Achalasia in Children—Clinical Presentation, Diagnosis, Long-Term Treatment Outcomes, and Quality of Life
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Evaluation of Diagnostic Methods
- Barium X-ray follow through was evaluated by one radiologist on a radiological scale of EA according to Rezende et al. [11]: grade I—slow esophagus emptying, peristaltic disorders—tertiary waves or a lack of peristalsis; grade II—a slight enlargement of the esophagus, and more intense tertiary waves; grade III—a significant widening of the esophagus, with a narrowing of the lower segment—a characteristic image of the “bird’s beak”, violent convulsive muscle spasms or a complete lack of peristalsis of the esophagus; grade IV — the image as in stage III and a very large dilation of the esophagus with the change of its axis;
- Gastroscopy results were evaluated according to the presence of the following: residual food in the esophagus, an enlargement of the esophagus, changes in the mucosa of the esophagus (resulting from long-residual food in the esophagus), stomach cardia—sometimes with a resistance passing the endoscope;
- In the manometry, four basic features of EA were assessed: an increased resting pressure of the LES > 45 mmHg, a lack or incomplete LES relaxation in response to the incoming bite of food (LES > 8 mmHg), a lack of esophageal motility, and the positive pressure in the esophagus.
2.3. Treatment
2.3.1. Endoscopic Pneumatic Dilatation
2.3.2. Heller Myotomy
2.3.3. Treatment Outcome
2.4. Statistical Analysis
3. Results
3.1. Demographics
3.2. Clinical Presentation at the Onset
3.3. Diagnostic Investigations
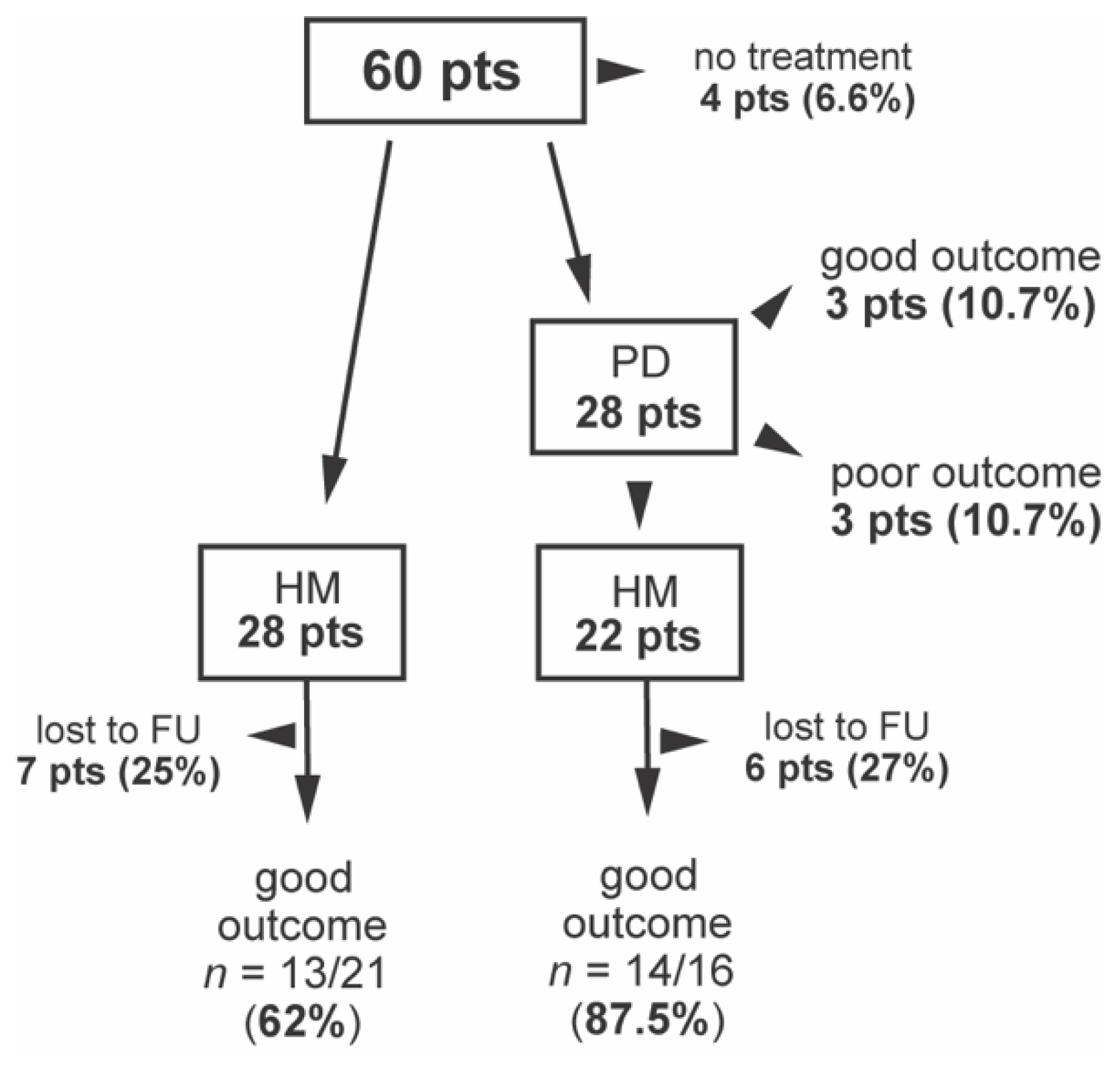
3.4. Treatment
3.5. Long-Term Outcome and Prognostic Factors
3.6. Quality of Life
3.7. AAA Syndrome Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rudolph, C.D.; Sood, M.R. Achalasia and other motor disorders. In Pediatric Gastrointestinal and Liver Disease; Wyllie, R., Hyams, J.S., Kay, M., Eds.; Elsevier: Philadelphia, PA, USA, 2011; pp. 248–254. [Google Scholar]
- Franklin, A.L.; Petrosyan, M.; Kane, T.D. Childhood achalasia: A comprehensive review of disease, diagnosis and therapeutic management. World J. Gastrointest. Endosc. 2014, 6, 105–111. [Google Scholar] [CrossRef]
- Marlais, M.; Fishman, J.R.; Fell, J.M.E.; Haddad, M.J.; Rawat, D.J. UK incidence of achalasia: An 11-year national epidemiological study. Arch. Dis. Child. 2010, 96, 192–194. [Google Scholar] [CrossRef]
- Ikeda, M.; Hirano, M.; Shinoda, K.; Katsumata, N.; Furutama, D.; Nakamura, K.; Ikeda, S.-I.; Tanaka, T.; Hanafusa, T.; Kitajima, H.; et al. Triple a syndrome in Japan. Muscle Nerve 2013, 48, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Boeckxstaens, G.E.; Annese, V.; Varannes, S.B.D.; Chaussade, S.; Costantini, M.; Cuttitta, A.; Elizalde, J.I.; Fumagalli, U.; Gaudric, M.; Rohof, W.O.; et al. Pneumatic dilation versus laparoscopic Heller’s myotomy for idiopathic achalasia. N. Engl. J. Med. 2011, 364, 1807–1816. [Google Scholar] [CrossRef] [Green Version]
- Eckardt, V.F. Clinical presentation and complications of achalasia. Gastrointest. Endosc. Clin. N. Am. 2001, 11, 281–292. [Google Scholar] [CrossRef]
- Caldaro, T.; Familiari, P.; Romeo, E.F.; Gigante, G.; Marchese, M.; Contini, A.C.I.; di Abriola, G.F.; Cucchiara, S.; De Angelis, P.; Torroni, F.; et al. Treatment of esophageal achalasia in children: Today and tomorrow. J. Pediatr. Surg. 2015, 50, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Minami, H.; Kobayashi, Y.; Sato, Y.; Kaga, M.; Suzuki, M.; Satodate, H.; Odaka, N.; Itoh, H.; Kudo, S. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy 2010, 42, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Goneidy, A.; Cory-Wright, J.; Zhu, L.; Malakounides, G. Surgical management of esophageal achalasia in pediatrics: A systematic review. Eur. J. Pediatr. Surg. 2019, 30, 013–020. [Google Scholar] [CrossRef]
- Zaninotto, G.; Bennett, C.; Boeckxstaens, G.; Costantini, M.; Ferguson, M.K.; Pandolfino, J.E.; Patti, M.G.; Ribeiro, U.; Richter, J.; Swanstrom, L.; et al. The 2018 ISDE achalasia guidelines. Dis. Esophagus 2018, 31, doy071. [Google Scholar] [CrossRef] [Green Version]
- Rezende, J.M. Classificaçåo radiológica do megaesôfago/A radiologic classification of the megaesophagus (achalasia of the esophagus). Rev. Goiana Med. 1982, 28, 187–191. [Google Scholar]
- Heller, E. Extramucose cardioplastie beim chronischen cardiospasmus mit dilatation des oesophagus. Mitt Grengeb Med. Chir 1913, 2, 141–149. [Google Scholar]
- Urbach, D.R.; Tomlinson, G.A.; Harnish, J.L.; Martino, R.; Diamant, N.E. A measure of disease-specific health-related quality of life for achalasia. Am. J. Gastroenterol. 2005, 100, 1668–1676. [Google Scholar] [CrossRef]
- Boeckxstaens, G.E.; Zaninotto, G.; Richter, J.E. Achalasia. Lancet 2014, 383, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Niebisch, S.; Hadzijusufovic, E.; Mehdorn, M.; Müller, M.; Scheuermann, U.; Lyros, O.; Schulz, H.G.; Jansen-Winkeln, B.; Lang, H.; Gockel, I. Achalasia—An unnecessary long way to diagnosis. Dis. Esophagus 2017, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hallal, C.; Kieling, C.O.; Nunes, D.L.; Ferreira, C.T.; Peterson, G.; Barros, S.G.S.; Arruda, C.A.; Fraga, J.C.; Goldani, H.A.S. Diagnosis, misdiagnosis, and associated diseases of achalasia in children and adolescents: A twelve-year single center experience. Pediatr. Surg. Int. 2012, 28, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- El-Takli, I.; O’Brien, P.; Paterson, W.G. Clinical diagnosis of achalasia: How reliable is the barium X-Ray? Can. J. Gastroenterol. 2006, 20, 335–337. [Google Scholar] [CrossRef]
- Boeckxstaens, G. Achalasia. Best Pr. Res. Clin. Gastroenterol. 2007, 21, 595–608. [Google Scholar] [CrossRef]
- Islam, S. Achalasia. Semin. Pediatr. Surg. 2017, 26, 116–120. [Google Scholar] [CrossRef]
- Pastor, A.C.; Mills, J.; Marcon, M.A.; Himidan, S.; Kim, P.C. A single center 26-year experience with treatment of esophageal achalasia: Is there an optimal method? J. Pediatr. Surg. 2009, 44, 1349–1354. [Google Scholar] [CrossRef]
- Lee, C.W.; Kays, D.W.; Chen, M.K.; Islam, S. Outcomes of treatment of childhood achalasia. J. Pediatr. Surg. 2010, 45, 1173–1177. [Google Scholar] [CrossRef] [Green Version]
- Zagory, J.A.; Golden, J.M.; Demeter, N.E.; Nguyen, Y.; Ford, H.R.; Nguyen, N.X. Heller myotomy is superior to balloon dilatation or botulinum injection in children with achalasia: A two-center review. J. Laparoendosc. Adv. Surg. Tech. 2016, 26, 483–487. [Google Scholar] [CrossRef]
- Allaix, M.E.; Patti, M.G. What is the best primary therapy for achalasia: Medical or surgical treatment? who owns achalasia? J. Gastrointest. Surg. 2013, 17, 1547–1549. [Google Scholar] [CrossRef]
- Saliakellis, E.; Thapar, N.; Roebuck, D.; Cristofori, F.; Cross, K.; Kiely, E.; Curry, J.; Lindley, K.; Borrelli, O. Long-Term outcomes of Heller’s myotomy and balloon dilatation in childhood achalasia. Eur. J. Nucl. Med. Mol. Imaging 2017, 176, 899–907. [Google Scholar] [CrossRef]
- Babu, R.; Grier, D.; Cusick, E.; Spicer, R.D. Pneumatic dilatation for childhood achalasia. Pediatr. Surg. Int. 2001, 17, 505–507. [Google Scholar] [CrossRef]
- Jung, C.; Michaud, L.; Mougenot, J.-F.; Lamblin, M.-D.; Philippe-Chomette, P.; Cargill, G.; Bonnevalle, M.; Boige, N.; Bellaiche, M.; Viala, J.; et al. Treatments for pediatric achalasia: Heller myotomy or pneumatic dilatation? Gastroenterol. Clin. Biol. 2010, 34, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Pandian, T.K.; Naik, N.D.; Fahy, A.S.; Arghami, A.; Farley, D.R.; Ishitani, M.B.; Moir, C.R. Laparoscopic esophagomyotomy for achalasia in children: A review. World J. Gastrointest. Endosc. 2016, 8, 56–66. [Google Scholar] [CrossRef]
- Avanoğlu, A.; Mutaf, O. Surgical treatment of achalasia in children: Is an added antireflux procedure necessary? Pediatr. Surg. Int. 1996, 11, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Corda, L.; Pacilli, M.; Clarke, S.; Fell, J.M.; Rawat, D.; Haddad, M. Laparoscopic oesophageal cardiomyotomy without fundoplica-tion in children with achalasia: A 10-year experience: A retrospective review of the results of laparoscopic oesophageal car-diomyotomy without an anti-reflux procedure in children with achalasia. Surg. Endosc. 2010, 24, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Pachl, M.J.; Rex, D.; Decoppi, P.; Cross, K.; Kiely, E.M.; Drake, D.; Pierro, A.; Curry, J.I. Paediatric laparoscopic Heller’s cardiomyoto-my: A single centre series. J. Pediatr. Surg. 2014, 49, 289–292. [Google Scholar] [CrossRef]
- Alhussaini, B.; Gottrand, F.; Goutet, J.-M.; Scaillon, M.; Michaud, L.; Spyckerelle, C.; Viola, S.; Lamblin, M.-D. Clinical and manometric characteristics of allgrove syndrome. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 271–274. [Google Scholar] [CrossRef]
- Milenkovic, T.; Zdravkovic, D.; Savic, N.; Todorovic, S.; Mitrovic, K.; Koehler, K.; Huebner, A. Triple A syndrome: 32 years experience of a single centre (1977–2008). Eur. J. Nucl. Med. Mol. Imaging 2010, 169, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Flokas, M.E.; Tomani, M.; Agdere, L.; Brown, B. Triple a syndrome (allgrove syndrome): Improving outcomes with a multidis-ciplinary approach. Pediatr. Health Med. Ther. 2019, 10, 99–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| 1. How much has achalasia limited the types of food you have been able to eat in the last month? (Please check one.) | |||||
| Not limited at all (I can eat and drink all the foods that I would like to). (1) | Somewhat limited (I can eat and drink most of the foods that I would like to). (2) | Moderately or severely limited (I can eat and drink very few of the foods that I would like to). (3) | |||
| 2. Raw hard fruits and vegetables: (please circle one.) | |||||
| Can swallow with no problem (1) | Can swallow, but with a little difficulty (2) | Can swallow with great difficulty or not at all (3) | |||
| 3. Rice: (Please circle one.) | |||||
| Can swallow with no problem (1) | Can swallow, but with a little difficulty (2) | Can swallow with great difficulty or not at all (3) | |||
| 4. Clear fluids (water, juice, coffee, tea): (please circle one.) | |||||
| Can swallow with no problem (1) | Can swallow, but with a little difficulty (2) | Can swallow with great difficulty or not at all (3) | |||
| 5. How often in the past month have you needed to drink water while eating to deal with food caught in your esophagus? (Please circle one.) | |||||
| Never/Rarely (1) | Sometimes (2) | Frequently/Every time I eat (3) | |||
| 6. How often have you experienced pain when eating during the past month? (Please circle one.) | |||||
| Never (1) | Rarely (2) | Sometimes (3) | Frequently/Every time I eat (4) | ||
| 7. During the past month, how much of a problem for you was heartburn (a burning pain behind the lower part of the chest)? (Please circle one.) | |||||
| No problem (1) | Mild problem (2) | Moderate problem (3) | Severe problem (4) | Very severe problem (5) | |
| 8. When you sit down to eat a meal, are you bothered by how long it takes you to finish eating? (Please check one.) | |||||
| No, I eat as quickly as I like. (1) | Yes, I am bothered by how long it takes me to eat. (2) | ||||
| 9. Has having achalasia limited your lifestyle? (Please check one.) | |||||
| No, it is not at all limiting (My daily activities have not changed.) (1) | Yes, it has limited my lifestyle (It has affected some areas, and I can no longer participate in all the activities I want to do.) (2) | ||||
| 10. How much do you agree with the following statement about how satisfied you are with your health in regard to achalasia? (Please circle the number that best describes your feelings.) I am satisfied with my health in regard to achalasia. | |||||
| Strongly agree (1) | Agree (2) | Neither agree or disagree (3) | Disagree (4) | Strongly disagree (5) | |
| Patient Data | Number/Mean (Median, Range) |
|---|---|
| Sex (male/female) | 34/26 |
| Type of EA | |
| Isolated | 51 (85%) |
| AAA | 9 (15%) |
| Age at first EA symptoms median/range (years) | 9.4 (0.1–17.5) |
| Age at diagnosis median/range (years) | 12.0 (1–17) |
| Time to EA diagnosis median/range (weeks) | 1.0 (0.5–2.0) |
| Age at surgery (years) | 11.7 (12, 3–18) |
| Age at the moment of follow-up contact (years) | 22.9 (6–41) |
| Follow-up time (years) | 12.1 (0.7–26.6) |
| Co-morbidities | 37 (61.7%) |
| GI | 19 (31.6%) |
| H. pylori infection | 9 (47%) |
| Gastritis | 3 (16%) |
| Duodennitis | 2 (10%) |
| Celiac disease | 2 (10%) |
| Pylorostenosis | 2 (10%) |
| Gilbert syndrome | 1 (5%) |
| Esophageal diverticula | 1 (5%) |
| Neurological disorders | 9 (15%) |
| Epilepsy | 5 (55%) |
| Psychomotor retardation | 4 (44%) |
| CMV infection with changes in EEG | 1 (11%) |
| Muscular hypotonia | 1 (11%) |
| Down syndrome | 2 (3%) |
| Symptoms | n = 46 |
| Dysphagia | 39 (84.8%) |
| Regurgitation | 42 (91.3%) |
| Retrosternal chest pain while eating | 22 (47.8%) |
| Heartburn | 10 (21.7%) |
| Coughing or choking while eating | 17 (37%) |
| Tolerated diet | n = 58 |
| Liquid | 30 (51.7%) |
| Pulpy | 16 (27.6%) |
| Ordinary | 6 (10.3%) |
| Ordinary with the need for drinking while eating | 6 (10.3%) |
| BMI | |
| Mean ±SD | 16.3 ± 3.8 |
| BMI z-score mean ± SD | −1.7 ± 3.4 |
| <10th percentile | 25 (41.6%) |
| <3rd percentile | 17 (28.3%) |
| X-ray follow through | n = 51 |
| EA features | 51 (100%) |
| “bird’s beak” sign | 42 (82.4%) |
| Esophageal dilatation | 48 (94.1%) |
| Slow contrast transition | 49 (96.1%) |
| Contrast retention in esophagus | 38 (74.5%) |
| Esophageal peristalsis disorders | 32 (62.7%) |
| Gastroscopy | n = 53 |
| Any EA feature | 46 (86.8%) |
| Residual food in the esophagus | 40 (75.5%) |
| Esophageal enlargement | 31 (58.5%) |
| Closed stomach cardia | 39 (73.6%) |
| Esophageal mucosa lesions | 15 (28.3%) |
| Manometry | n = 41 |
| EA features | 39 (95.1%) |
| LES > 45 mmHg | 24 (58.5%) |
| Lack or incomplete LES relaxation | 32 (78%) |
| Lack of esophageal motility | 39 (95.1%) |
| Positive pressure in the esophagus | 28 (68.3%) |
| Regurgitation | Dysphagia | Chest Pain | |
|---|---|---|---|
| Every Day | 80.9% | 64.70% | 35.90% |
| Sporadic | 10.4% | 20.10% | 11.90% |
| Absent | 8.7% | 15.20% | 52.20% |
| Variable | Good (n = 27) | Poor (n = 10) | 95% CI | p-Value |
|---|---|---|---|---|
| Gender: female | 13 (48%) | 5 (50%) | 0.63–1.49 | 0.99 |
| AAA syndrome | 3 (11%) | 2 (20%) | 0.30–1.30 | 0.59 |
| Age at first GI symptoms | ||||
| years, median (range) | 9.0 (0.1–15.5) | 6.4 (0.7–14.7) | −5.7–1.7 | 0.35 |
| Age at surgery | ||||
| years, median (range) | 12.7 (3.0–18.7) | 13.7 (5.2–17.2) | –2.9–3.6 | 0.80 |
| Time from GI symptoms to initial treatment | ||||
| years, median (range) | 2.0 (0.02–7.2) | 4.6 (0.2–9.1) | –0.48–5.59 | 0.11 |
| Manometry (n = 27) | ||||
| LES pressure >45 mmHg | 12 (57%) | 6 (100%) | 0.43–0.99 | 0.07 |
| Barium X-ray follow through (n = 33) | ||||
| Rezende III or IV grade | 18 (75%) | 5 (55%) | 0.82–2.56 | 0.39 |
| Endoscopy (n = 34) | ||||
| Mucosal changes | 7 (26%) | 3 (42%) | 0.46–1.21 | 0.39 |
| Residual food | 17 (63%) | 6 (85%) | 0.57–1.23 | 0.38 |
| BMI z-score at surgery | ||||
| <3 percentile | 8 (29%) | 3 (30%) | 0.57–1.47 | 0.99 |
| Treatment | ||||
| Endoscopic PD before HM | 9 (33%) | 1 (10%) | 0.85–1.93 | 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarzębicka, D.; Czubkowski, P.; Sieczkowska-Gołub, J.; Kierkuś, J.; Kowalski, A.; Stefanowicz, M.; Oracz, G. Achalasia in Children—Clinical Presentation, Diagnosis, Long-Term Treatment Outcomes, and Quality of Life. J. Clin. Med. 2021, 10, 3917. https://doi.org/10.3390/jcm10173917
Jarzębicka D, Czubkowski P, Sieczkowska-Gołub J, Kierkuś J, Kowalski A, Stefanowicz M, Oracz G. Achalasia in Children—Clinical Presentation, Diagnosis, Long-Term Treatment Outcomes, and Quality of Life. Journal of Clinical Medicine. 2021; 10(17):3917. https://doi.org/10.3390/jcm10173917
Chicago/Turabian StyleJarzębicka, Dorota, Piotr Czubkowski, Joanna Sieczkowska-Gołub, Jarosław Kierkuś, Adam Kowalski, Marek Stefanowicz, and Grzegorz Oracz. 2021. "Achalasia in Children—Clinical Presentation, Diagnosis, Long-Term Treatment Outcomes, and Quality of Life" Journal of Clinical Medicine 10, no. 17: 3917. https://doi.org/10.3390/jcm10173917
APA StyleJarzębicka, D., Czubkowski, P., Sieczkowska-Gołub, J., Kierkuś, J., Kowalski, A., Stefanowicz, M., & Oracz, G. (2021). Achalasia in Children—Clinical Presentation, Diagnosis, Long-Term Treatment Outcomes, and Quality of Life. Journal of Clinical Medicine, 10(17), 3917. https://doi.org/10.3390/jcm10173917






