The Association between Foot and Ulcer Microcirculation Measured with Laser Speckle Contrast Imaging and Healing of Diabetic Foot Ulcers
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
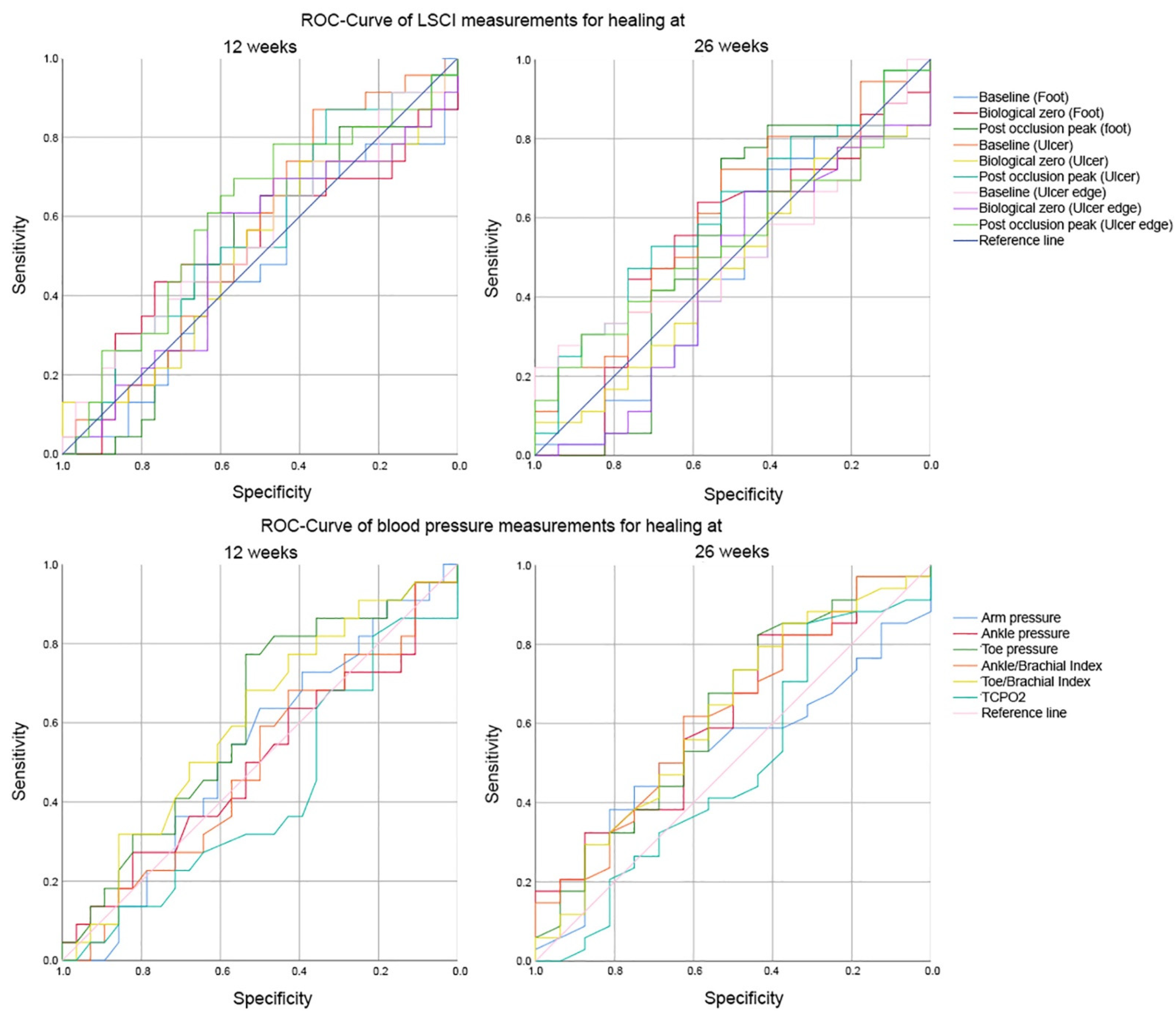
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IDF Diabetes Atlas 9th Edition 2019. Available online: https://www.diabetesatlas.org/en/ (accessed on 7 February 2021).
- Boulton, A.J.; Vileikyte, L.; Ragnarson-Tennvall, G.; Apelqvist, J. The global burden of diabetic foot disease. Lancet 2005, 366, 1719–1724. [Google Scholar] [CrossRef]
- Kerr, M.; Rayman, G.; Jeffcoate, W.J. Cost of diabetic foot disease to the National Health Service in England. Diabet. Med. 2014, 31, 1498–1504. [Google Scholar] [CrossRef] [PubMed]
- Nabuurs-Franssen, M.H.; Huijberts, M.S.P.; Kruseman, A.C.N.; Willems, J.; Schaper, N. Health-related quality of life of diabetic foot ulcer patients and their caregivers. Diabetol. 2005, 48, 1906–1910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jupiter, D.C.; Thorud, J.C.; Buckley, C.J.; Shibuya, N. The impact of foot ulceration and amputation on mortality in diabetic patients. I: From ulceration to death, a systematic review. Int. Wound J. 2016, 13, 892–903. [Google Scholar] [CrossRef] [PubMed]
- Hinchliffe, R.J.; Brownrigg, J.R.W.; Apelqvist, J.; Boyko, E.; Fitridge, R.; Mills, J.; Reekers, J.; Shearman, C.P.; Zierler, R.E.; Schaper, N.; et al. IWGDF guidance on the diagnosis, prognosis and management of peripheral artery disease in patients with foot ulcers in diabetes. Diabetes/Metab. Res. Rev. 2016, 32, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Prompers, L.; Schaper, N.; Apelqvist, J.; Edmonds, M.; Jude, E.; Mauricio, D.; Uccioli, L.; Urbancic, V.; Bakker, K.; Holstein, P.; et al. Prediction of outcome in individuals with diabetic foot ulcers: Focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia 2008, 51, 747–755. [Google Scholar] [CrossRef] [Green Version]
- Forsythe, R.O.; Apelqvist, J.; Boyko, E.J.; Fitridge, R.; Hong, J.P.; Katsanos, K.; Mills, J.L.; Nikol, S.; Reekers, J.; Venermo, M.; et al. Performance of prognostic markers in the prediction of wound healing or amputation among patients with foot ulcers in diabetes: A systematic review. Diabetes/Metab. Res. Rev. 2020, 36, e3278. [Google Scholar] [CrossRef]
- Jones, D.W.; Wyers, M.C. Lower Extremity Arterial Reconstruction in Patients with Diabetes Mellitus: Principles of Treatment. In The Diabetic Foot; Humana: Cham, Switzerland, 2018; pp. 327–343. [Google Scholar]
- Spångéus, A.; Wijkman, M.; Lindström, T.; Engvall, J.E.; Östgren, C.J.; Nystrom, F.H.; Länne, T. Toe brachial index in middle aged patients with diabetes mellitus type 2: Not just a peripheral issue. Diabetes Res. Clin. Pract. 2013, 100, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Faglia, E.; Clerici, G.; Caminiti, M.; Quarantiello, A.; Curci, V.; Morabito, A. Predictive Values of Transcutaneous Oxygen Tension for Above-the-ankle Amputation in Diabetic Patients with Critical Limb Ischemia. Eur. J. Vasc. Endovasc. Surg. 2007, 33, 731–736. [Google Scholar] [CrossRef] [Green Version]
- Mills, J.L. Open bypass and endoluminal therapy: Complementary techniques for revascularization in diabetic patients with critical limb ischaemia. Diabetes/Metab. Res. Rev. 2008, 24, S34–S39. [Google Scholar] [CrossRef]
- Lukkari-Rautiarinen, E.; Lepäntalo, M.; Pietilä, J. Reproducibility of skin blood flow, perfusion pressure and oxygen tension measurements in advanced lower limb ischaemia. Eur. J. Vasc. Surg. 1989, 3, 345–350. [Google Scholar] [CrossRef]
- Mennes, O.A.; van Netten, J.J.; Slart, R.H.; Steenbergen, W. Novel Optical Techniques for Imaging Microcirculation in the Diabetic Foot. Curr. Pharm. Des. 2018, 24, 1304–1316. [Google Scholar] [CrossRef] [Green Version]
- Briers, J.D. Laser Doppler, speckle and related techniques for blood perfusion mapping and imagingLaser Doppler, speckle and related techniques for blood perfusion mapping and imaging. Physiol. Meas. 2001, 22, R35–R66. [Google Scholar] [CrossRef] [PubMed]
- Boas, D.A.; Dunn, A.K. Laser speckle contrast imaging in biomedical optics. J. Biomed. Opt. 2010, 15, 011109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roustit, M.; Millet, C.; Blaise, S.; Dufournet, B.; Cracowski, J. Excellent reproducibility of laser speckle contrast imaging to assess skin microvascular reactivity. Microvasc. Res. 2010, 80, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Millet, C.; Roustit, M.; Blaise, S.; Cracowski, J. Comparison between laser speckle contrast imaging and laser Doppler imaging to assess skin blood flow in humans. Microvasc. Res. 2011, 82, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Mahé, G.; Humeau-Heurtier, A.; Durand, S.; Leftheriotis, G.; Abraham, P. Assessment of Skin Microvascular Function and Dysfunction With Laser Speckle Contrast Imaging. Circ. Cardiovasc. Imaging 2012, 5, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Iredahl, F.; Löfberg, A.; Sjöberg, F.; Farnebo, S.; Tesselaar, E. Non-Invasive Measurement of Skin Microvascular Response during Pharmacological and Physiological Provocations. Connes P, editor. PLoS ONE 2015, 10, e0133760. [Google Scholar] [CrossRef]
- Mennes, O.A.; van Netten, J.J.; Van Baal, J.G.; Steenbergen, W. Assessment of microcirculation in the diabetic foot with laser speckle contrast imaging. Physiol. Meas. 2019, 40, 065002. [Google Scholar] [CrossRef]
- Van Netten, J.J.; Bus, S.A.; Apelqvist, J.; Lipsky, B.A.; Hinchliffe, R.J.; Game, F.; Rayman, G.; Lazzarini, P.A.; Forsythe, R.O.; Peters, E.; et al. Definitions and criteria for diabetic foot disease. Diabetes/Metab. Res. Rev. 2020, 36, e3268. [Google Scholar] [CrossRef] [Green Version]
- Lipsky, B.A.; Senneville, É.; Abbas, Z.G.; Aragón-Sánchez, J.; Diggle, M.; Embil, J.M.; International Working Group on the Diabetic Foot (IWGDF). Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes/Metab. Res. Rev. 2020, 36, e3280. [Google Scholar] [CrossRef] [Green Version]
- Startpagina Diabetische Voet—Richtlijn—Richtlijnendatabase. Available online: https://richtlijnendatabase.nl/richtlijn/diabetische_voet/startpagina_diabetische_voet.html (accessed on 7 February 2021).
- Schaper, N.C.; Van Netten, J.J.; Apelqvist, J.; Bus, S.A.; Hinchliffe, R.J.; Lipsky, B.A.; IWGDF Editorial Board. Practical Guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes/Metab. Res. Rev. 2020, 36, e3266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinchliffe, R.J.; Forsythe, R.O.; Apelqvist, J.; Boyko, E.J.; Fitridge, R.; Hong, J.P.; Katsanos, K.; Mills, J.L.; Nikol, S.; Reekers, J.; et al. Guidelines on diagnosis, prognosis, and management of peripheral artery disease in patients with foot ulcers and diabetes (IWGDF 2019 update). Diabetes/Metab. Res. Rev. 2020, 36, e3276. [Google Scholar] [CrossRef] [PubMed]
- Lavery, L.A.; Armstrong, D.G.; Harkless, L.B. Classification of diabetic foot wounds. J. Foot Ankle Surg. 1996, 35, 528–531. [Google Scholar] [CrossRef]
- Elgzyri, T.; Larsson, J.; Thörne, J.; Eriksson, K.-F.; Apelqvist, J. Outcome of Ischemic Foot Ulcer in Diabetic Patients Who Had no Invasive Vascular Intervention. Eur. J. Vasc. Endovasc. Surg. 2013, 46, 110–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gershater, M.A.; Löndahl, M.; Nyberg, P.; Larsson, J.; Thörne, J.; Eneroth, M.; Apelqvist, J. Complexity of factors related to outcome of neuropathic and neuroischaemic/ischaemic diabetic foot ulcers: A cohort study. Diabetologia 2008, 52, 398–407. [Google Scholar] [CrossRef] [Green Version]
- Wallin, L.; Björnsson, H.; Stenström, A. Fluorescein angiography for predicting healing of foot ulcers. Acta Orthop. Scand. 1989, 60, 40–44. [Google Scholar] [CrossRef] [Green Version]
- Holstein, P.; Lassen, N.A. Healing of ulcers on the feet correlated with distal blood pressure measurements in occlusive arterial disease. Acta Orthop. Scand. 1980, 51, 995–1006. [Google Scholar] [CrossRef]
- Kalani, M.; Brismar, K.; Fagrell, B.; Ostergren, J.; Jorneskog, G. Transcutaneous oxygen tension and toe blood pressure as predictors for outcome of diabetic foot ulcers. Diabetes Care 1999, 22, 147–151. [Google Scholar] [CrossRef]
- Wang, Z.; Hasan, R.; Firwana, B.; Elraiyah, T.; Tsapas, A.; Prokop, L.; Mills, J.; Murad, M.H. A systematic review and meta-analysis of tests to predict wound healing in diabetic foot. J. Vasc. Surg. 2016, 63, 29S–36S.e2. [Google Scholar] [CrossRef] [Green Version]
- Fagher, K.; Katzman, P.; Londahl, M. Transcutaneous oxygen pressure as a predictor for short-term survival in patients with type 2 diabetes and foot ulcers: A comparison with ankle–brachial index and toe blood pressure. Acta Diabetol. 2018, 55, 781–788. [Google Scholar] [CrossRef] [Green Version]
- Rajagopalan, C.; Viswanathan, V.; Rajsekar, S.; Selvaraj, B.; Daniel, L. Diabetic foot ulcers—comparison of performance of ankle-brachial index and transcutaneous partial oxygen pressure in predicting outcome. Int. J. Diabetes Dev. Ctries. 2018, 38, 179–184. [Google Scholar] [CrossRef]
- Jeffcoate, W.; Game, F.; Morbach, S.; Narres, M.; Van Acker, K.; Icks, A. Assessing data on the incidence of lower limb amputation in diabetes. Diabetologia 2021, 64, 1442–1446. [Google Scholar] [CrossRef] [PubMed]
- Jeffcoate, W.J.; A Bus, S.; Game, F.L.; Hinchliffe, R.J.; E Price, P.; Schaper, N.C. Reporting standards of studies and papers on the prevention and management of foot ulcers in diabetes: Required details and markers of good quality. Lancet Diabetes Endocrinol. 2016, 4, 781–788. [Google Scholar] [CrossRef] [Green Version]


| Variable | Baseline | 12 Weeks | 26 Weeks | |||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD N (%) | Healed Mean ± SD | Non-Healed Mean ± SD | p-Value | Healed Mean ± SD | Non-Healed Mean ± SD | p-Value | ||
| Patient Characteristics | 53 (100%) | 23 (43.4%) | 30 (56.6%) | 36 (67.9%) | 17 (32.1%) | |||
| Age (Years) | 66.7 ± 12.8 | 68.9 ± 13.1 | 65.1 ± 12.7 | 0.300 | 67.3 ± 11.9 | 65.7 ± 15.1 | 0.679 | |
| Gender | Male | 42 (79.2%) | 18 (78.3%) | 24 (80%) | 0.877 | 30 (83.3%) | 12 (70.6%) | 0.286 |
| Female | 11 (20.8%) | 5 (21.7%) | 6 (20%) | 6 (16.7%) | 5 (29.4%) | |||
| Height (cm) | 179.4 ± 9.6 | 179.1 ± 11.1 | 179.7 ± 8.4 | 0.858 | 179.7 ± 10.7 | 178.7 ± 6.6 | 0.744 | |
| Weight (kg) | 96.0 ± 19.9 | 98.0 ± 21.4 | 94.4 ± 18.8 | 0.546 | 96.9 ± 20.1 | 93.9 ± 20.0 | 0.660 | |
| BMI | 29.7 ± 5.5 | 30.4 ± 5.5 | 29.2 ± 5.6 | 0.475 | 29.9 ± 5.2 | 29.4 ± 6.4 | 0.808 | |
| HbA1c (mmol/mol) | 63.6 ± 21.0 | 59.5 ± 14.4 | 67.6 ± 25.6 | 0.221 | 63.0 ± 15.9 | 65.7 ± 33.4 | 0.729 | |
| Smoking | Yes | 11 (52.4%) | 3 (42.9%) | 8 (57.1%) | 0.590 | 6 (46.2%) | 5 (62.5%) | 0.436 |
| No | 4 (19.0%) | 1 (14.3%) | 3 (21.4%) | 2 (16.4%) | 2 (25.0%) | |||
| Stopped | 6 (28.6%) | 3 (42.9%) | 3 (21.4%) | 5 (38.5%) | 1 (12.5%) | |||
| Unknown | 32 | 16 | 16 | 23 | 9 | |||
| Diabetes Type | 1 | 3 (5.7%) | 1 (4.3%) | 2 (6.7%) | 0.717 | 2 (5.6%) | 1 (5.9%) | 0.962 |
| 2 | 50 (94.3%) | 22 (95.7%) | 28 (90.0%) | 34 (94.4%) | 16 (88.2%) | |||
| Diabetes Duration | ≤10 years | 20 (43.5%) | 8 (38.1%) | 12 (48.0%) | 0.394 | 12 (36.4%) | 8 (61.5%) | 0.180 |
| >10 years | 26 (56.5%) | 13 (61.9%) | 13 (52.0%) | 21 (63.6%) | 5 (38.5%) | |||
| Unknown | 7 | 2 | 5 | 3 | 4 | |||
| Dialysis | Yes | 3 (5.7%) | 1 (4.3%) | 2 (6.7%) | 0.266 | 2 (5.6%) | 1 (5.9%) | 0.998 |
| No | 47 (88.7%) | 22 (95.7%) | 25 (83.3%) | 32 (88.9%) | 15 (88.2%) | |||
| In the past | 3 (5.7%) | 0 (0.0%) | 3 (10.0%) | 2 (5.6%) | 1 (5.9%) | |||
| Infections | Yes | 8 (15.1%) | 4 (17.4%) | 4 (13.3%) | 0.683 | 5 (13.9%) | 3 (17.6%) | 0.721 |
| No | 45 (84.9%) | 19 (82.6%) | 26 (86.7%) | 31 (86.1%) | 14 (82.4%) | |||
| Neuropathy | Yes | 48 (96.0%) | 22 (100.0%) | 26 (92.9%) | 0.201 | 35 (100.0%) | 13 (86.7%) | 0.027 * |
| No | 2 (4.0%) | 0 (0.0%) | 2 (7.1%) | 0 (0.0%) | 2 (13.3%) | |||
| Unknown | 3 | 1 | 2 | 1 | 2 | |||
| UT-classification | 0.776 | 0.704 | ||||||
| 0A | 4 (7.5%) | 2 (8.7%) | 2 (6.7%) | 3 (8.3%) | 1 (5.9%) | |||
| 1A | 30 (56.6%) | 15 (65.2%) | 15 (50.0%) | 22 (62.9%) | 8 (47.1%) | |||
| 1B | 1 (1.9%) | 0 (0.0%) | 1 (3.3%) | 0 (0.0%) | 1 (5.9%) | |||
| 1C | 1 (1.9%) | 0 (0.0%) | 1 (3.3%) | 1 (2.8%) | 0 (0.0%) | |||
| 2A | 5 (9.4%) | 1 (4.3%) | 4 (13.3%) | 3 (8.3%) | 2 (11.8%) | |||
| 2B | 5 (9.4%) | 2 (8.7%) | 3 (10.0%) | 3 (8.3%) | 2 (11.8%) | |||
| 3A | 3 (5.7%) | 1 (4.3%) | 2 (6.7%) | 2 (5.6%) | 1 (5.9%) | |||
| 3B | 3 (5.7%) | 2 (8.7%) | 1 (3.3%) | 2 (5.6%) | 1 (5.9%) | |||
| 3C | 1 (1.9%) | 0 (0.0%) | 1 (3.3%) | 0 (0.0%) | 1 (5.9%) | |||
| History of Ulcers | Yes | 31 (58.5%) | 15 (65.2%) | 16 (53.3%) | 0.384 | 20 (55.6%) | 11 (64.7%) | 0.528 |
| No | 22 (41.5%) | 8 (34.8%) | 14 (46.7%) | 16 (44.4%) | 6 (35.3%) | |||
| Minor Amputation | Yes | 8 (15.1%) | 2 (8.7%) | 6 (20.0%) | 0.255 | 5 (13.9%) | 3 (17.6%) | 0.721 |
| No | 45 (84.9%) | 21 (91.3%) | 24 (80.0%) | 31 (86.1%) | 14 (82.4%) | |||
| Vascular Status | Non- ischemic | 7 (13.2%) | 1 (4.3%) | 6 (20.0%) | 0.925 | 4 (11.1%) | 3 (17.6%) | 0.275 |
| Ischemic | 28 (52.8%) | 16 (69.6%) | 12 (40.0%) | 23 (63.9%) | 5 (29.4%) | |||
| Critical- ischemic | 18 (34.0%) | 6 (26.1%) | 12 (40.0%) | 9 (25.0%) | 9 (52.9%) | |||
| Variable | Baseline | 12 Weeks | 26 Weeks | |||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD N (%) | Healed Mean ± SD | Non-Healed Mean ± SD | p-Value | Healed Mean ± SD | Non-Healed Mean ± SD | p-Value | ||
| Laser Speckle Contrast Imaging (PU) | ||||||||
| Foot | ||||||||
| Baseline | 50.3 ± 14.6 | 49.3 ± 15.1 | 51.1 ± 14.5 | 0.654 | 49.4 ± 13.9 | 52.3 ± 16.3 | 0.508 | |
| Biological zero | 12.8 ± 7.7 | 12.7 ± 7.3 | 12.8 ± 8.1 | 0.959 | 12.5 ± 6.5 | 13.5 ± 10 | 0.637 | |
| Post occlusion peak | 77.3 ± 26.6 | 76.7 ± 24.4 | 77.8 ± 28.6 | 0.889 | 77.4 ± 23.2 | 77.2 ± 33.6 | 0.983 | |
| Ulcer | ||||||||
| Baseline | 104.8 ± 34.6 | 108.8 ± 33 | 101.8 ± 36.1 | 0.467 | 109.1 ± 35.7 | 95.8 ± 31.2 | 0.197 | |
| Biological zero | 25.2 ± 15.3 | 26.4 ± 17.9 | 24.3 ± 13.2 | 0.631 | 25 ± 16.3 | 25.7 ± 13.4 | 0.884 | |
| Post occlusion peak | 104.0 ± 33.4 | 107.8 ± 32.6 | 101.1 ± 34.3 | 0.473 | 108.2 ± 35.2 | 95.2 ± 28.2 | 0.190 | |
| Ulcer Edge | ||||||||
| Baseline | 92.2 ± 30.7 | 96.3 ± 33.4 | 89.1 ± 28.6 | 0.402 | 94.2 ± 33.8 | 88.1 ± 23 | 0.509 | |
| Biological zero | 20.1 ± 10.7 | 20.5 ± 10.8 | 19.8 ± 10.9 | 0.840 | 19.4 ± 10 | 21.7 ± 12.3 | 0.465 | |
| Post occlusion peak | 102.0 ± 32.9 | 108.1 ± 33.9 | 97.3 ± 31.9 | 0.239 | 104.8 ± 35.3 | 96 ± 27.4 | 0.373 | |
| Non-invasive Blood Pressure Measurements (mmHg) | ||||||||
| Ankle | 121.9 ± 41.0 | 121.3 ± 46.3 | 122.3 ± 37.2 | 0.931 | 126.9 ± 41.4 | 110.4 ± 39 | 0.183 | |
| Toe | 88.7 ± 45.3 | 97.7 ± 45.1 | 81.8 ± 45.1 | 0.220 | 95.4 ± 45.2 | 75.2 ± 43.8 | 0.136 | |
| ABI | 0.90 ± 0.31 | 0.88 ± 0.3 | 0.92 ± 0.32 | 0.698 | 0.94 ± 0.32 | 0.82 ± 0.3 | 0.188 | |
| TBI | 0.68 ± 0.37 | 0.73 ± 0.32 | 0.64 ± 0.41 | 0.410 | 0.73 ± 0.38 | 0.57 ± 0.34 | 0.151 | |
| TcpO2 | 47.9 ± 17.5 | 44.8 ± 15.1 | 50.3 ± 19.1 | 0.262 | 46.8 ± 14.1 | 50.1 ± 23.6 | 0.526 | |
| 12 Weeks | Threshold | AUC | Sensitivity | Specificity | LLR+ | LLR– |
| Laser speckle contrast imaging (PU) | ||||||
| Foot | ||||||
| Baseline | 43.5 PU | 0.467 | 0.696 | 0.400 | 1.16 | 0.76 |
| Biological zero | 14.3 PU | 0.528 | 0.435 | 0.767 | 1.86 | 0.74 |
| Post occlusion peak | 73.5 PU | 0.517 | 0.609 | 0.567 | 1.40 | 0.69 |
| Ulcer | ||||||
| Baseline | 84.3 PU | 0.558 | 0.870 | 0.367 | 1.37 | 0.36 ** |
| Biological zero | 15.7 PU | 0.510 | 0.739 | 0.400 | 1.23 | 0.65 |
| Post occlusion peak | 89.4 PU | 0.561 | 0.870 | 0.333 | 1.30 | 0.39 ** |
| Ulcer edge | ||||||
| Baseline | 103.0 PU | 0.552 | 0.435 | 0.700 | 1.45 | 0.81 |
| Biological zero | 19.9 PU | 0.519 | 0.609 | 0.633 | 1.66 | 0.62 |
| Post occlusion peak | 96.6 PU | 0.603 | 0.696 | 0.567 | 1.61 | 0.54 |
| Non-invasive blood pressure measurements (mmHg) | ||||||
| Arm pressure | 130.5 mmHg | 0.528 | 0.636 | 0.500 | 1.27 | 0.73 |
| Ankle pressure | 153.0 mmHg | 0.500 | 0.273 | 0.821 | 1.53 | 0.89 |
| Toe pressure | 77.5 mmHg | 0.608 | 0.773 | 0.536 | 1.66 | 0.42 ** |
| Ankle brachial index | 0.83 | 0.494 | 0.682 | 0.429 | 1.19 | 0.74 |
| Toe brachial index | 0.57 | 0.599 | 0.682 | 0.536 | 1.47 | 0.59 |
| TcpO2 | 30.5 mmHg | 0.416 | 0.818 | 0.214 | 1.04 | 0.85 |
| 26 weeks | Threshold | Sensitivity | Specificity | LLR+ | LLR– | |
| Laser speckle contrast imaging (PU) | ||||||
| Foot | ||||||
| Baseline | 41.9 PU | 0.454 | 0.722 | 0.412 | 1.23 | 0.67 |
| Biological zero | 10.9 PU | 0.540 | 0.639 | 0.588 | 1.55 | 0.61 |
| Post occlusion peak | 62.3 PU | 0.541 | 0.750 | 0.529 | 1.59 | 0.47 * |
| Ulcer | ||||||
| Baseline | 92.3 PU | 0.606 | 0.722 | 0.529 | 1.53 | 0.52 |
| Biological zero | 12.7 PU | 0.469 | 0.750 | 0.294 | 1.06 | 0.85 |
| Post occlusion peak | 109.5 PU | 0.609 | 0.472 | 0.765 | 2.01 * | 0.69 |
| Ulcer edge | ||||||
| Baseline | 118.5 PU | 0.525 | 0.278 | 0.941 | 4.72 * | 0.77 |
| Biological zero | 14.0 PU | 0.455 | 0.667 | 0.471 | 1.26 | 0.71 |
| Post occlusion peak | 123.0 PU | 0.547 | 0.306 | 0.882 | 2.60 * | 0.79 |
| Non-invasive blood pressure measurements (mmHg) | ||||||
| Ankle pressure | 96.0 mmHg | 0.619 | 0.824 | 0.438 | 1.46 | 0.40 * |
| Toe pressure | 54.0 mmHg | 0.626 | 0.824 | 0.438 | 1.46 | 0.40 * |
| Ankle brachial index | 0.89 | 0.619 | 0.618 | 0.625 | 1.65 | 0.61 |
| Toe brachial index | 0.51 | 0.618 | 0.735 | 0.500 | 1.47 | 0.53 |
| TcpO2 | 30.5 mmHg | 0.484 | 0.853 | 0.313 | 1.24 | 0.47 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mennes, O.A.; van Netten, J.J.; van Baal, J.G.; Slart, R.H.J.A.; Steenbergen, W. The Association between Foot and Ulcer Microcirculation Measured with Laser Speckle Contrast Imaging and Healing of Diabetic Foot Ulcers. J. Clin. Med. 2021, 10, 3844. https://doi.org/10.3390/jcm10173844
Mennes OA, van Netten JJ, van Baal JG, Slart RHJA, Steenbergen W. The Association between Foot and Ulcer Microcirculation Measured with Laser Speckle Contrast Imaging and Healing of Diabetic Foot Ulcers. Journal of Clinical Medicine. 2021; 10(17):3844. https://doi.org/10.3390/jcm10173844
Chicago/Turabian StyleMennes, Onno A., Jaap J. van Netten, Jeff G. van Baal, Riemer H. J. A. Slart, and Wiendelt Steenbergen. 2021. "The Association between Foot and Ulcer Microcirculation Measured with Laser Speckle Contrast Imaging and Healing of Diabetic Foot Ulcers" Journal of Clinical Medicine 10, no. 17: 3844. https://doi.org/10.3390/jcm10173844
APA StyleMennes, O. A., van Netten, J. J., van Baal, J. G., Slart, R. H. J. A., & Steenbergen, W. (2021). The Association between Foot and Ulcer Microcirculation Measured with Laser Speckle Contrast Imaging and Healing of Diabetic Foot Ulcers. Journal of Clinical Medicine, 10(17), 3844. https://doi.org/10.3390/jcm10173844







