea, J.G.; et al. Long-term effect of thymectomy plus prednisone versus prednisone alone in patients with non-thymomatous myasthenia gravis: 2-year extension of the MGTX randomised trial. Lancet Neurol. 2019, 18, 259–268. [Google Scholar] [CrossRef]
- Sanders, D.B.; Wolfe, D.B.; Benatar, M.; Evoli, A.; Gilhus, N.E.; Illa, I.; Kuntz, N.; Massey, J.M.; Melms, A.; Murai, H.; et al. International consensus guidance for management of myasthenia gravis: Executive summary. Neurology 2016, 87, 419–425. [Google Scholar]
- Warburton, D.E.; Nicol, C.W.; Bredin, S.S. Health benefits of physical activity: The evidence. CMAJ 2006, 174, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.R.; Bredin, S.S.D. Health benefits of physical activity: A systematic review of current systematic reviews. Curr. Opin. Cardiol. 2017, 32, 541–556. [Google Scholar] [CrossRef]
- Yu, J. The etiology and exercise implications of sarcopenia in the elderly. Int. J. Nurs. Sci. 2015, 2, 199–203. [Google Scholar] [CrossRef][Green Version]
- Cass, S. Myasthenia gravis and sports participation. Curr. Sports Med. Rep. 2013, 12, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Cup, E.H.; Pieterse, A.J.; Broek-Pastoor, J.M.T.; Munneke, M.; van Engelen, B.G.; Hendricks, H.T.; van der Wilt, G.J.; Oostendorp, R.A. Exercise Therapy and Other Types of Physical Therapy for Patients with Neuromuscular Diseases: A Systematic Review. Arch. Phys. Med. Rehabil. 2007, 88, 1452–1464. [Google Scholar] [CrossRef] [PubMed]
- Elsais, A.; Johansen, B.; Kerty, E. Airway limitation and exercise intolerance in well-regulated myasthenia gravis patients. Acta Neurol. Scand. Suppl. 2010, 190, 12–17. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.-K.; Fielding, R.A.; Martin, F.C.; Michel, J.-P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Shigemoto, K.; Kubo, S.; Mori, S.; Yamada, S.; Akiyoshi, T.; Miyazaki, T. Muscle weakness and neuromuscular junctions in aging and disease. Geriatr. Gerontol. Int. 2010, 10, S137–S147. [Google Scholar] [CrossRef]
- Zalesin, K.C.; Franklin, B.A.; Miller, W.M.; Peterson, E.D.; McCullough, P.A. Impact of obesity on cardiovascular disease. Med. Clin. N. Am. 2011, 95, 919–937. [Google Scholar] [CrossRef] [PubMed]
- Braz, N.F.T.; Rocha, N.P.; Vieira, É.L.M.; Gomez, R.S.; Kakehasi, A.M.; Teixeira, A.L. Body composition and adipokines plasma levels in patients with myasthenia gravis treated with high cumulative glucocorticoid dose. J. Neurol. Sci. 2017, 381, 169–175. [Google Scholar] [CrossRef]
- Mori, S.; Koshi, K.; Shigemoto, K. The important role of the neuromuscular junction in maintaining muscle mass and strength. J. Phys. Fit. Sports Med. 2014, 3, 111–114. [Google Scholar] [CrossRef]
- Hangartner, T.N.; Warner, S.; Braillon, P.; Jankowski, L.; Shepherd, J. The Official Positions of the International Society for Clinical Densitometry: Acquisition of Dual-Energy X-Ray Absorptiometry Body Composition and Considerations Regarding Analysis and Repeatability of Measures. J. Clin. Densitom. 2013, 16, 520–536. [Google Scholar] [CrossRef] [PubMed]
- Doran, D.A.; Mc Geever, S.; Collins, K.D.; Quinn, C.; McElhone, R.; Scott, M. The Validity of Commonly Used Adipose Tissue Body Composition Equations Relative to Dual Energy X-ray Absorptiometry (DXA) in Gaelic Games Players. Int. J. Sports Med. 2013, 35, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Rahbek, M.A.; Mikkelsen, E.E.; Overgaard, K.; Vinge, L.; Andersen, H.; Dalgas, U. Exercise in myasthenia gravis: A feasibility study of aerobic and resistance training. Muscle Nerve 2017, 56, 700–709. [Google Scholar] [CrossRef]
- Westerberg, E.; Molin, C.J.; Lindblad, I.; Emtner, M.; Punga, A.R. Physical exercise in myasthenia gravis is safe and improves neuromuscular parameters and physical performance-based measures: A pilot study. Muscle Nerve 2017, 56, 207–214. [Google Scholar] [CrossRef]
- Westerberg, E.; Molin, C.J.; Sporndly Nees, S.; Widenfalk, J.; Punga, A.R. The impact of physical exercise on neuromuscular function in Myasthenia gravis patients: A single-subject design study. Medicine 2018, 97, e11510. [Google Scholar] [CrossRef]
- Stout, J.R.; Eckerson, J.M.; May, E.; Coulter, C.; Bradley-Popovich, G.E. Effects of resistance exercise and creatine supplementation on myasthenia gravis: A case study. Med. Sci. Sports Exerc. 2001, 33, 869–872. [Google Scholar] [CrossRef]
- Jaretzki, A., III; Barohn, R.J.; Ernstoff, R.M.; Kaminski, H.J.; Keesey, J.C.; Penn, A.S.; Sanders, D.B. Myasthenia gravis: Recommendations for clinical research standards. Task Force of the Medical Scien-tific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology 2000, 55, 16–23. [Google Scholar]
- Wong, S.H.; Nitz, J.C.; Williams, K.; Brauer, S.G. Effects of balance strategy training in myasthenia gravis: A case study series. Muscle Nerve 2014, 49, 654–660. [Google Scholar] [CrossRef]
- Nelson, M.E.; Rejeski, W.J.; Blair, S.N.; Duncan, P.W.; Judge, J.O.; King, A.C.; Macera, C.A.; Castaneda-Sceppa, C. Physical activity and public health in older adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc. 2007, 39, 1435–1445. [Google Scholar] [CrossRef] [PubMed]
- McRae, G.; Payne, A.; Zelt, J.G.E.; Scribbans, T.D.; Jung, M.E.; Little, J.P.; Gurd, B.J. Extremely low volume, whole-body aerobic–resistance training improves aerobic fitness and muscular endurance in females. Appl. Physiol. Nutr. Metab. 2012, 37, 1124–1131. [Google Scholar] [CrossRef]
- Bedlack, R.S.; Simel, D.L.; Bosworth, H.; Samsa, G.; Tucker-Lipscomb, B.; Sanders, D.B. Quantitative myasthenia gravis score: Assessment of responsiveness and longitudinal validity. Neurology 2005, 64, 1968–1970. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Murai, H.; Imai, T.; Nagane, Y.; Masuda, M.; Tsuda, E.; Konno, S.; Oji, S.; Nakane, S.; Motomura, M.; et al. Quality of life in purely ocular myasthenia in Japan. BMC Neurol. 2014, 14, 142. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-K.; Liu, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Bahyah, K.S.; Chou, M.-Y.; Chen, L.-Y.; Hsu, P.-S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus Report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- The Examination Committee of Criteria for ‘Obesity Disease’ in Japan; Japan Society for the Study of Obesity. New criteria for ’obesity disease’ in Japan. Circ. J. 2002, 66, 987–992. [Google Scholar] [CrossRef]
- McDonald, C.M. Physical activity, health impairments, and disability in neuromuscular disease. Am. J. Phys. Med. Rehabil. 2002, 81, S108–S120. [Google Scholar] [CrossRef] [PubMed]
- Davidson, L.; Mulligan, H.; Certifi, P.-G. Exercise prescription in the physiotherapeutic management of Myasthenia Gravis: A case report. N. Z. J. Physiotherapy 2005, 33, 13–18. [Google Scholar]
- Cheng, Y.J.; A Macera, C.; Addy, C.L.; Sy, F.S.; Wieland, D.; Blair, S.N. Effects of physical activity on exercise tests and respiratory function. Br. J. Sports Med. 2003, 37, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Calik-Kutukcu, E.; Salci, Y.; Karanfil, E.; Fil-Balkan, A.; Bekircan-Kurt, C.E.; Armutlu, K. Expiratory muscle strength as a predictor of functional exercise capacity in generalized myasthenia gravis. Neuroscience 2019, 24, 95–100. [Google Scholar] [CrossRef]
- Molin, C.J.; Punga, A.R. Compound Motor Action Potential: Electrophysiological Marker for Muscle Training. J. Clin. Neuro-Physiol. 2016, 33, 340–345. [Google Scholar] [CrossRef]
- Lohi, E.L.; Lindberg, C. Andersen O Physical training effects in myasthenia gravis. Arch. Phys. Med. Rehabil. 1993, 74, 1178–1180. [Google Scholar] [PubMed]
- Duez, L.; Qerama, E.; Fuglsang-Frederiksen, A.; Bangsbo, J.; Jensen, T.S. Electrophysiological characteristics of motor units and muscle fibers in trained and untrained young male subjects. Muscle Nerve 2010, 42, 177–183. [Google Scholar] [CrossRef]
- Cureton, K.J.; Collins, M.A.; Hill, D.W.; McElhannon, F.M., Jr. Muscle hypertrophy in men and women. Med. Sci. Sports Exerc. 1988, 20, 338–344. [Google Scholar] [CrossRef]
- Wilmore, J.H. Alterations in strength, body composition and anthropometric measurements consequent to a 10-week weight training program. Med. Sci. Sports 1974, 6, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Sharif, K.; Watad, A.; Bragazzi, N.L.; Lichtbroun, M.; Amital, H.; Shoenfeld, Y. Physical activity and autoimmune diseases: Get moving and manage the disease. Autoimmun. Rev. 2018, 17, 53–72. [Google Scholar] [CrossRef]
- Lundberg, I.E.; Vencovsky, J.; Alexanderson, H. Therapy of myositis: Biological and physical. Curr. Opin. Rheumatol. 2014, 26, 704–711. [Google Scholar] [CrossRef]
- Graham, R.C.; Hughes, R.A.; White, C.M. A prospective study of physiotherapist prescribed community based exercise in inflammatory peripheral neuropathy. J. Neurol. 2007, 254, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Millstein, R.A. Measuring Outcomes in Adult Weight Loss Studies That Include Diet and Physical Activity: A Systematic Review. J. Nutr. Metab. 2014, 2014, 421423. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, L.; Westerberg, E.; Punga, A.R. Myasthenia Gravis and Physical Exercise: A Novel Paradigm. Front. Neurol. 2020, 11, 675. [Google Scholar] [CrossRef] [PubMed]
| Variable | n (%) or Mean ± Standard Deviation |
|---|---|
| Male sex | 13 (37.1) |
| Age (year) | 56.1 ± 8.6 |
| Age group | |
| 40–49 yrs. | 10 (28.6) |
| 50–59 yrs. | 10 (28.6) |
| 60–70 yrs. | 15 (42.9) |
| Disease duration (year) | 12.3 ± 10.6 |
| Obesity | 14 (40.0) |
| Sarcopenia | 8 (22.9) |
| Sarcopenic obesity | 1 (2.9) |
| Use of corticosteroids in the last six months | 14 (40.0) |
| Corticosteroid daily dose in recent six months (mg) | 5.3 ± 5.7 |
| Immunosuppressant used | 10 (28.6) |
| MGFA type | |
| Type II | 21 (60) |
| Type III | 14 (40) |
| Variable | Pre-Test (n = 34) | Post-Test (n = 34) | Mean Difference (95% CI) | p |
|---|---|---|---|---|
| Body mass index (kg/m2) | 24.80 ± 4.62 | 24.86 ± 4.50 | 0.06 (−0.29, 0.41) | 0.733 |
| Fat mass (kg) | ||||
| Arms | 2.05 ± 0.92 | 2.07 ± 0.88 | 0.01 (−0.12, 0.15) | 0.819 |
| Legs | 6.68 ± 2.71 | 6.85 ± 3.29 | 0.17 (−0.52, 0.86) | 0.623 |
| Appendicular | 8.73 ± 3.42 | 8.91 ± 3.95 | 0.18 (−0.53, 0.90) | 0.605 |
| Muscle mass(kg) | ||||
| Arms | 4.29 ± 1.49 | 4.28 ± 1.30 | −0.01 (−0.19, 0.17) | 0.925 |
| Legs | 13.70 ± 3.11 | 13.56 ± 3.31 | −0.13 (−0.42, 0.16) | 0.357 |
| Appendicular | 17.99 ± 4.43 | 17.85 ± 4.53 | −0.14 (−0.54, 0.26) | 0.478 |
| Fat adiposity (%) | ||||
| Android | 43.26 ± 9.45 | 44.84 ± 9.98 | 1.58 (−0.20, 3.35) | 0.079 |
| Gynoid | 39.78 ± 7.59 | 38.76 ± 9.25 | −1.02 (−2.63, 0.60) | 0.209 |
| Muscle (%) | ||||
| Arms | 67.91 ± 10.79 | 67.85 ± 10.72 | −0.06 (−0.98, 0.85) | 0.888 |
| Legs | 67.80 ± 8.50 | 67.28 ± 10.38 | −0.52 (−2.42, 1.37) | 0.579 |
| Appendicular | 67.77 ± 8.63 | 67.29 ± 10.15 | −0.48 (−2.13, 1.16) | 0.556 |
| Android | 56.74 ± 9.45 | 55.16 ± 9.98 | −1.58 (−3.35, 0.20) | 0.079 |
| Gynoid | 60.22 ± 7.59 | 61.24 ± 9.25 | 1.02 (−0.60, 2.63) | 0.209 |
| Whole body | 65.51 ± 9.10 | 65.72 ± 8.09 | 0.21 (−1.39, 1.82) | 0.787 |
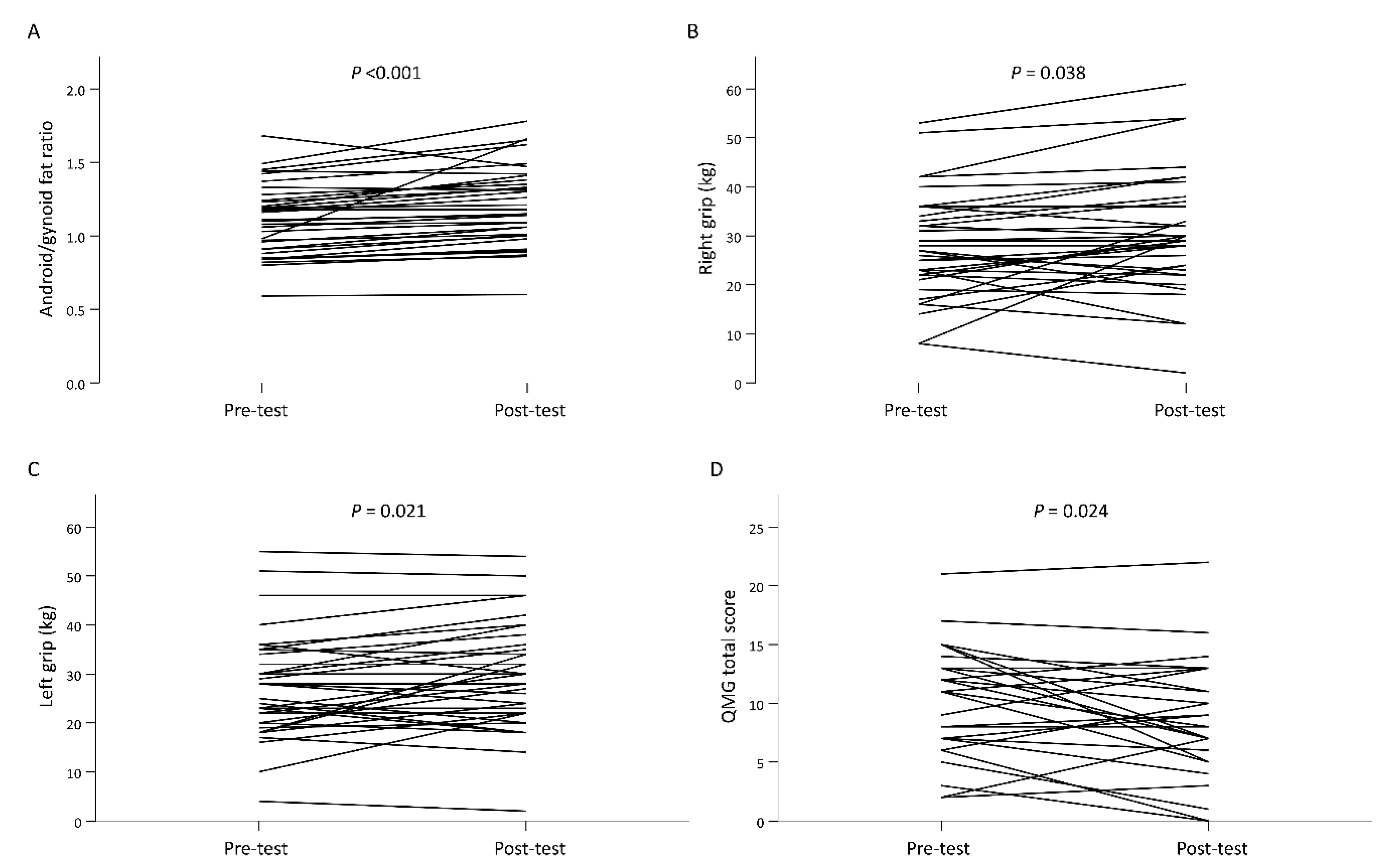
| Android/gynoid fat ratio | 1.11 ± 0.24 | 1.20 ± 0.27 | 0.10 (0.05, 0.14) | <0.001 * |
| Body fat percentage (%) | 34.10 ± 8.47 | 34.33 ± 8.06 | 0.23 (−0.60, 1.06) | 0.576 |
| ASMI | 6.66 ± 1.21 | 6.59 ± 1.20 | −0.06 (−0.20, 0.07) | 0.340 |
| Forced vital capacity (%) | 72.64 ± 18.48 | 77.42 ± 16.28 | 4.79 (−2.36, 11.93) | 0.182 |
| Walk speed (m/s) | 1.10 ± 0.25 | 1.10 ± 0.28 | −0.01 (−0.08, 0.07) | 0.852 |
| Right grip (kg) | 27.82 ± 10.58 | 30.35 ± 12.25 | 2.53 (0.15, 4.91) | 0.038 * |
| Left grip (kg) | 27.21 ± 10.79 | 29.56 ± 11.02 | 2.35 (0.37, 4.34) | 0.021 * |
| Quality of life score (n = 32) | 14.91 ± 11.29 | 11.41 ± 12.24 | −3.50 (−7.51, 0.51) | 0.085 |
| QMG total score (n = 32) | 10.47 ± 4.78 | 9.00 ± 5.22 | −1.47 (−2.73, −0.21) | 0.024 * |
| Variable | High Exercise Group (≥56.3 Min/Week) | Low Exercise Group (<56.3 Min/Week) | Mean Difference (95% CI) | p for Interaction | ||||
|---|---|---|---|---|---|---|---|---|
| Pre-Test (n = 17) | Post-Test (n = 17) | p | Pre-Test (n = 17) | Post-Test (n = 17) | p | |||
| Body mass index (kg/m2) | 23.85 ± 4.46 | 23.91 ± 4.40 | 0.862 | 25.74 ± 4.71 | 25.81 ± 4.52 | 0.718 | −0.01 (−0.68, 0.66) | 0.972 |
| Fat mass (kg) | ||||||||
| Arms | 1.81 ± 0.91 | 1.78 ± 0.85 | 0.685 | 2.30 ± 0.88 | 2.35 ± 0.83 | 0.619 | −0.08 (−0.33, 0.16) | 0.505 |
| Legs | 6.30 ± 2.54 | 6.78 ± 3.73 | 0.477 | 7.06 ± 2.89 | 6.92 ± 2.90 | 0.366 | 0.63 (−0.67, 1.92) | 0.342 |
| Appendicular | 8.11 ± 3.35 | 8.56 ± 4.38 | 0.507 | 9.36 ± 3.46 | 9.27 ± 3.57 | 0.701 | 0.54 (−0.80, 1.88) | 0.429 |
| Muscle mass (kg) | ||||||||
| Arms | 4.70 ± 1.52 | 4.52 ± 1.44 | 0.074 | 3.88 ± 1.37 | 4.05 ± 1.15 | 0.251 | −0.35 (−0.68, −0.03) | 0.033 * |
| Legs | 14.29 ± 3.26 | 14.07 ± 3.69 | 0.399 | 13.10 ± 2.92 | 13.06 ± 2.90 | 0.743 | −0.18 (−0.72, 0.36) | 0.511 |
| Appendicular | 18.99 ± 4.73 | 18.58 ± 5.06 | 0.204 | 16.98 ± 4.00 | 17.11 ± 3.94 | 0.589 | −0.54 (−1.27, 0.20) | 0.152 |
| Fat adiposity (%) | ||||||||
| Android | 38.84 ± 9.11 | 41.33 ± 11.01 | 0.149 | 47.67 ± 7.74 | 48.34 ± 7.64 | 0.258 | 1.83 (−1.48, 5.14) | 0.278 |
| Gynoid | 37.40 ± 7.17 | 36.99 ± 10.37 | 0.783 | 42.16 ± 7.45 | 40.54 ± 7.88 | 0.013 | 1.20 (−1.84, 4.24) | 0.439 |
| Muscle (%) | ||||||||
| Arms | 73.23 ± 9.60 | 72.35 ± 10.31 | 0.215 | 62.59 ± 9.37 | 63.34 ± 9.35 | 0.187 | −1.62 (−3.28, 0.03) | 0.054 |
| Legs | 69.94 ± 8.31 | 68.48 ± 12.24 | 0.430 | 65.65 ± 8.39 | 66.07 ± 8.32 | 0.363 | −1.89 (−5.43, 1.66) | 0.297 |
| Appendicular | 70.65 ± 8.38 | 69.25 ± 11.72 | 0.382 | 64.88 ± 8.10 | 65.32 ± 8.18 | 0.298 | −1.84 (−4.90, 1.22) | 0.239 |
| Android | 61.16 ± 9.11 | 58.67 ± 11.01 | 0.149 | 52.33 ± 7.74 | 51.66 ± 7.64 | 0.258 | −1.83 (−5.14, 1.48) | 0.278 |
| Gynoid | 62.60 ± 7.17 | 63.01 ± 10.37 | 0.783 | 57.84 ± 7.45 | 59.46 ± 7.88 | 0.013 | −1.20 (−4.24, 1.84) | 0.439 |
| Whole body | 69.21 ± 8.00 | 69.04 ± 7.37 | 0.776 | 61.80 ± 8.81 | 62.40 ± 7.56 | 0.689 | −0.77 (−3.80, 2.25) | 0.616 |
| Android/gynoid fat ratio | 1.06 ± 0.27 | 1.14 ± 0.27 | 0.003 | 1.15 ± 0.21 | 1.26 ± 0.26 | 0.012 | −0.03 (−0.12, 0.05) | 0.457 |
| Body fat percentage (%) | 31.08 ± 7.68 | 31.06 ± 7.37 | 0.974 | 37.12 ± 8.35 | 37.59 ± 7.55 | 0.452 | −0.49 (−2.06, 1.07) | 0.535 |
| ASMI | 6.88 ± 1.25 | 6.76 ± 1.29 | 0.161 | 6.43 ± 1.16 | 6.42 ± 1.12 | 0.967 | −0.12 (−0.38, 0.13) | 0.354 |
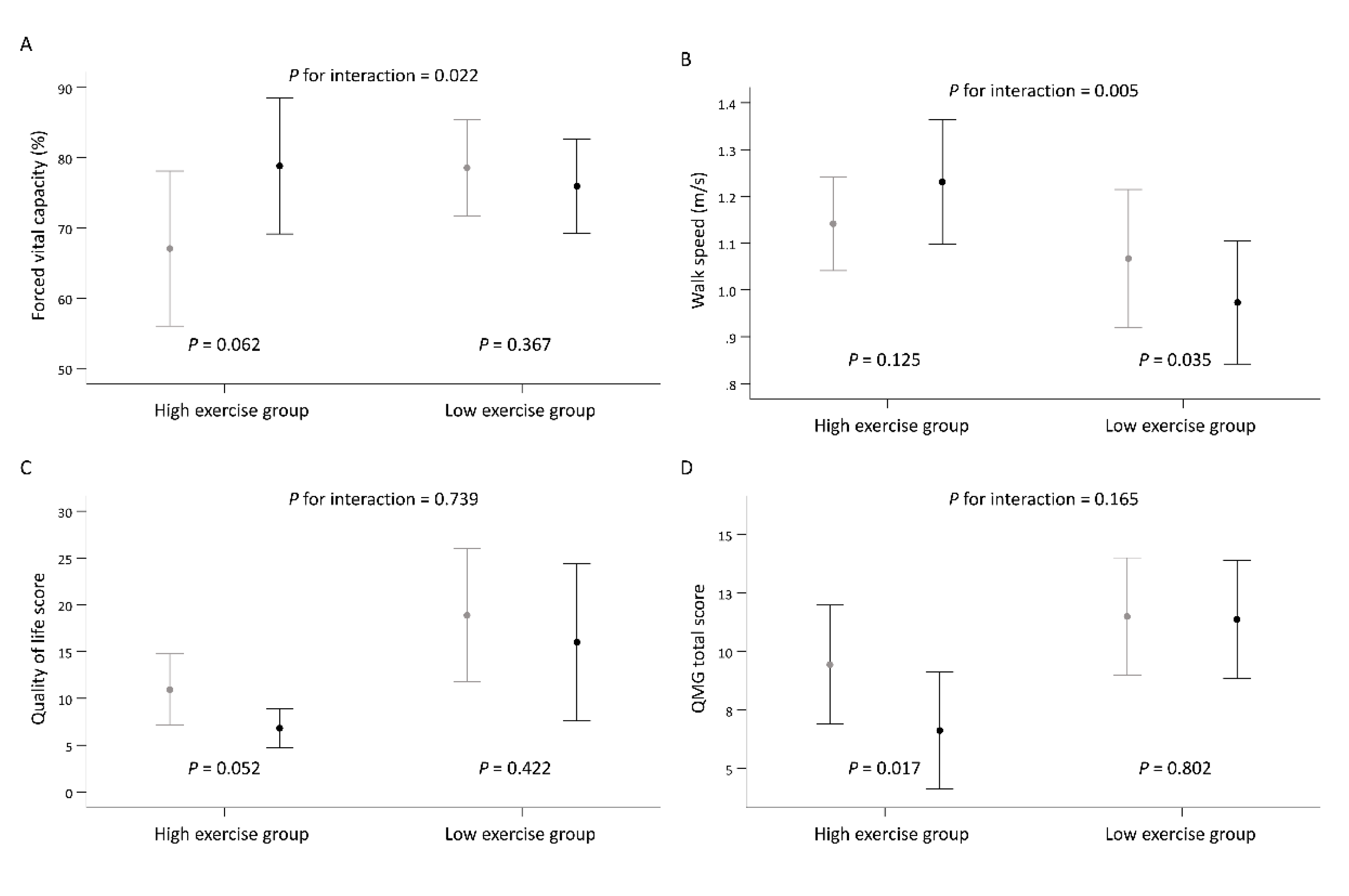
| Forced vital capacity (%) | 67.06 ± 21.47 | 78.82 ± 18.83 | 0.062 | 78.56 ± 12.82 | 75.94 ± 13.52 | 0.367 | 14.39 (2.05, 26.72) | 0.022 * |
| Walk speed (m/s) | 1.14 ± 0.20 | 1.23 ± 0.25 | 0.125 | 1.07 ± 0.29 | 0.97 ± 0.26 | 0.035 | 0.18 (0.05, 0.31) | 0.005 * |
| Right grip (kg) | 29.65 ± 11.30 | 31.53 ± 13.82 | 0.247 | 26.00 ± 9.80 | 29.18 ± 10.77 | 0.093 | −1.29 (−5.80, 3.21) | 0.573 |
| Left grip (kg) | 28.94 ± 11.87 | 29.94 ± 12.46 | 0.552 | 25.47 ± 9.63 | 29.18 ± 9.74 | 0.002 | −2.71 (−6.36, 0.95) | 0.146 |
| Quality of life score (n = 32) | 10.94 ± 7.16 | 6.81 ± 3.89 | 0.052 | 18.88 ± 13.36 | 16.00 ± 15.80 | 0.422 | −1.24 (−8.51, 6.04) | 0.739 |
| QMG total score (n = 32) | 9.44 ± 4.77 | 6.63 ± 4.67 | 0.017 | 11.50 ± 4.70 | 11.38 ± 4.73 | 0.802 | −1.76 (−4.26, 0.73) | 0.165 |
| Variable | Sarcopenia | Non-Sarcopenia | Mean Difference (95% CI) | p for Interaction | ||||
|---|---|---|---|---|---|---|---|---|
| Pre-Test (n = 8) | Post-Test (n = 8) | p | Pre-Test (n = 26) | Post-Test (n = 26) | p | |||
| Body mass index (kg/m2) | 21.71 ± 2.32 | 21.50 ± 2.51 | 0.617 | 25.75 ± 4.76 | 25.89 ± 4.49 | 0.465 | −0.35 (−1.18, 0.47) | 0.401 |
| Fat mass (kg) | ||||||||
| Arms | 1.58 ± 0.53 | 1.66 ± 0.57 | 0.197 | 2.20 ± 0.97 | 2.19 ± 0.92 | 0.933 | 0.09 (−0.10, 0.29) | 0.345 |
| Legs | 4.99 ± 1.32 | 5.15 ± 1.29 | 0.503 | 7.20 ± 2.83 | 7.37 ± 3.55 | 0.700 | −0.01 (−0.95, 0.92) | 0.975 |
| Appendicular | 6.57 ± 1.38 | 6.81 ± 1.42 | 0.394 | 9.40 ± 3.59 | 9.56 ± 4.26 | 0.720 | 0.08 (−0.92, 1.08) | 0.879 |
| Muscle mass(kg) | ||||||||
| Arms | 3.37 ± 0.57 | 3.50 ± 0.61 | 0.088 | 4.58 ± 1.57 | 4.52 ± 1.37 | 0.652 | 0.19 (−0.07, 0.44) | 0.149 |
| Legs | 11.39 ± 1.53 | 11.46 ± 1.44 | 0.631 | 14.40 ± 3.14 | 14.21 ± 3.47 | 0.291 | 0.26 (−0.16, 0.69) | 0.228 |
| Appendicular | 14.76 ± 2.01 | 14.96 ± 2.01 | 0.252 | 18.98 ± 4.52 | 18.73 ± 4.74 | 0.332 | 0.45 (−0.11, 1.01) | 0.118 |
| Fat adiposity (%) | ||||||||
| Android | 40.29 ± 11.29 | 41.87 ± 10.21 | 0.305 | 44.17 ± 8.86 | 45.75 ± 9.94 | 0.151 | 0.003 (−3.33, 3.33) | 0.999 |
| Gynoid | 38.69 ± 7.24 | 37.36 ± 7.08 | 0.268 | 40.12 ± 7.81 | 39.20 ± 9.90 | 0.361 | −0.41 (−3.18, 2.37) | 0.774 |
| Muscle (%) | ||||||||
| Arms | 68.15 ± 9.65 | 67.88 ± 9.87 | 0.783 | 67.84 ± 11.29 | 67.84 ± 11.15 | 0.998 | −0.28 (−2.30, 1.75) | 0.789 |
| Legs | 69.56 ± 7.71 | 69.07 ± 7.37 | 0.621 | 67.26 ± 8.80 | 66.72 ± 11.21 | 0.659 | −0.04 (−2.84, 2.92) | 0.979 |
| Appendicular | 69.05 ± 7.11 | 68.59 ± 7.00 | 0.646 | 67.37 ± 9.14 | 66.88 ± 11.02 | 0.638 | 0.03 (−2.60, 2.66) | 0.982 |
| Android | 59.71 ± 11.29 | 58.13 ± 10.21 | 0.305 | 55.83 ± 8.86 | 54.25 ± 9.94 | 0.151 | −0.003 (−3.33, 3.33) | 0.999 |
| Gynoid | 61.31 ± 7.24 | 62.64 ± 7.08 | 0.268 | 59.88 ± 7.81 | 60.80 ± 9.90 | 0.361 | 0.41 (−2.37, 3.18) | 0.774 |
| Whole body | 66.14 ± 11.04 | 67.45 ± 7.99 | 0.689 | 65.31 ± 8.67 | 65.19 ± 8.19 | 0.786 | 1.43 (−4.39, 7.25) | 0.629 |
| Android/gynoid fat ratio | 1.06 ± 0.26 | 1.14 ± 0.30 | 0.041 | 1.12 ± 0.24 | 1.22 ± 0.26 | 0.002 | −0.01 (−0.10, 0.07) | 0.765 |
| Body fat percentage (%) | 32.23 ± 8.12 | 32.64 ± 7.96 | 0.689 | 34.67 ± 8.65 | 34.85 ± 8.17 | 0.702 | 0.24 (−1.77, 2.25) | 0.815 |
| ASMI | 5.63 ± 0.67 | 5.74 ± 0.58 | 0.241 | 6.97 ± 1.17 | 6.85 ± 1.23 | 0.160 | 0.23 (0.01, 0.45) | 0.044 |
| Forced vital capacity (%) | 76.29 ± 12.83 | 82.14 ± 9.77 | 0.160 | 71.65 ± 19.82 | 76.15 ± 17.57 | 0.313 | 0.86 (−9.86, 11.57) | 0.876 |
| Walk speed (m/s) | 1.06 ± 0.25 | 1.15 ± 0.42 | 0.322 | 1.12 ± 0.25 | 1.08 ± 0.23 | 0.343 | 0.13 (−0.04, 0.30) | 0.146 |
| Right grip (kg) | 26.75 ± 6.80 | 29.25 ± 6.02 | 0.140 | 28.15 ± 11.59 | 30.69 ± 13.70 | 0.098 | −0.04 (−3.99, 3.91) | 0.985 |
| Left grip (kg) | 26.00 ± 6.93 | 27.63 ± 5.71 | 0.368 | 27.58 ± 11.82 | 30.15 ± 12.23 | 0.038 | −0.95 (−4.79, 2.88) | 0.627 |
| Quality of life score (n = 32) | 11.29 ± 7.30 | 11.43 ± 11.13 | 0.957 | 15.92 ± 12.10 | 11.40 ± 12.75 | 0.071 | 3.47 (−3.18, 10.12) | 0.306 |
| QMG total score (n = 32) | 7.57 ± 4.20 | 8.29 ± 4.99 | 0.593 | 11.28 ± 4.69 | 9.20 ± 5.36 | 0.005 | 1.78 (−0.94, 4.50) | 0.201 |
| Variable | Obesity | Non-Obesity | Mean Difference (95% CI) | p for Interaction | ||||
|---|---|---|---|---|---|---|---|---|
| Pre-Test (n = 14) | Post-Test (n = 14) | p | Pre-Test (n = 20) | Post-Test (n = 20) | p | |||
| Body mass index (kg/m2) | 29.11 ± 3.67 | 29.12 ± 3.29 | 0.977 | 21.77 ± 2.13 | 21.87 ± 2.21 | 0.650 | −0.09 (−0.79, 0.62) | 0.812 |
| Fat mass (kg) | ||||||||
| Arms | 2.74 ± 0.77 | 2.71 ± 0.72 | 0.838 | 1.57 ± 0.68 | 1.62 ± 0.68 | 0.136 | −0.08 (−0.37, 0.21) | 0.597 |
| Legs | 8.32 ± 2.41 | 8.14 ± 2.39 | 0.339 | 5.53 ± 2.33 | 5.94 ± 3.57 | 0.473 | −0.59 (−1.72, 0.53) | 0.303 |
| Appendicular | 11.06 ± 2.81 | 10.85 ± 2.89 | 0.472 | 7.10 ± 2.84 | 7.56 ± 4.09 | 0.423 | −0.67 (−1.87, 0.53) | 0.273 |
| Muscle mass(kg) | ||||||||
| Arms | 5.05 ± 1.90 | 5.01 ± 1.51 | 0.805 | 3.76 ± 0.79 | 3.78 ± 0.86 | 0.837 | −0.06 (−0.45, 0.32) | 0.743 |
| Legs | 15.80 ± 3.26 | 15.83 ± 3.44 | 0.808 | 12.23 ± 1.99 | 11.97 ± 2.12 | 0.256 | 0.29 (−0.21, 0.79) | 0.259 |
| Appendicular | 20.85 ± 4.91 | 20.84 ± 4.83 | 0.975 | 15.98 ± 2.70 | 15.75 ± 2.90 | 0.415 | 0.22 (−0.51, 0.96) | 0.549 |
| Fat adiposity (%) | ||||||||
| Android | 50.94 ± 4.98 | 51.00 ± 4.41 | 0.921 | 37.88 ± 8.02 | 40.53 ± 10.60 | 0.073 | −2.59 (−5.46, 0.29) | 0.078 |
| Gynoid | 41.36 ± 6.98 | 39.76 ± 7.32 | 0.030 | 38.68 ± 7.98 | 38.07 ± 10.52 | 0.639 | −0.99 (−3.73, 1.75) | 0.478 |
| Muscle (%) | ||||||||
| Arms | 63.37 ± 10.62 | 64.22 ± 10.15 | 0.143 | 71.09 ± 9.96 | 70.39 ± 10.61 | 0.281 | 1.56 (−0.04, 3.15) | 0.055 |
| Legs | 65.48 ± 7.29 | 65.94 ± 7.43 | 0.283 | 69.42 ± 9.08 | 68.21 ± 12.12 | 0.445 | 1.68 (−1.39, 4.75) | 0.284 |
| Appendicular | 65.01 ± 7.86 | 65.46 ± 7.90 | 0.197 | 69.70 ± 8.81 | 68.56 ± 11.49 | 0.412 | 1.59 (−1.07, 4.24) | 0.242 |
| Android | 49.06 ± 4.98 | 49.00 ± 4.41 | 0.921 | 62.12 ± 8.02 | 59.47 ± 10.60 | 0.073 | 2.59 (−0.29, 5.46) | 0.078 |
| Gynoid | 58.64 ± 6.98 | 60.24 ± 7.32 | 0.030 | 61.32 ± 7.98 | 61.93 ± 10.52 | 0.639 | 0.99 (−1.75, 3.73) | 0.478 |
| Whole body | 60.45 ± 5.98 | 60.71 ± 5.89 | 0.573 | 69.04 ± 9.35 | 69.23 ± 7.64 | 0.891 | 0.07 (−2.58, 2.73) | 0.957 |
| Android/gynoid fat ratio | 1.25 ± 0.18 | 1.31 ± 0.16 | 0.065 | 1.00 ± 0.23 | 1.13 ± 0.31 | 0.001 | −0.07 (−0.15, 0.01) | 0.105 |
| Body fat percentage (%) | 39.52 ± 5.96 | 39.29 ± 5.89 | 0.600 | 30.30 ± 7.97 | 30.86 ± 7.63 | 0.381 | −0.79 (−2.23, 0.65) | 0.283 |
| ASMI | 7.43 ± 1.39 | 7.40 ± 1.35 | 0.733 | 6.11 ± 0.68 | 6.02 ± 0.67 | 0.359 | 0.05 (−0.21, 0.31) | 0.706 |
| Forced vital capacity (%) | 74.93 ± 16.19 | 78.00 ± 10.32 | 0.418 | 70.95 ± 20.27 | 77.00 ± 19.85 | 0.288 | −2.95 (−15.44, 9.55) | 0.644 |
| Walk speed (m/s) | 1.06 ± 0.30 | 1.00 ± 0.25 | 0.130 | 1.14 ± 0.21 | 1.17 ± 0.29 | 0.507 | −0.10 (−0.23, 0.03) | 0.116 |
| Right grip (kg) | 29.93 ± 12.42 | 34.07 ± 14.77 | 0.012 | 26.35 ± 9.12 | 27.75 ± 9.71 | 0.424 | 2.74 (−1.48, 6.96) | 0.203 |
| Left grip (kg) | 27.21 ± 12.44 | 32.21 ± 12.89 | 0.001 | 27.20 ± 9.81 | 27.70 ± 9.40 | 0.707 | 4.50 (1.17, 7.83) | 0.008 |
| Quality of life score (n = 32) | 17.69 ± 13.09 | 12.46 ± 14.05 | 0.259 | 13.00 ± 9.79 | 10.68 ± 11.19 | 0.132 | −2.99 (−11.26, 5.29) | 0.480 |
| QMG total score (n = 32) | 11.85 ± 4.72 | 9.69 ± 4.85 | 0.063 | 9.53 ± 4.71 | 8.53 ± 5.53 | 0.205 | −0.21 (−2.97, 2.55) | 0.883 |
| Variable | QMG Score ≤ 10 | QMG Score > 11 | Mean Difference (95% CI) | p for Interaction | ||||
|---|---|---|---|---|---|---|---|---|
| Pre-Test (n = 14) | Post-Test (n = 14) | p | Pre-Test (n = 20) | Post-Test (n = 20) | p | |||
| Body mass index (kg/m2) | 24.94 ± 5.48 | 24.60 ± 5.27 | 0.266 | 24.70 ± 4.06 | 25.04 ± 4.00 | 0.100 | 0.68 (0.01, 1.35) | 0.046 |
| Fat mass (kg) | ||||||||
| Arms | 1.86 ± 0.86 | 1.99 ± 0.93 | 0.296 | 2.18 ± 0.95 | 2.12 ± 0.86 | 0.339 | −0.20 (−0.46, 0.06) | 0.139 |
| Legs | 6.58 ± 3.33 | 6.26 ± 3.06 | 0.144 | 6.75 ± 2.27 | 7.27 ± 3.45 | 0.363 | 0.84 (−0.28, 1.96) | 0.143 |
| Appendicular | 8.44 ± 3.96 | 8.25 ± 3.85 | 0.501 | 8.93 ± 3.07 | 9.38 ± 4.05 | 0.436 | 0.64 (−0.56, 1.84) | 0.294 |
| Muscle mass(kg) | ||||||||
| Arms | 4.31 ± 1.85 | 4.47 ± 1.57 | 0.392 | 4.27 ± 1.22 | 4.15 ± 1.11 | 0.156 | −0.28 (−0.65, 0.09) | 0.138 |
| Legs | 14.21 ± 3.38 | 14.03 ± 3.63 | 0.381 | 13.33 ± 2.93 | 13.24 ± 3.12 | 0.638 | 0.09 (−0.45, 0.63) | 0.736 |
| Appendicular | 18.53 ± 4.99 | 18.50 ± 5.09 | 0.930 | 17.61 ± 4.09 | 17.39 ± 4.16 | 0.389 | −0.19 (−0.97, 0.59) | 0.634 |
| Fat adiposity (%) | ||||||||
| Android | 41.64 ± 8.96 | 42.97 ± 8.43 | 0.135 | 44.39 ± 9.85 | 46.15 ± 10.96 | 0.219 | 0.43 (−2.64, 3.51) | 0.782 |
| Gynoid | 38.25 ± 9.08 | 36.66 ± 9.59 | 0.050 | 40.86 ± 6.39 | 40.24 ± 8.95 | 0.631 | 0.98 (−1.79, 3.76) | 0.488 |
| Muscle (%) | ||||||||
| Arms | 69.28 ± 11.17 | 69.36 ± 10.26 | 0.919 | 66.95 ± 10.70 | 66.79 ± 11.16 | 0.762 | −0.25 (−2.08, 1.58) | 0.789 |
| Legs | 69.23 ± 10.33 | 69.80 ± 9.13 | 0.441 | 66.79 ± 7.07 | 65.51 ± 11.04 | 0.400 | −1.86 (−5.03, 1.31) | 0.249 |
| Appendicular | 69.27 ± 10.26 | 69.65 ± 9.22 | 0.586 | 66.72 ± 7.38 | 65.63 ± 10.67 | 0.411 | −1.46 (−4.24, 1.32) | 0.302 |
| Android | 58.36 ± 8.96 | 57.03 ± 8.43 | 0.135 | 55.61 ± 9.85 | 53.85 ± 10.96 | 0.219 | −0.43 (−3.51, 2.64) | 0.782 |
| Gynoid | 61.75 ± 9.08 | 63.34 ± 9.59 | 0.050 | 59.14 ± 6.39 | 59.76 ± 8.95 | 0.631 | −0.98 (−3.76, 1.79) | 0.488 |
| Whole body | 66.12 ± 10.17 | 67.67 ± 8.07 | 0.384 | 65.08 ± 8.52 | 64.35 ± 8.01 | 0.191 | −2.28 (−5.70, 1.14) | 0.191 |
| Android/gynoid fat ratio | 1.12 ± 0.21 | 1.22 ± 0.26 | <0.001 | 1.10 ± 0.27 | 1.19 ± 0.28 | 0.018 | −0.002 (−0.08, 0.08) | 0.959 |
| Body fat percentage (%) | 32.59 ± 9.06 | 32.37 ± 8.06 | 0.760 | 35.16 ± 8.10 | 35.70 ± 7.97 | 0.293 | 0.75 (−0.86, 2.37) | 0.359 |
| ASMI | 6.71 ± 1.39 | 6.65 ± 1.44 | 0.653 | 6.62 ± 1.10 | 6.55 ± 1.04 | 0.335 | −0.005 (−0.29, 0.28) | 0.973 |
| Forced vital capacity (%) | 74.29 ± 13.50 | 77.64 ± 12.42 | 0.241 | 71.42 ± 21.72 | 77.26 ± 18.97 | 0.329 | 2.44 (−9.67, 14.56) | 0.692 |
| Walk speed (m/s) | 1.14 ± 0.12 | 1.24 ± 0.24 | 0.101 | 1.08 ± 0.30 | 1.01 ± 0.27 | 0.065 | −0.18 (−0.32, −0.05) | 0.007 |
| Right grip (kg) | 33.93 ± 9.48 | 35.86 ± 13.11 | 0.217 | 23.55 ± 9.28 | 26.50 ± 10.26 | 0.103 | 1.02 (−3.31, 5.35) | 0.644 |
| Left grip (kg) | 34.29 ± 10.58 | 34.71 ± 10.82 | 0.699 | 22.25 ± 7.93 | 25.95 ± 9.88 | 0.017 | 3.27 (−0.12, 6.67) | 0.059 |
| Quality of life score (n = 32) | 10.79 ± 8.75 | 6.07 ± 8.22 | 0.068 | 18.11 ± 12.20 | 15.56 ± 13.41 | 0.408 | 1.16 (−5.80, 8.12) | 0.743 |
| QMG total score (n = 32) | 6.07 ± 2.27 | 6.00 ± 3.94 | 0.935 | 13.89 ± 3.07 | 11.33 ± 4.96 | 0.005 | −1.98 (−4.38, 0.43) | 0.107 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-C.; Chen, Y.-K.; Chiu, H.-C.; Yeh, J.-H. Changes in Physical Fitness and Body Composition Associated with Physical Exercise in Patients with Myasthenia Gravis: A Longitudinal Prospective Study. J. Clin. Med. 2021, 10, 4031. https://doi.org/10.3390/jcm10174031
Chang C-C, Chen Y-K, Chiu H-C, Yeh J-H. Changes in Physical Fitness and Body Composition Associated with Physical Exercise in Patients with Myasthenia Gravis: A Longitudinal Prospective Study. Journal of Clinical Medicine. 2021; 10(17):4031. https://doi.org/10.3390/jcm10174031
Chicago/Turabian StyleChang, Che-Cheng, Yen-Kung Chen, Hou-Chang Chiu, and Jiann-Horng Yeh. 2021. "Changes in Physical Fitness and Body Composition Associated with Physical Exercise in Patients with Myasthenia Gravis: A Longitudinal Prospective Study" Journal of Clinical Medicine 10, no. 17: 4031. https://doi.org/10.3390/jcm10174031
APA StyleChang, C.-C., Chen, Y.-K., Chiu, H.-C., & Yeh, J.-H. (2021). Changes in Physical Fitness and Body Composition Associated with Physical Exercise in Patients with Myasthenia Gravis: A Longitudinal Prospective Study. Journal of Clinical Medicine, 10(17), 4031. https://doi.org/10.3390/jcm10174031






