Clinical Significance of Variable Histomorphologic Findings Related to Mucosal Inflammation in Negative Appendectomy
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
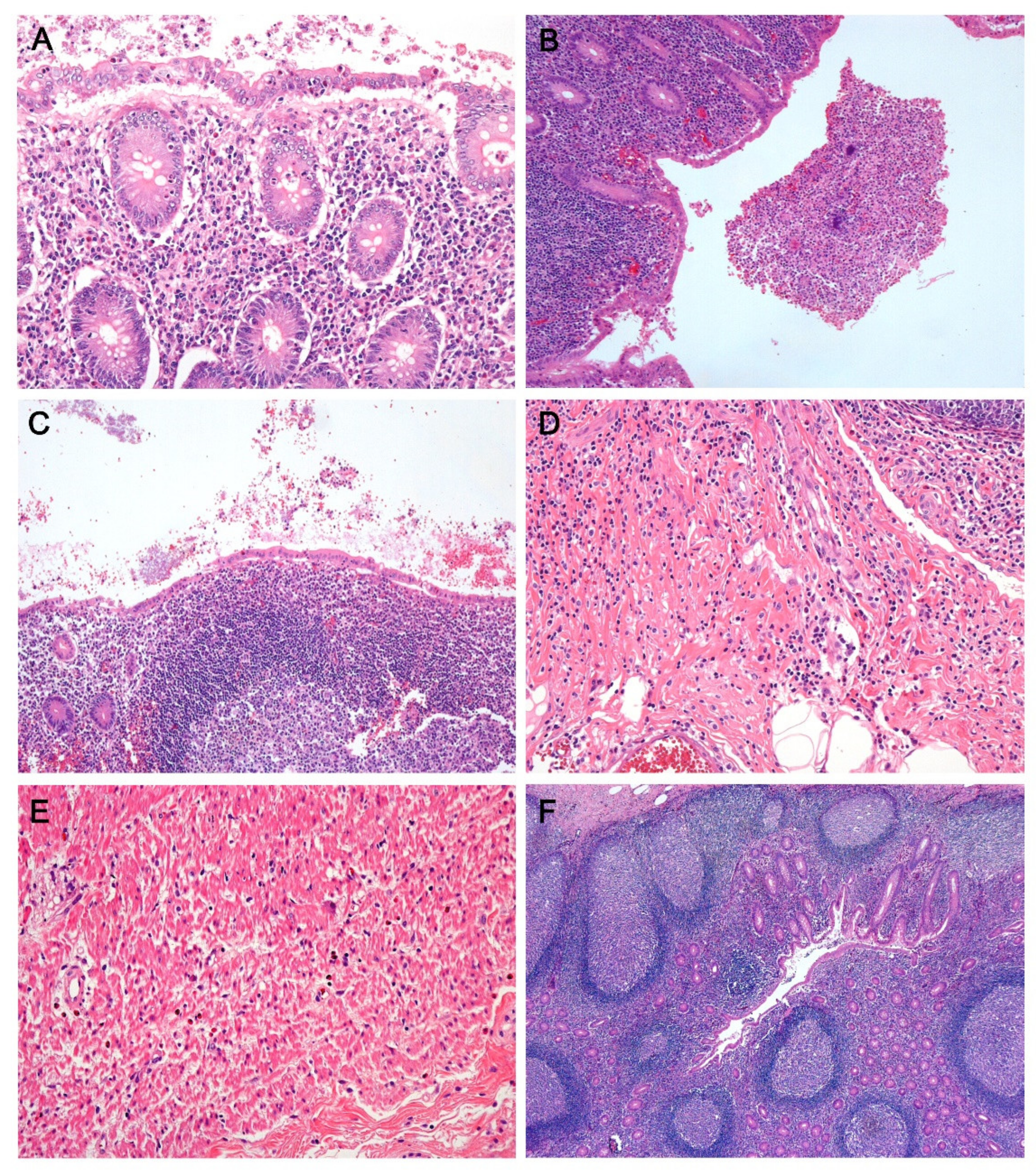
2.2. Histologic Evaluation
2.3. Radiologic Findings
2.4. Statistical Analysis
3. Results
3.1. Patient Baseline Characteristics
3.2. Correlation between Histomorphologic Findings and AIR Score-Based Risk Stratification in Negative Appendectomy
3.3. Correlation between Histomorpholgic Findngs and Appendiceal Diameter in Negative Appendcetomy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ohle, R.; O’Reilly, F.; O’Brien, K.K.; Fahey, T.; Dimitrov, B.D. The Alvarado score for predicting acute appendicitis: A systematic review. BMC Med. 2011, 9, 139. [Google Scholar] [CrossRef]
- Ebell, M.H.; Shinholser, J. What are the most clinically useful cutoffs for the Alvarado and Pediatric Appendicitis Scores? A systematic review. Ann. Emerg. Med. 2014, 64, 365–372. [Google Scholar] [CrossRef] [PubMed]
- De Castro, S.M.; Unlu, C.; Steller, E.P.; van Wagensveld, B.A.; Vrouenraets, B.C. Evaluation of the appendicitis inflammatory response score for patients with acute appendicitis. World J. Surg. 2012, 36, 1540–1545. [Google Scholar] [CrossRef]
- Andersson, M.; Andersson, R.E. The appendicitis inflammatory response score: A tool for the diagnosis of acute appendicitis that outperforms the Alvarado score. World J. Surg. 2008, 32, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Gopalam, P.R.; Konidala, M.V.S.S. Comparison of acute inflammatory score and Alvarado score in diagnosis of acute appendicitis at a tertiary care hospital. Int. Surg. J. 2017, 4, 4034–4038. [Google Scholar] [CrossRef]
- Scott, A.J.; Mason, S.E.; Arunakirinathan, M.; Reissis, Y.; Kinross, J.M.; Smith, J.J. Risk stratification by the Appendicitis Inflammatory Response score to guide decision-making in patients with suspected appendicitis. Br. J. Surg. 2015, 102, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Carr, N.J. The pathology of acute appendicitis. Ann. Diagn. Pathol. 2000, 4, 46–58. [Google Scholar] [CrossRef]
- Detmer, D.E.; Nevers, L.E.; Sikes, E.D. Regional results of acute appendicitis care. JAMA 1981, 246, 1318–1320. [Google Scholar] [CrossRef]
- Raja, A.S.; Wright, C.; Sodickson, A.D.; Zane, R.D.; Schiff, G.D.; Hanson, R.; Baeyens, P.F.; Khorasani, R. Negative appendectomy rate in the era of CT: An 18-year perspective. Radiology 2010, 256, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Bendeck, S.E.; Nino-Murcia, M.; Berry, G.J.; Jeffrey, R.B., Jr. Imaging for suspected appendicitis: Negative appendectomy and perforation rates. Radiology 2002, 225, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Blitman, N.M.; Anwar, M.; Brady, K.B.; Taragin, B.H.; Freeman, K. Value of focused appendicitis ultrasound and Alvarado score in predicting appendicitis in children: Can we reduce the use of CT? AJR Am. J. Roentgenol. 2015, 204, W707–W712. [Google Scholar] [CrossRef][Green Version]
- Greenson, J.K. Diagnostic Pathology Gastrointestinal, 2nd ed.; Elsevier: Salt Lake City, UT, USA, 2016; pp. 302–305. [Google Scholar]
- Marudanayagam, R.; Williams, G.T.; Rees, B.I. Review of the pathological results of 2660 appendicectomy specimens. J. Gastroenterol. 2006, 41, 745–749. [Google Scholar] [CrossRef]
- Noffsinger, A.E. Fenoglio-Preiser’s Gastrointestinal Pathology, 4th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2017; pp. 444–446. [Google Scholar]
- Robert, D.; Odze, J.R.G. Surgical Pathology of the GI Tract, Liver, Biliary Tract, and Pancreas, 2nd ed.; Elsevier: Salt Lake City, UT, USA, 2009; pp. 395–397. [Google Scholar]
- Pieper, R.; Kager, L.; Näsman, P. Clinical significance of mucosal inflammation of the vermiform appendix. Ann. Surg. 1983, 197, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Bhangu, A.; Søreide, K.; Di Saverio, S.; Assarsson, J.H.; Drake, F.T. Acute appendicitis: Modern understanding of pathogenesis, diagnosis, and management. Lancet 2015, 386, 1278–1287. [Google Scholar] [CrossRef]
- Barcia, J.J.; Reissenweber, N. Neutrophil count in the normal appendix and early appendicitis: Diagnostic index of real acute inflammation. Ann. Diagn. Pathol. 2002, 6, 352–356. [Google Scholar] [CrossRef]
- Xu, Y.; Jeffrey, R.B.; DiMaio, M.A.; Olcott, E.W. Lymphoid hyperplasia of the appendix: A potential pitfall in the sonographic diagnosis of appendicitis. AJR Am. J. Roentgenol. 2016, 206, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.C.; Olcott, E.W.; Poullos, P.D.; Jeffrey, R.B.; Thompson, M.O.; Rosenberg, J.; Shin, L.K. Predictors of appendicitis on computed tomography among cases with borderline appendix size. Emerg. Radiol. 2015, 22, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Singhal, V.; Jadhav, V. Acute appendicitis: Are we over diagnosing it? Ann. R. Coll. Surg. Engl. 2007, 89, 766–769. [Google Scholar] [CrossRef][Green Version]
- Joshi, M.K.; Joshi, R.; Alam, S.E.; Agarwal, S.; Kumar, S. Negative appendectomy: An audit of resident-performed surgery. How can its incidence be minimized? Indian J. Surg. 2015, 77, 913–917. [Google Scholar] [CrossRef]
- Mizumoto, R.; Cristaudo, A.T.; Lai, N.K.; Premaratne, G.; Hendahewa, R. Dilemma of mucosal appendicitis: A clinico-pathological entity? A retrospective cohort study. ANZ J. Surg. 2018, 88, E284–E288. [Google Scholar] [CrossRef]
- Aravindan, K.; Vijayaraghavan, D.; Manipadam, M. Acute eosinophilic appendicitis and the significance of eosinophil—Edema lesion. Indian J. Pathol. Microbiol. 2010, 53, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.; Shrestha, A.; Tiwari, M.; Ranabhat, S.; Maharjan, S. Role of eosinophils in acute appendicitis. JNMA J. Nepal Med. Assoc. 2015, 53, 12–17. [Google Scholar] [CrossRef]
- Ring-Mrozik, E.; Hecker, W.C.; Wiebecke, B.; Hansmann, A.; Trammer, A. New morphological findings in so-called negative appendectomies. Eur. J. Pediatr. Surg. 1993, 3, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Wagner, P.L.; Eachempati, S.R.; Soe, K.; Pieracci, F.M.; Shou, J.; Barie, P.S. Defining the current negative appendectomy rate: For whom is preoperative computed tomography making an impact? Surgery 2008, 144, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Orscheln, E.S.; Trout, A.T. Appendiceal diameter: CT versus sonographic measurements. Pediatr. Radiol. 2016, 46, 316–321. [Google Scholar] [CrossRef]

| NA | |||||
|---|---|---|---|---|---|
| Variables, n (%) | IA | NA without MI | NA with MI | p Value | |
| (n = 24) | (n = 24) | (n = 94) | a | b | |
| Age, years (median, range) | 13 (0–65) | 20 (4–71) | 18.5 (4–67) | ||
| Sex | 0.155 | 0.72 | |||
| Male | 17 (71%) | 14 (58%) | 51 (54%) | ||
| Female | 7 (29%) | 10 (42%) | 43 (46%) | ||
| Risk according to AIR score | 0.328 | 0.016 * | |||
| Low | 22 (92%) | 24 (100%) | 75 (80%) | ||
| Intermediate | 2 (8%) | 0 | 19 (20%) | ||
| Mucosal inflammation | 0.002 * | <0.001 * | |||
| Absent | 12 (50%) | 24 (100%) | 0 | ||
| Present | 12 (50%) | 0 | 94 (100%) | ||
| Luminal inflammation | 0.729 | 0.02 * | |||
| Absent | 21 (87%) | 24 (100%) | 76 (81%) | ||
| Present | 3 (13%) | 0 | 18 (19%) | ||
| Submucosal inflammation | 0.225 | 0.002* | |||
| Absent | 21 (87%) | 24 (100%) | 66 (70%) | ||
| Present | 3 (13%) | 0 | 28 (30%) | ||
| Mucosal neutrophil count | 0.003 * | <0.001 * | |||
| Low (<10/5 HPF) | 17 (71%) | 24 (100%) | 21 (22%) | ||
| High (≥10/5 HPF) | 7 (29%) | 0 | 73 (78%) | ||
| Intramuscular eosinophil count | 0.172 | 0.031 * | |||
| Low (≤10/4 HPF) | 21 (87%) | 22 (92%) | 66 (70%) | ||
| High (>10/4 HPF) | 3 (13%) | 2 (8%) | 28 (30%) | ||
| Mucosal erosion | 0.197 | 0.009 * | |||
| Absent | 21 (87%) | 23 (96%) | 66 (70%) | ||
| Present | 3 (13%) | 1 (4%) | 28 (30%) | ||
| Surface epithelial flattening | 0.012 * | <0.001 * | |||
| Absent | 12 (50%) | 15 (62%) | 14 (12%) | ||
| Present | 12 (50%) | 9 (38%) | 80 (68%) | ||
| LFH | 0.303 | 0.213 | |||
| Absent | 19 (79%) | 19 (79%) | 62 (66%) | ||
| Present | 5 (21%) | 5 (21%) | 32 (34%) | ||
| Hyperplastic epithelial change | 0.878 | 0.852 | |||
| Absent | 22 (92%) | 22 (92%) | 85 (90%) | ||
| Present | 2 (8%) | 2 (8%) | 9 (10%) | ||
| Variables, n (%) | Appendiceal Diameter | p Value | ||
|---|---|---|---|---|
| <6 mm | 6–6.9 mm | 7–9 mm | ||
| (n = 13) | (n = 27) | (n = 37) | ||
| Risk according to AIR score | 0.144 | |||
| Low risk (1–4) | 13 (100%) | 23 (85%) | 30 (81%) | |
| Intermediate risk (5–7) | 0 | 4 (15%) | 7 (19%) | |
| Age | 0.185 | |||
| Pediatric (3–18 years) | 5 (38%) | 18 (67%) | 24 (65%) | |
| Adult | 8 (62%) | 9 (33%) | 13 (35%) | |
| Histologic parameters | ||||
| Mucosal inflammation | 0.116 | |||
| Absent | 4 (31%) | 8 (30%) | 4 (11%) | |
| Present | 9 (69%) | 19 (70%) | 33 (89%) | |
| Luminal inflammation | 0.029 * | |||
| Absent | 13 (100%) | 24 (89%) | 26 (70%) | |
| Present | 0 | 3 (11%) | 11 (30%) | |
| Submucosal inflammation | 0.105 | |||
| Absent | 8 (62%) | 24 (89%) | 26 (70%) | |
| Present | 5 (38%) | 3 (11%) | 11 (30%) | |
| Mucosal neutrophil count | 0.034 * | |||
| Low (<10/5 HPF) | 6 (46%) | 15 (56%) | 9 (24%) | |
| High (≥10/5 HPF) | 7 (54%) | 12 (44%) | 28 (76%) | |
| Intramuscular eosinophil count | 0.21 | |||
| Low (≤10/4 HPF) | 12 (92%) | 18 (67%) | 26 (70%) | |
| High (>10/4 HPF) | 1 (8%) | 9 (33%) | 11 (30%) | |
| Mucosal erosion | 0.408 | |||
| Absent | 11 (85%) | 19 (70%) | 24 (65%) | |
| Present | 2 (15%) | 8 (30%) | 13 (35%) | |
| Surface epithelial flattening | 0.004 * | |||
| Absent | 8 (62%) | 8 (30%) | 5 (14%) | |
| Present | 5 (38%) | 19 (70%) | 32 (86%) | |
| LFH | 0.028 * | |||
| Absent | 10 (77%) | 22 (81%) | 19 (51%) | |
| Present | 3 (23%) | 5 (19%) | 18 (49%) | |
| Hyperplastic epithelial change | 0.995 | |||
| Absent | 12 (92%) | 25 (93%) | 34 (92%) | |
| Present | 1 (8%) | 2 (7%) | 3 (8%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, E.; Yoo, Y.; Kim, J.M.; Sung, S.H.; Lee, D.; Park, S. Clinical Significance of Variable Histomorphologic Findings Related to Mucosal Inflammation in Negative Appendectomy. J. Clin. Med. 2021, 10, 4030. https://doi.org/10.3390/jcm10174030
Choi E, Yoo Y, Kim JM, Sung SH, Lee D, Park S. Clinical Significance of Variable Histomorphologic Findings Related to Mucosal Inflammation in Negative Appendectomy. Journal of Clinical Medicine. 2021; 10(17):4030. https://doi.org/10.3390/jcm10174030
Chicago/Turabian StyleChoi, Euno, Youngeun Yoo, Ji Min Kim, Sun Hee Sung, Dakeun Lee, and Sanghui Park. 2021. "Clinical Significance of Variable Histomorphologic Findings Related to Mucosal Inflammation in Negative Appendectomy" Journal of Clinical Medicine 10, no. 17: 4030. https://doi.org/10.3390/jcm10174030
APA StyleChoi, E., Yoo, Y., Kim, J. M., Sung, S. H., Lee, D., & Park, S. (2021). Clinical Significance of Variable Histomorphologic Findings Related to Mucosal Inflammation in Negative Appendectomy. Journal of Clinical Medicine, 10(17), 4030. https://doi.org/10.3390/jcm10174030






