Circulating Osteopontin Levels and Outcomes in Patients Hospitalized for COVID-19
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design
2.2. Healthy Cohort
2.3. Outcomes and Measures
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Study Cohort
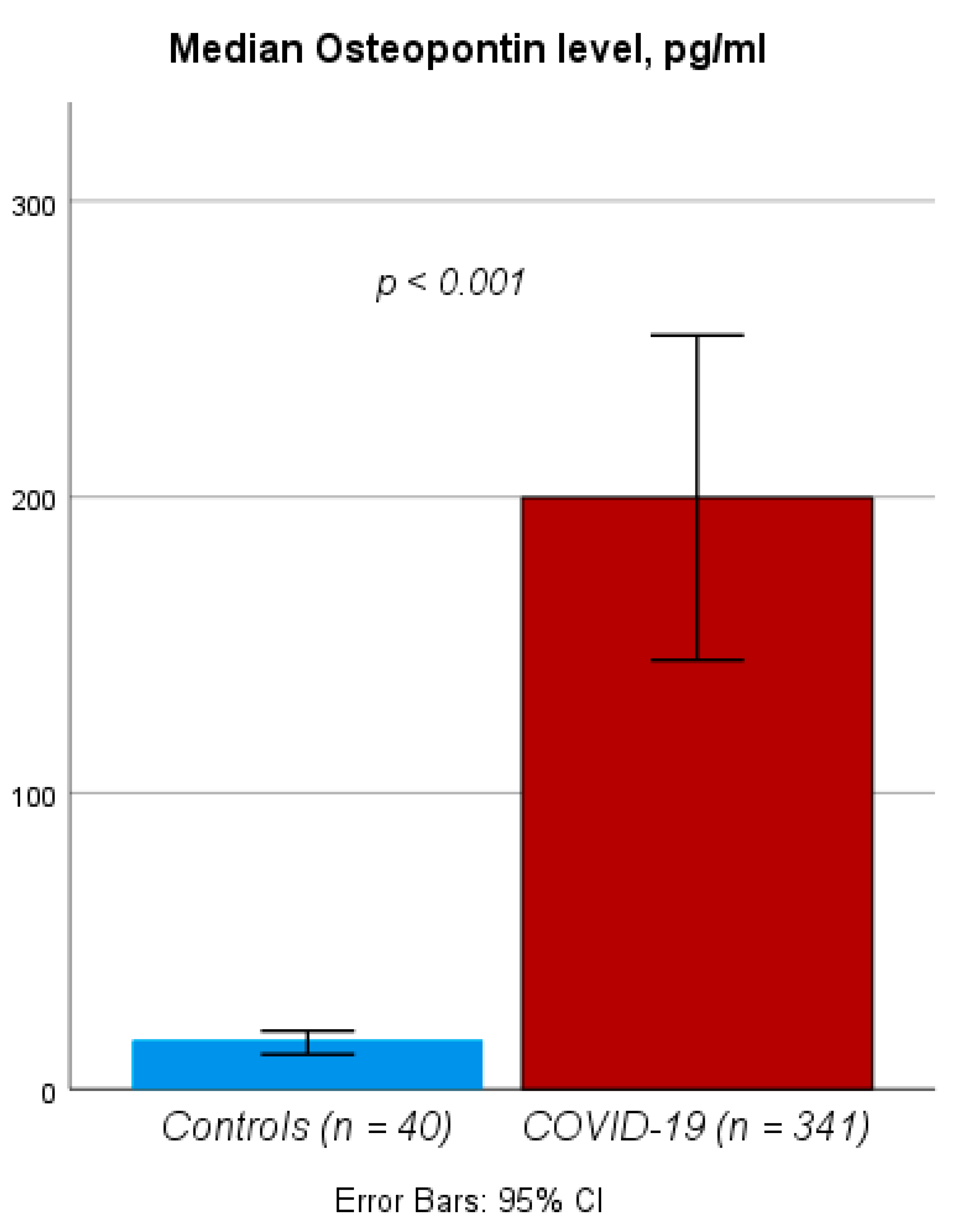
3.2. Circulating Levels of OPN Are Elevated in Patients Hospitalized for COVID-19
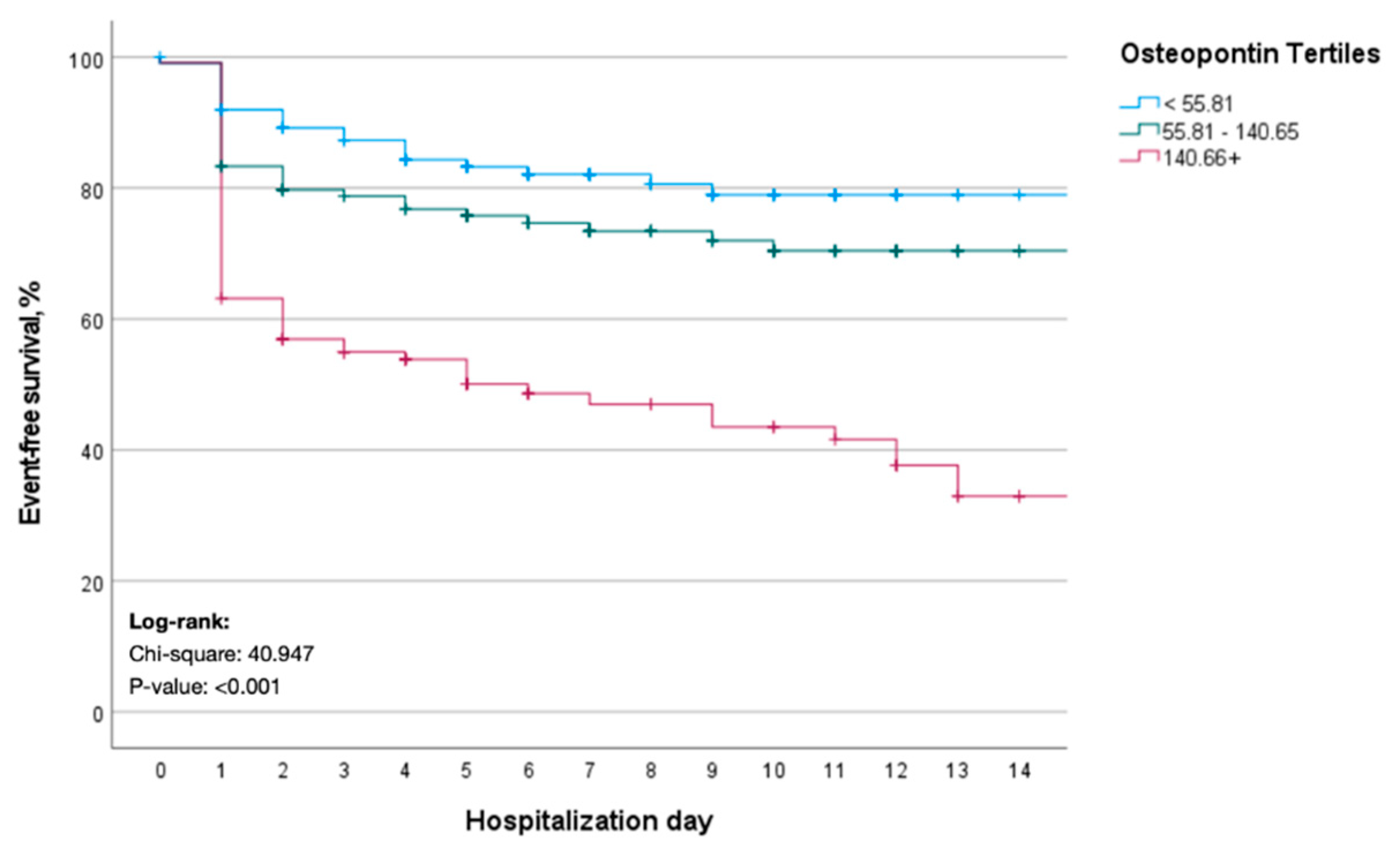
3.3. Elevated Levels of OPN Are Associated with an Increased Risk of Death and Need of Mechanical Ventilation
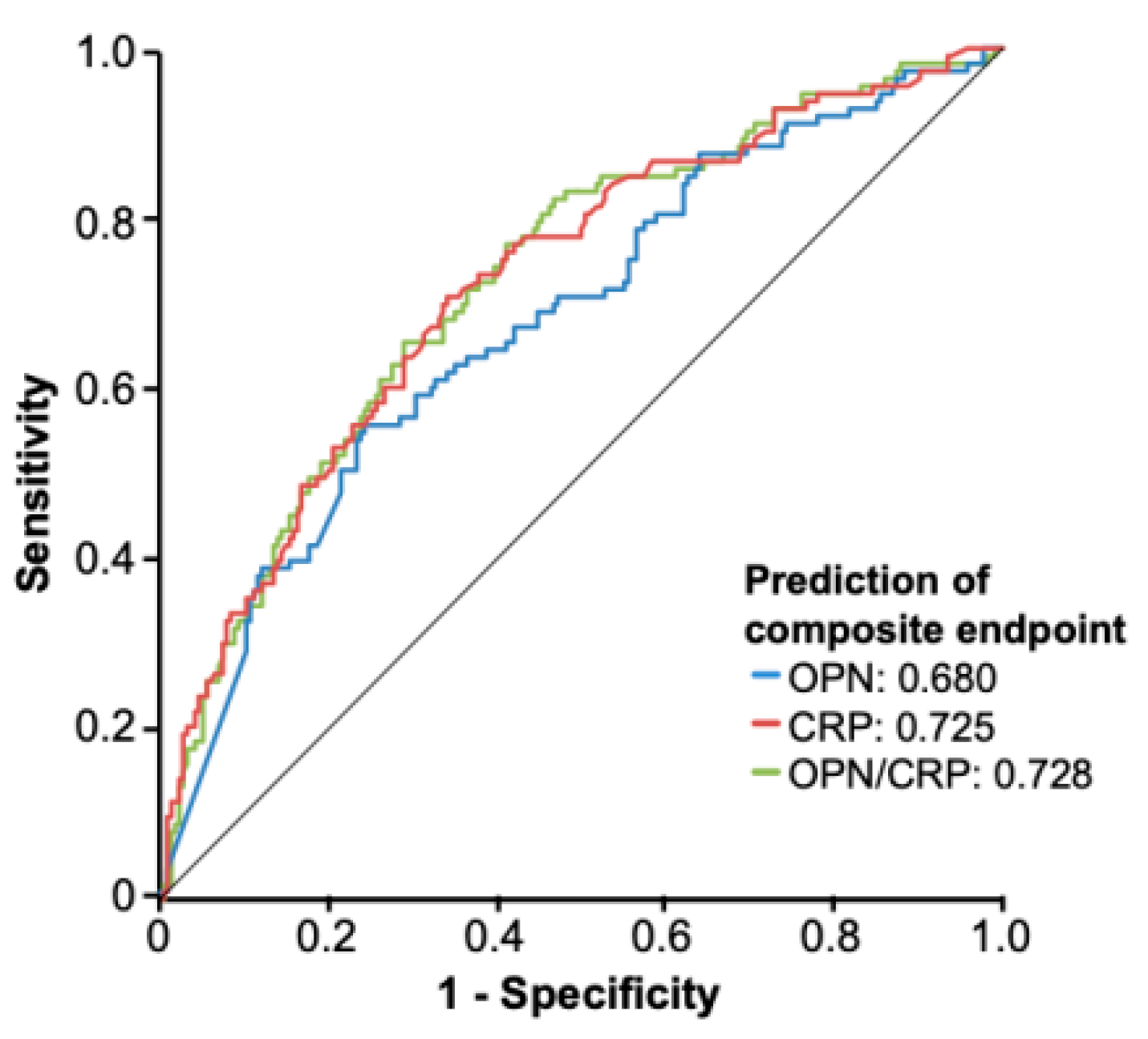
3.4. Circulating OPN as a Predictor of Severe Clinical Course of Disease
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Huang, F.; Yang, Y.; Wang, F.; Yuan, J.; Zhang, Z.; Qin, Y.; Li, X.; Zhao, D.; et al. Elevated plasma levels of selective cytokines in COVID-19 patients reflect viral load and lung injury. Natl. Sci. Rev. 2020, 7, 1003–1011. [Google Scholar] [CrossRef]
- Henry, B.M.; de Oliveira, M.H.S.; Benoit, S.; Plebani, M.; Lippi, G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A meta-analysis. Clin. Chem. Lab. Med. 2020, 58, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Garg, J.; Houghton, D.E.; Murad, M.H.; Kondur, A.; Chaudhary, R.; Wysokinski, W.E.; McBane, R.D. Thromboinflammatory Biomarkers in COVID-19: Systematic Review and Meta-analysis of 17,052 Patients. Mayo Clin. Proc. Innov. Qual. Outcomes 2021, 5, 388–402. [Google Scholar] [CrossRef]
- Wang, K.X.; Denhardt, D.T. Osteopontin: Role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008, 19, 333–345. [Google Scholar] [CrossRef]
- Roderburg, C.; Benz, F.; Cardenas, D.V.; Lutz, M.; Hippe, H.-J.; Luedde, T.; Trautwein, C.; Frey, N.; Koch, A.; Tacke, F.; et al. Persistently elevated osteopontin serum levels predict mortality in critically ill patients. Crit. Care 2015, 19, 271. [Google Scholar] [CrossRef]
- Castello, L.M.; Baldrighi, M.; Molinari, L.; Salmi, L.; Cantaluppi, V.; Vaschetto, R.; Zunino, G.; Quaglia, M.; Bellan, M.; Gavelli, F.; et al. The Role of Osteopontin as a Diagnostic and Prognostic Biomarker in Sepsis and Septic Shock. Cells 2019, 8, 174. [Google Scholar] [CrossRef]
- Carbone, F.; Bonaventura, A.; Vecchiè, A.; Meessen, J.; Minetti, S.; Elia, E.; Ferrara, D.; Ansaldo, A.M.; Tulli, G.; Guarducci, D.; et al. Early osteopontin levels predict mortality in patients with septic shock. Eur. J. Intern. Med. 2020, 78, 113–120. [Google Scholar] [CrossRef]
- Cappellano, G.; Abreu, H.; Raineri, D.; Scotti, L.; Castello, L.; Vaschetto, R.; Chiocchetti, A. High levels of circulating osteopontin in inflammatory lung disease regardless of SARS-CoV-2 infection. EMBO Mol. Med. 2021, 13, e14124. [Google Scholar] [CrossRef]
- Azam, T.U.; Shadid, H.R.; Blakely, P.; O’Hayer, P.; Berlin, H.; Pan, M.; Zhao, P.; Zhao, L.; Pennathur, S.; Pop-Busui, R.; et al. Soluble Urokinase Receptor (SuPAR) in COVID-19–Related AKI. J. Am. Soc. Nephrol. 2020, 31, 2725–2735. [Google Scholar] [CrossRef]
- Weiss, P.; Murdoch, D.R. Clinical course and mortality risk of severe COVID-19. Lancet 2020, 395, 1014–1015. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Leisman, D.E.; Ronner, L.; Pinotti, R.; Taylor, M.D.; Sinha, P.; Calfee, C.S.; Hirayama, A.V.; Mastroiani, F.; Turtle, C.J.; Harhay, M.O.; et al. Cytokine elevation in severe and critical COVID-19: A rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 2020, 8, 1233–1244. [Google Scholar] [CrossRef]
- Interleukin-6 Receptor Antagonists in Critically Ill Patients with COVID-19. N. Engl. J.Med. 2021, 384, 1491–1502. [CrossRef]
- Soin, A.S.; Kumar, K.; Choudhary, N.S.; Sharma, P.; Mehta, Y.; Kataria, S.; Govil, D.; Deswal, V.; Chaudhry, D.; Singh, P.K.; et al. Tocilizumab plus standard care versus standard care in patients in India with moderate to severe COVID-19-associated cytokine release syndrome (COVINTOC): An open-label, multicentre, randomised, controlled, phase 3 trial. Lancet Respir. Med. 2021, 9, 511–521. [Google Scholar] [CrossRef]
- Varım, C.; Demirci, T.; Cengiz, H.; Hacıbekiroğlu, I.; Tuncer, F.B.; Çokluk, E.; Toptan, H.; Karabay, O.; Yıldırım, I. Relationship between serum osteopontin levels and the severity of COVID-19 infection. Wien. Klin. Wochenschr. 2021, 133, 298–302. [Google Scholar] [CrossRef]
- Loosen, S.H.; Heise, D.; DeJong, C.H.; Roy, S.; Tacke, F.; Trautwein, C.; Roderburg, C.; Luedde, T.; Neumann, U.P.; Binnebösel, M. Circulating Levels of Osteopontin Predict Patients’ Outcome after Resection of Colorectal Liver Metastases. J. Clin. Med. 2018, 7, 390. [Google Scholar] [CrossRef]
- Loosen, S.H.; Roderburg, C.; Kauertz, K.L.; Pombeiro, I.; Leyh, C.; Benz, F.; Vucur, M.; Longerich, T.; Koch, A.; Braunschweig, T.; et al. Elevated levels of circulating osteopontin are associated with a poor survival after resection of cholangiocarcinoma. J. Hepatol. 2017, 67, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Kadota, J.; Mizunoe, S.; Mito, K.; Mukae, H.; Yoshioka, S.; Kawakami, K.; Koguchi, Y.; Fukushima, K.; Kon, S.; Kohno, S.; et al. High plasma concentrations of osteopontin in patients with interstitial pneumonia. Respir. Med. 2005, 99, 111–117. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ueno, T.; Miyazaki, E.; Ando, M.; Nureki, S.-I.; Kumamoto, T. Osteopontin levels are elevated in patients with eosinophilic pneumonia. Respirology 2010, 15, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Clemente, N.; Comi, C.; Raineri, D.; Cappellano, G.; Vecchio, D.; Orilieri, E.; Gigliotti, C.L.; Boggio, E.; Dianzani, C.; Sorosina, M.; et al. Role of Anti-Osteopontin Antibodies in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. Front. Immunol. 2017, 8, 321. [Google Scholar] [CrossRef] [PubMed]
| All (n = 341) | |
|---|---|
| Clinical characteristics | |
| Age in years (mean, SD) | 61 (16) |
| <45, n (%) | 52 (15.2%) |
| 45–64, n (%) | 150 (44.0%) |
| 65–79, n (%) | 95 (27.9%) |
| ≥80, n (%) | 44 (12.9%) |
| Male, n (%) | 211 (61.9%) |
| White race, n (%) | 224 (65.7%) |
| Black race, n (%) | 92 (27.0%) |
| BMI kg/m2, mean (SD) | 30.79 (7.58) |
| Current or former smoker, n (%) | 112 (32.8%) |
| Diabetes mellitus, n (%) | 116 (34.0%) |
| Hypertension, n (%) | 206 (60.4%) |
| Coronary artery disease, n (%) | 47 (13.8%) |
| Congestive heart failure, n (%) | 47 (13.8) |
| Laboratory parameters | |
| Admission eGFR, mL/min/1.73 m2, mean (SD) | 70.61 (33.02) |
| White cell count, 103 ×, mean (SD) | 7.6 (4.2) |
| Absolute neutrophil count, 103 ×, mean (SD) | 5.8 (3.6) |
| Absolute lymphocyte count, 103 ×, mean (SD) | 1.1 (1.7) |
| Aspartate aminotransferase, IU/L, mean (SD) | 56.85 (49.53) |
| Alanine aminotransferase, IU/L, mean (SD) | 41.6 (51.3) |
| Bilirubin, mg/dl, mean (SD) | 0.65 (0.39) |
| Alkaline phosphatase, IU/L, mean (SD) | 81 (46) |
| C-reactive protein, mg/dl, median (IQR) | 8.3 (3.6–17.3) |
| D-dimer, FEU mg/l, median (IQR) | 1.085 (0.57–2.18) |
| Ferritin, ng/mL, median (IQR) | 613.15 (268.6–1321.7) |
| Procalcitonin, ng/mL, median (IQR) | 0.2 (0.1–0.77) |
| OPN, ng/mL, median (IQR) | 80.42 (43.44–164.0) |
| Outcomes | |
| Need for MV, n (%) | 104 (30.5%) |
| Need for RRT, n (%) | 35 (10.3%) |
| Death, n (%) | 53 (15.5%) |
| Parameter | β (95%CI) | p-Value |
|---|---|---|
| Age | −6.49 (−38.03–25.05) | 0.260 |
| Male sex | 20.13 (2.95–37.32) | 0.690 |
| Black race | −1.39 (−3.58–0.80) | 0.022 |
| BMI | −0.94 (−1.53–0.36) | 0.210 |
| eGFR | 3.22 (−0.51–6.94) | 0.002 |
| WBC | 0.63 (−0.02–1.27) | 0.090 |
| CRP | 28.66 (−6.66–63.99) | 0.060 |
| Diabetes mellitus | −3.35 (−41.45–34.76) | 0.110 |
| Hypertension | −22.22 (−70.73–26.30) | 0.860 |
| Coronary artery disease | 38.66 (−9.98–87.29) | 0.370 |
| Congestive heart failure | −6.49 (−38.03–25.05) | 0.120 |
| 1st Tertile (OPN < 55.81 ng/mL, n = 113) | 2nd Tertile (OPN 55.81–140.65 ng/mL, n = 114) | 3rd Tertile (OPN ≥ 140.66 ng/mk, n = 114) | p-Value | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age in years (mean, SD) | 58 (16) | 62 (15) | 61 (16) | 0.120 |
| <45, n (%) | 19 (16.8%) | 16 (14.0%) | 17 (14.9%) | 0.380 |
| 45–64, n (%) | 57 (50.4%) | 46 (40.4%) | 47 (41.2%) | 0.240 |
| 65–79, n (%) | 28 (24.8%) | 36 (31.6%) | 31 (27.2%) | 0.510 |
| ≥80, n (%) | 9 (8.0%) | 16 (14.0%) | 19 (16.7%) | 0.130 |
| Male, n (%) | 64 (56.6%) | 74 (64.9%) | 73 (64.0%) | 0.370 |
| White race, n (%) | 86 (76.1%) | 78 (68.4%) | 60 (52.6%) | 0.022 |
| Black race, n (%) | 20 (17.7%) | 29 (25.4%) | 43 (37.7%) | 0.022 |
| BMI kg/m2, mean (SD) | 30.95 (7.68) | 30.7 (7.67) | 30.75 (7.46) | 0.970 |
| Current or former smoker, n (%) | 31 (27.4%) | 40 (35.1%) | 41 (36.0%) | 0.175 |
| Diabetes mellitus, n (%) | 28 (24.8%) | 43 (37.7%) | 45 (39.5%) | 0.039 |
| Hypertension, n (%) | 59 (52.2%) | 71 (62.3%) | 76 (66.7%) | 0.074 |
| Coronary artery disease, n (%) | 14 (12.4%) | 16 (14.0%) | 17 (14.9%) | 0.850 |
| Congestive heart failure, n (%) | 11 (9.7%) | 14 (12.3%) | 22 (19.3%) | 0.096 |
| Laboratory parameters | ||||
| Admission eGFR, mL/min/1.73 m2, mean (SD) | 76.77 (31.5) | 72.85 (30.83) | 62.26 (35.11) | 0.003 |
| White cell count, 103×, mean (SD) | 6.9 (3.4) | 7.4 (4.1) | 8.6 (4.9) | 0.005 |
| Absolute neutrophil count, 103×, mean (SD) | 5.1 (3.0) | 5.6 (3.7) | 6.8 (3.9) | 0.001 |
| Absolute lymphocyte count, 103×, mean (SD) | 1.1 (0.5) | 1.1 (0.5) | 1.1 (2.8) | 0.970 |
| Aspartate aminotransferase, IU/L, mean (SD) | 50.29 (61.74) | 54.47 (35.21) | 65.67 (46.96) | 0.054 |
| Alanine aminotransferase, IU/L, mean (SD) | 43.8 (78.9) | 40.5 (28) | 40.5 (30.1) | 0.855 |
| Bilirubin, mg/dl, mean (SD) | 0.61 (0.35) | 40.5 (0.39) | 0.62 (0.42) | 0.079 |
| Alkaline phosphatase, IU/L, mean (SD) | 75 (43) | 85 (49) | 84 (46) | 0.266 |
| C-reactive protein, mg/dl, median (IQR) | 4.6 (2.1–11.0) | 7.86 (3.9–16.2) | 14.35 (7.28–23.1) | <0.001 |
| D-dimer, FEU mg/l, median (IQR) | 0.7 (0.48–2.025) | 0.965 (0.56–1.985) | 1.33 (0.69–2.97) | 0.013 |
| Ferritin, ng/mL, median (IQR) | 356.65 (198–1094.5) | 625.5 (316.3–1260.3) | 904.9 (353.9–1530.6) | <0.001 |
| Procalcitonin, ng/mL, median (IQR) | 0.135 (0.07–0.305) | 0.19 (0.1–0.42) | 0.54 (0.15–2.07) | <0.001 |
| OPN, ng/mL, median (IQR) | 28.17 (19.87–43.17) | 80.23 (66.91–105.61) | 372.53 (164–400) | <0.001 |
| Outcomes | ||||
| Need for MV, n (%) | 18 (15.9%) | 29 (25.4%) | 57 (50.0%) | <0.001 |
| Need for RRT, n (%) | 10 (8.8%) | 8 (7.0%) | 17 (14.9%) | 0.121 |
| Death, n (%) | 12 (10.6%) | 10 (8.8%) | 31 (27.2%) | 0.001 |
| Death (n = 53) | Need for Mechanical Ventilation (n = 104) | Need for Renal Replacement Therapy (n = 35) | Composite Endpoint (n = 192) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p | OR | 95%CI | p | OR | 95%CI | p | OR | 95%CI | p | OR | 95%CI | |||||
| Age (10 year increase) | 0.001 | 1.710 | 1.238 | 2.360 | 0.489 | 0.924 | 0.738 | 1.156 | 0.357 | 0.850 | 0.601 | 1.201 | 0.393 | 1.106 | 0.878 | 1.393 |
| Male sex | 0.186 | 1.680 | 0.778 | 3.650 | 0.163 | 1.50 | 0.848 | 2.647 | 0.002 | 5.444 | 1.830 | 16.18 | 0.339 | 1.328 | 0.743 | 2.371 |
| BMI (per 5 units) | 0.147 | 1.221 | 0.933 | 1.598 | 0.018 | 1.259 | 1.040 | 1.525 | 0.874 | 1.024 | 0.765 | 1.371 | 0.918 | 0.967 | 0.514 | 1.821 |
| Blacks (compared to non-blacks) | 0.001 | 0.184 | 0.066 | 0.507 | 0.459 | 1.260 | 0.683 | 2.323 | 0.406 | 1.499 | 0.577 | 3.893 | 0.054 | 1.218 | 0.997 | 1.488 |
| Current or former smoker | 0.034 | 2.479 | 1.069 | 5.749 | 0.078 | 1.757 | 0.938 | 3.291 | 0.982 | 0.988 | 0.357 | 2.733 | 0.018 | 0.941 | 0.894 | 0.990 |
| Diabetes mellitus | 0.023 | 2.455 | 1.133 | 5.321 | 0.047 | 1.817 | 1.008 | 3.275 | 0.749 | 0.862 | 0.348 | 2.138 | 0.004 | 1.111 | 1.034 | 1.193 |
| Hypertension | 0.476 | 0.724 | 0.297 | 1.763 | 0.573 | 1.213 | 0.621 | 2.370 | 0.493 | 1.519 | 0.460 | 5.017 | 0.087 | 1.011 | 0.998 | 1.024 |
| Coronary artery disease | 0.063 | 0.365 | 0.126 | 1.054 | 0.118 | 0.495 | 0.205 | 1.195 | 0.426 | 0.612 | 0.182 | 2.053 | 0.012 | 2.154 | 1.187 | 3.910 |
| Congestive heart failure | 0.175 | 1.972 | 0.740 | 5.258 | 0.377 | 0.685 | 0.295 | 1.587 | 0.541 | 0.707 | 0.232 | 2.154 | 0.677 | 1.158 | 0.580 | 2.314 |
| eGFR | 0.080 | 0.941 | 0.879 | 1.007 | 0.105 | 0.959 | 0.911 | 1.009 | <0.001 | 0.794 | 0.729 | 0.866 | 0.085 | 0.480 | 0.208 | 1.107 |
| OPN < 55.81 ng/mL | Reference | |||||||||||||||
| OPN 55.81–140.65 ng/mL | 0.357 | 0.623 | 0.227 | 1.706 | 0.112 | 1.775 | 0.874 | 3.603 | 0.582 | 0.730 | 0.238 | 2.240 | 0.626 | 1.190 | 0,592 | 2.393 |
| OPN > 140.66 ng/mL | 0.006 | 3.450 | 1.439 | 8.273 | <0.001 | 4.931 | 2.480 | 9.804 | 0.89 | 0.930 | 0.329 | 2.626 | 0.001 | 3.232 | 1.619 | 6.450 |
| OPN (per 100% increase) | 0.008 | 1.415 | 1.095 | 1.827 | <0.001 | 1.598 | 1.308 | 1.950 | 0.639 | 1.077 | 0.789 | 1.470 | ||||
| HR, 95%CI | p-Value | |
|---|---|---|
| OPN < 55.81 ng/mL | Reference | |
| OPN 55.81–140.65 ng/mL | 1.170 (0.669–2.046) | 0.582 |
| OPN > 140.65 ng/mL | 1.834 (1.071–3.139) | 0.027 |
| Age | 0.996 (0.980–1.012) | 0.629 |
| Male Sex | 0.902 (0.609–1.337) | 0.607 |
| Black Race | 0.965 (0.625–1.488) | 0.871 |
| BMI | 1.029 (1.002–1.057) | 0.034 |
| eGFR | 0.992 (0.985–0.998) | 0.014 |
| Diabetes mellitus | 1.526 (1.014–2.298) | 0.043 |
| Hypertension | 1.204 (0.728–1.992) | 0.470 |
| Coronary artery disease | 0.807 (0.454–1.435) | 0.466 |
| Congestive heart failure | 1.256 (0.752–2.096) | 0.383 |
| CRP | 1.312 (1.145–1.504) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayek, S.S.; Roderburg, C.; Blakely, P.; Launius, C.; Eugen-Olsen, J.; Tacke, F.; Ktena, S.; Keitel, V.; Luedde, M.; Giamarellos-Bourboulis, E.J.; et al. Circulating Osteopontin Levels and Outcomes in Patients Hospitalized for COVID-19. J. Clin. Med. 2021, 10, 3907. https://doi.org/10.3390/jcm10173907
Hayek SS, Roderburg C, Blakely P, Launius C, Eugen-Olsen J, Tacke F, Ktena S, Keitel V, Luedde M, Giamarellos-Bourboulis EJ, et al. Circulating Osteopontin Levels and Outcomes in Patients Hospitalized for COVID-19. Journal of Clinical Medicine. 2021; 10(17):3907. https://doi.org/10.3390/jcm10173907
Chicago/Turabian StyleHayek, Salim S., Christoph Roderburg, Pennelope Blakely, Christopher Launius, Jesper Eugen-Olsen, Frank Tacke, Sofia Ktena, Verena Keitel, Mark Luedde, Evangelos J. Giamarellos-Bourboulis, and et al. 2021. "Circulating Osteopontin Levels and Outcomes in Patients Hospitalized for COVID-19" Journal of Clinical Medicine 10, no. 17: 3907. https://doi.org/10.3390/jcm10173907
APA StyleHayek, S. S., Roderburg, C., Blakely, P., Launius, C., Eugen-Olsen, J., Tacke, F., Ktena, S., Keitel, V., Luedde, M., Giamarellos-Bourboulis, E. J., Luedde, T., & Loosen, S. H. (2021). Circulating Osteopontin Levels and Outcomes in Patients Hospitalized for COVID-19. Journal of Clinical Medicine, 10(17), 3907. https://doi.org/10.3390/jcm10173907









