Thyroid Function in Adults with Prader–Willi Syndrome; a Cohort Study and Literature Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Review
2.2. Statistical Analysis
3. Results
3.1. Baseline
3.2. Hypo- and Hyperthyroidism
3.3. Thyroid Hormone Levels
3.4. Psychotropic Drugs
3.5. Literature Review
3.6. Clinical Recommendations
4. Discussion
4.1. Vulnerability of the Patients
4.1.1. Exercise Intolerance and Cardiovascular Risk
4.1.2. Brain Function
4.2. Diagnostic Challenges
4.2.1. Patients’ Delay
4.2.2. Doctors’ Delay
4.2.3. Unreliability of TSH
4.3. Altered Thyroid Hormone Metabolism
4.3.1. Psychotropic Drugs
4.3.2. GH Treatment
4.3.3. Leptin and Ghrelin
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cassidy, S.B.; Schwartz, S.; Miller, J.L.; Driscoll, D.J. Prader-Willi syndrome. Genet. Med. 2012, 14, 10–26. [Google Scholar] [CrossRef] [Green Version]
- Cassidy, S.B.; Driscoll, D.J. Prader-Willi syndrome. Eur. J. Hum. Genet. 2009, 17, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Cheon, C.K. Genetics of Prader-Willi syndrome and Prader-Will-Like syndrome. Ann. Pediatric Endocrinol. Metab. 2016, 21, 126–135. [Google Scholar] [CrossRef]
- Mackay, J.; McCallum, Z.; Ambler, G.R.; Vora, K.; Nixon, G.; Bergman, P.; Shields, N.; Milner, K.; Kapur, N.; Crock, P.; et al. Requirements for improving health and well-being of children with Prader-Willi syndrome and their families. J. Paediatr. Child. Health 2019, 55, 1029–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muscogiuri, G.; Formoso, G.; Pugliese, G.; Ruggeri, R.M.; Scarano, E.; Colao, A. Prader- Willi syndrome: An uptodate on endocrine and metabolic complications. Rev. Endocr. Metab. Disord. 2019, 20, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Angulo, M.A.; Butler, M.G.; Cataletto, M.E. Prader-Willi syndrome: A review of clinical, genetic, and endocrine findings. J. Endocrinol. Invest. 2015, 38, 1249–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swaab, D.F. Prader-Willi syndrome and the hypothalamus. Acta Paediatr. 1997, 423, 50–54. [Google Scholar] [CrossRef] [Green Version]
- Burman, P.; Ritzen, E.M.; Lindgren, A.C. Endocrine dysfunction in Prader-Willi syndrome: A review with special reference to GH. Endocr. Rev. 2001, 22, 787–799. [Google Scholar] [CrossRef]
- Holm, V.A.; Cassidy, S.B.; Butler, M.G.; Hanchett, J.M.; Greenswag, L.R.; Whitman, B.Y.; Greenberg, F. Prader-Willi syndrome: Consensus diagnostic criteria. Pediatrics 1993, 91, 398–402. [Google Scholar]
- De Geronimo, V.; Cannarella, R.; La Vignera, S. Thyroid Function and Obesity: From Mechanisms to the Benefits of Levothyroxine in Obese Patient. Endocr. Metab. Immune Disord. Drug Targets 2021. [Google Scholar] [CrossRef]
- Akici, N.; Onal, Z.E.; Gurbuz, T.; Sag, C.; Kilinc, S. Atherogenic Indices in the Assessment of Cardiovascular Disease Risk in Children with Obesity and Subclinical Hypothyroidism. Acta Endocrinol. 2020, 16, 334–338. [Google Scholar] [CrossRef]
- Chaker, L.; Bianco, A.C.; Jonklaas, J.; Peeters, R.P. Hypothyroidism. Lancet 2017, 390, 1550–1562. [Google Scholar] [CrossRef]
- Butler, M.G.; Manzardo, A.M.; Heinemann, J.; Loker, C.; Loker, J. Causes of death in Prader-Willi syndrome: Prader-Willi Syndrome Association (USA) 40-year mortality survey. Genet. Med. 2017, 19, 635–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacoricona Alfaro, D.L.; Lemoine, P.; Ehlinger, V.; Molinas, C.; Diene, G.; Valette, M.; Pinto, G.; Coupaye, M.; Poitou-Bernert, C.; Thuilleaux, D.; et al. Causes of death in Prader-Willi syndrome: Lessons from 11 years’ experience of a national reference center. Orphanet J. Rare Dis. 2019, 14, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whittington, J.E.; Holland, A.J.; Webb, T.; Butler, J.; Clarke, D.; Boer, H. Population prevalence and estimated birth incidence and mortality rate for people with Prader-Willi syndrome in one UK Health Region. J. Med. Genet. 2001, 38, 792–798. [Google Scholar] [CrossRef] [Green Version]
- Klein, I.; Danzi, S. Thyroid Disease and the Heart. Curr. Probl. Cardiol. 2016, 41, 65–92. [Google Scholar] [CrossRef] [Green Version]
- Rotondi, M.; Magri, F.; Chiovato, L. Risk of coronary heart disease and mortality for adults with subclinical hypothyroidism. JAMA 2010, 304, 2481–2482. [Google Scholar] [CrossRef]
- Pellikaan, K.; Rosenberg, A.G.W.; Kattentidt-Mouravieva, A.A.; Kersseboom, R.; Bos-Roubos, A.G.; Veen-Roelofs, J.M.C.; van Wieringen, N.; Hoekstra, F.M.E.; van den Berg, S.A.A.; van der Lely, A.J.; et al. Missed Diagnoses and Health Problems in Adults With Prader-Willi Syndrome: Recommendations for Screening and Treatment. J. Clin. Endocrinol. Metab. 2020, 105, e4671–e4687. [Google Scholar] [CrossRef]
- Sanyal, D.; Raychaudhuri, M. Hypothyroidism and obesity: An intriguing link. Indian J. Endocrinol. Metab. 2016, 20, 554–557. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, X.; Fu, C.; Su, M.; Jiang, F.; Xu, D.; Li, R.; Qian, J.; Wang, N.; Chen, Y.; et al. Thyroid Stimulating Hormone (TSH) Is Associated With General and Abdominal Obesity: A Cohort Study in School-Aged Girls During Puberty in East China. Front. Endocrinol. 2020, 11, 620. [Google Scholar] [CrossRef]
- Chen, X.; Deng, S.; Sena, C.; Zhou, C.; Thaker, V.V. Relationship of TSH Levels with Cardiometabolic Risk Factors in US Youth and Reference Percentiles for Thyroid Function. J. Clin. Endocrinol. Metab. 2021, 106, e1221–e1230. [Google Scholar] [CrossRef]
- Knudsen, N.; Laurberg, P.; Rasmussen, L.B.; Bulow, I.; Perrild, H.; Ovesen, L.; Jorgensen, T. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J. Clin. Endocrinol. Metab. 2005, 90, 4019–4024. [Google Scholar] [CrossRef]
- Waring, A.C.; Arnold, A.M.; Newman, A.B.; Buzkova, P.; Hirsch, C.; Cappola, A.R. Longitudinal changes in thyroid function in the oldest old and survival: The cardiovascular health study all-stars study. J. Clin. Endocrinol. Metab. 2012, 97, 3944–3950. [Google Scholar] [CrossRef] [Green Version]
- Bremner, A.P.; Feddema, P.; Leedman, P.J.; Brown, S.J.; Beilby, J.P.; Lim, E.M.; Wilson, S.G.; O’Leary, P.C.; Walsh, J.P. Age-related changes in thyroid function: A longitudinal study of a community-based cohort. J. Clin. Endocrinol. Metab. 2012, 97, 1554–1562. [Google Scholar] [CrossRef] [Green Version]
- Festen, D.A.; Visser, T.J.; Otten, B.J.; Wit, J.M.; Duivenvoorden, H.J.; Hokken-Koelega, A.C. Thyroid hormone levels in children with Prader-Willi syndrome before and during growth hormone treatment. Clin. Endocrinol. 2007, 67, 449–456. [Google Scholar] [CrossRef]
- Michalaki, M.A.; Vagenakis, A.G.; Leonardou, A.S.; Argentou, M.N.; Habeos, I.G.; Makri, M.G.; Psyrogiannis, A.I.; Kalfarentzos, F.E.; Kyriazopoulou, V.E. Thyroid function in humans with morbid obesity. Thyroid 2006, 16, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, B.; Masserini, B.; Iorio, L.; Delnevo, A.; Malavazos, A.E.; Morricone, L.; Sburlati, L.F.; Orsi, E. Relationship of thyroid function with body mass index and insulin-resistance in euthyroid obese subjects. J. Endocrinol. Invest. 2010, 33, 640–643. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Platz, E.A.; Ladenson, P.W.; Mondul, A.M.; Menke, A.; de Gonzalez, A.B. Body fatness and markers of thyroid function among U.S. men and women. PLoS ONE 2012, 7, e34979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, J.; Zhang, L.; An, Y.; Duan, Y.; Liu, J.; Wang, G. Association Between Body Mass Index and Thyroid Function in Euthyroid Chinese Adults. Med. Sci Monit 2021, 27, e930865. [Google Scholar] [CrossRef]
- Meng, Z.; Liu, M.; Zhang, Q.; Liu, L.; Song, K.; Tan, J.; Jia, Q.; Zhang, G.; Wang, R.; He, Y.; et al. Gender and Age Impacts on the Association Between Thyroid Function and Metabolic Syndrome in Chinese. Medicine 2015, 94, e2193. [Google Scholar] [CrossRef]
- Iughetti, L.; Vivi, G.; Balsamo, A.; Corrias, A.; Crino, A.; Delvecchio, M.; Gargantini, L.; Greggio, N.A.; Grugni, G.; Hladnik, U.; et al. Thyroid function in patients with Prader-Willi syndrome: An Italian multicenter study of 339 patients. J. Pediatric Endocrinol. Metab. 2019, 32, 159–165. [Google Scholar] [CrossRef]
- Butler, M.G.; Theodoro, M.; Skouse, J.D. Thyroid function studies in Prader-Willi syndrome. Am. J. Med. Genet. A 2007, 143A, 488–492. [Google Scholar] [CrossRef]
- Tauber, M.; Barbeau, C.; Jouret, B.; Pienkowski, C.; Malzac, P.; Moncla, A.; Rochiccioli, P. Auxological and endocrine evolution of 28 children with Prader-Willi syndrome: Effect of GH therapy in 14 children. Horm. Res. Paediatr. 2000, 53, 279–287. [Google Scholar] [CrossRef]
- Vaiani, E.; Herzovich, V.; Chaler, E.; Chertkoff, L.; Rivarola, M.A.; Torrado, M.; Belgorosky, A. Thyroid axis dysfunction in patients with Prader-Willi syndrome during the first 2 years of life. Clin. Endocrinol. 2010, 73, 546–550. [Google Scholar] [CrossRef]
- Wong, K.; Levitsky, L.L.; Misra, M. Predictors and growth consequences of central hypothyroidism in pediatric patients receiving recombinant human growth hormone. J. Pediatric Endocrinol. Metab. 2010, 23, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Diene, G.; Mimoun, E.; Feigerlova, E.; Caula, S.; Molinas, C.; Grandjean, H.; Tauber, M.; French Reference Centre for PWS. Endocrine disorders in children with Prader-Willi syndrome--data from 142 children of the French database. Horm. Res. Paediatr. 2010, 74, 121–128. [Google Scholar] [CrossRef]
- Sharkia, M.; Michaud, S.; Berthier, M.T.; Giguere, Y.; Stewart, L.; Deladoey, J.; Deal, C.; Van Vliet, G.; Chanoine, J.P. Thyroid function from birth to adolescence in Prader-Willi syndrome. J. Pediatrics 2013, 163, 800–805. [Google Scholar] [CrossRef]
- Kim, Y.J.; Cheon, C.K. Prader-Willi syndrome: A single center’s experience in Korea. Korean J. Pediatrics 2014, 57, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Oto, Y.; Murakami, N.; Matsubara, K.; Saima, S.; Ogata, H.; Ihara, H.; Nagai, T.; Matsubara, T. Effects of growth hormone treatment on thyroid function in pediatric patients with Prader-Willi syndrome. Am. J. Med. Genet. A 2020, 182, 659–663. [Google Scholar] [CrossRef]
- Lu, A.; Luo, F.; Sun, C.; Zhang, X.; Wang, L.; Lu, W. Sleep-disordered breathing and genetic findings in children with Prader-Willi syndrome in China. Ann. Transl. Med. 2020, 8, 989. [Google Scholar] [CrossRef] [PubMed]
- Konishi, A.; Ida, S.; Shoji, Y.; Etani, Y.; Kawai, M. Central hypothyroidism improves with age in very young children with Prader-Willi syndrome. Clin. Endocrinol. 2020, 94, 384–391. [Google Scholar] [CrossRef]
- Dagdeviren Cakir, A.; Bas, F.; Akin, O.; Siklar, Z.; Ozcabi, B.; Berberoglu, M.; Kardelen, A.D.; Bayramoglu, E.; Poyrazoglu, S.; Aydin, M.; et al. Clinical Characteristics and Growth Hormone Treatment in Patients with Prader-Willi Syndrome. J. Clin. Res. Pediatric Endocrinol. 2021. [Google Scholar]
- Hoybye, C.; Hilding, A.; Jacobsson, H.; Thoren, M. Metabolic profile and body composition in adults with Prader-Willi syndrome and severe obesity. J. Clin. Endocrinol. Metab. 2002, 87, 3590–3597. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.L.; Goldstone, A.P.; Couch, J.A.; Shuster, J.; He, G.; Driscoll, D.J.; Liu, Y.; Schmalfuss, I.M. Pituitary abnormalities in Prader-Willi syndrome and early onset morbid obesity. Am. J. Med. Genet. A 2008, 146A, 570–577. [Google Scholar] [CrossRef]
- Mogul, H.R.; Lee, P.D.; Whitman, B.Y.; Zipf, W.B.; Frey, M.; Myers, S.; Cahan, M.; Pinyerd, B.; Southren, A.L. Growth hormone treatment of adults with Prader-Willi syndrome and growth hormone deficiency improves lean body mass, fractional body fat, and serum triiodothyronine without glucose impairment: Results from the United States multicenter trial. J. Clin. Endocrinol. Metab. 2008, 93, 1238–1245. [Google Scholar] [CrossRef] [Green Version]
- Farholt, S.; Sode-Carlsen, R.; Christiansen, J.S.; Østergaard, J.R.; Høybye, C. Normal cortisol response to high-dose synacthen and insulin tolerance test in children and adults with Prader-Willi syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E173–E180. [Google Scholar] [CrossRef]
- Laurier, V.; Lapeyrade, A.; Copet, P.; Demeer, G.; Silvie, M.; Bieth, E.; Coupaye, M.; Poitou, C.; Lorenzini, F.; Labrousse, F.; et al. Medical, psychological and social features in a large cohort of adults with Prader-Willi syndrome: Experience from a dedicated centre in France. J. Intellect. Disabil. Res. 2015, 59, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Coupaye, M.; Tauber, M.; Cuisset, L.; Laurier, V.; Bieth, E.; Lacorte, J.M.; Oppert, J.M.; Clement, K.; Poitou, C. Effect of Genotype and Previous GH Treatment on Adiposity in Adults With Prader-Willi Syndrome. J. Clin. Endocrinol. Metab. 2016, 101, 4895–4903. [Google Scholar] [CrossRef]
- Proffitt, J.; Osann, K.; McManus, B.; Kimonis, V.E.; Heinemann, J.; Butler, M.G.; Stevenson, D.A.; Gold, J.A. Contributing factors of mortality in Prader-Willi syndrome. Am. J. Med. Genet. A 2019, 179, 196–205. [Google Scholar] [CrossRef]
- Pemmasani, G.; Yandrapalli, S. Age-stratified prevalence of relevant comorbidities and etiologies for hospitalizations in Prader–Willi syndrome patients. Am. J. Med. Genet. Part A 2021, 185, 600–601. [Google Scholar] [CrossRef]
- van Nieuwpoort, I.C.; Sinnema, M.; Castelijns, J.A.; Twisk, J.W.; Curfs, L.M.; Drent, M.L. The GH/IGF-I axis and pituitary function and size in adults with Prader-Willi syndrome. Horm. Res. Paediatr. 2011, 75, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Sinnema, M.; Maaskant, M.A.; van Schrojenstein Lantman-de Valk, H.M.; van Nieuwpoort, I.C.; Drent, M.L.; Curfs, L.M.; Schrander-Stumpel, C.T. Physical health problems in adults with Prader-Willi syndrome. Am. J. Med. Genet. A 2011, 155A, 2112–2124. [Google Scholar] [CrossRef] [PubMed]
- Grugni, G.; Crino, A.; Bedogni, G.; Cappa, M.; Sartorio, A.; Corrias, A.; Di Candia, S.; Gargantini, L.; Iughetti, L.; Pagano, C.; et al. Metabolic syndrome in adult patients with Prader-Willi syndrome. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1134–1140. [Google Scholar] [CrossRef]
- Radetti, G.; Fanolla, A.; Lupi, F.; Sartorio, A.; Grugni, G. Accuracy of different indexes of body composition and adiposity in identifying metabolic syndrome in adult subjects with Prader-Willi syndrome. J. Clin. Med. 2020, 9, 1646. [Google Scholar] [CrossRef] [PubMed]
- Paepegaey, A.C.; Coupaye, M.; Jaziri, A.; Ménesguen, F.; Dubern, B.; Polak, M.; Oppert, J.M.; Tauber, M.; Pinto, G.; Poitou, C. Impact of transitional care on endocrine and anthropometric parameters in Prader-Willi syndrome. Endocr. Connect. 2018, 7, 663–672. [Google Scholar] [CrossRef] [Green Version]
- Garmendia Madariaga, A.; Santos Palacios, S.; Guillen-Grima, F.; Galofre, J.C. The incidence and prevalence of thyroid dysfunction in Europe: A meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 923–931. [Google Scholar] [CrossRef] [Green Version]
- Kaminsky, P.; Robin-Lherbier, B.; Brunotte, F.; Escanye, J.M.; Walker, P.; Klein, M.; Robert, J.; Duc, M. Energetic metabolism in hypothyroid skeletal muscle, as studied by phosphorus magnetic resonance spectroscopy. J. Clin. Endocrinol. Metab. 1992, 74, 124–129. [Google Scholar] [CrossRef]
- Yavuz, S.; Del Prado, S.S.N.; Celi, F.S. Thyroid Hormone Action and Energy Expenditure. J. Endocr. Soc. 2019, 3, 1345–1356. [Google Scholar] [CrossRef]
- Butler, M.G.; Theodoro, M.F.; Bittel, D.C.; Donnelly, J.E. Energy expenditure and physical activity in Prader-Willi syndrome: Comparison with obese subjects. Am. J. Med. Genet. A 2007, 143A, 449–459. [Google Scholar] [CrossRef] [Green Version]
- Schoeller, D.A.; Levitsky, L.L.; Bandini, L.G.; Dietz, W.W.; Walczak, A. Energy expenditure and body composition in Prader-Willi syndrome. Metabolism 1988, 37, 115–120. [Google Scholar] [CrossRef]
- Biondi, B. Thyroid and obesity: An intriguing relationship. J. Clin. Endocrinol. Metab. 2010, 95, 3614–3617. [Google Scholar] [CrossRef] [Green Version]
- Bernal, J. Thyroid hormones and brain development. Vitam. Horm. 2005, 71, 95–122. [Google Scholar] [CrossRef]
- Samuels, M.H. Psychiatric and cognitive manifestations of hypothyroidism. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 377–383. [Google Scholar] [CrossRef] [Green Version]
- Correia, N.; Mullally, S.; Cooke, G.; Tun, T.K.; Phelan, N.; Feeney, J.; Fitzgibbon, M.; Boran, G.; O’Mara, S.; Gibney, J. Evidence for a specific defect in hippocampal memory in overt and subclinical hypothyroidism. J. Clin. Endocrinol. Metab. 2009, 94, 3789–3797. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, L.A.; Ganguli, M.; Dodge, H.H.; Toczek, T.; DeKosky, S.T.; Nebes, R.D. Hypothyroidism and cognition: Preliminary evidence for a specific defect in memory. Thyroid 2001, 11, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.D.; Tremont, G. Neuropsychiatric aspects of hypothyroidism and treatment reversibility. Minerva Endocrinol. 2007, 32, 49–65. [Google Scholar]
- Gulseren, S.; Gulseren, L.; Hekimsoy, Z.; Cetinay, P.; Ozen, C.; Tokatlioglu, B. Depression, anxiety, health-related quality of life, and disability in patients with overt and subclinical thyroid dysfunction. Arch. Med. Res. 2006, 37, 133–139. [Google Scholar] [CrossRef]
- Constant, E.L.; Adam, S.; Seron, X.; Bruyer, R.; Seghers, A.; Daumerie, C. Anxiety and depression, attention, and executive functions in hypothyroidism. J. Int. Neuropsychol. Soc. 2005, 11, 535–544. [Google Scholar] [CrossRef]
- Kuhlmann, L.; Joensson, I.M.; Froekjaer, J.B.; Krogh, K.; Farholt, S. A descriptive study of colorectal function in adults with Prader-Willi Syndrome: High prevalence of constipation. BMC Gastroenterol. 2014, 14, 63. [Google Scholar] [CrossRef] [Green Version]
- Persani, L.; Ferretti, E.; Borgato, S.; Faglia, G.; Beck-Peccoz, P. Circulating thyrotropin bioactivity in sporadic central hypothyroidism. J. Clin. Endocrinol. Metab. 2000, 85, 3631–3635. [Google Scholar] [CrossRef]
- Horimoto, M.; Nishikawa, M.; Ishihara, T.; Yoshikawa, N.; Yoshimura, M.; Inada, M. Bioactivity of thyrotropin (TSH) in patients with central hypothyroidism: Comparison between in vivo 3,5,3′-triiodothyronine response to TSH and in vitro bioactivity of TSH. J. Clin. Endocrinol. Metab. 1995, 80, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Lania, A.; Persani, L.; Beck-Peccoz, P. Central hypothyroidism. Pituitary 2008, 11, 181. [Google Scholar] [CrossRef] [PubMed]
- Ramschak-Schwarzer, S.; Radkohl, W.; Stiegler, C.; Dimai, H.P.; Leb, G. Interaction between psychotropic drugs and thyroid hormone metabolism—An overview. Acta Med. Austriaca 2000, 27, 8–10. [Google Scholar] [CrossRef]
- Sauvage, M.F.; Marquet, P.; Rousseau, A.; Raby, C.; Buxeraud, J.; Lachatre, G. Relationship between psychotropic drugs and thyroid function: A review. Toxicol. Appl. Pharmacol. 1998, 149, 127–135. [Google Scholar] [CrossRef]
- Bonnot, O.; Cohen, D.; Thuilleaux, D.; Consoli, A.; Cabal, S.; Tauber, M. Psychotropic treatments in Prader-Willi syndrome: A critical review of published literature. Eur. J. Pediatrics 2016, 175, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Bou Khalil, R.; Richa, S. Thyroid adverse effects of psychotropic drugs: A review. Clin. Neuropharmacol. 2011, 34, 248–255. [Google Scholar] [CrossRef] [Green Version]
- van der Spek, A.H.; Fliers, E.; Boelen, A. The classic pathways of thyroid hormone metabolism. Mol. Cell Endocrinol. 2017, 458, 29–38. [Google Scholar] [CrossRef]
- Bianco, A.C.; Salvatore, D.; Gereben, B.; Berry, M.J.; Larsen, P.R. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr. Rev. 2002, 23, 38–89. [Google Scholar] [CrossRef]
- Mullur, R.; Liu, Y.Y.; Brent, G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef] [Green Version]
- Rezvani, I.; DiGeorge, A.M.; Dowshen, S.A.; Bourdony, C.J. Action of human growth hormone (hGH) on extrathyroidal conversion of thyroxine (T4) to triiodothyronine (T3) in children with hypopituitarism. Pediatric Res. 1981, 15, 6–9. [Google Scholar] [CrossRef] [Green Version]
- Jorgensen, J.O.; Pedersen, S.A.; Laurberg, P.; Weeke, J.; Skakkebaek, N.E.; Christiansen, J.S. Effects of growth hormone therapy on thyroid function of growth hormone-deficient adults with and without concomitant thyroxine-substituted central hypothyroidism. J. Clin. Endocrinol. Metab. 1989, 69, 1127–1132. [Google Scholar] [CrossRef]
- Behan, L.A.; Monson, J.P.; Agha, A. The interaction between growth hormone and the thyroid axis in hypopituitary patients. Clin. Endocrinol. 2011, 74, 281–288. [Google Scholar] [CrossRef]
- Pirazzoli, P.; Cacciari, E.; Mandini, M.; Sganga, T.; Capelli, M.; Cicognani, A.; Gualandi, S. Growth and thyroid function in children treated with growth hormone. J. Pediatrics 1992, 121, 210–213. [Google Scholar] [CrossRef]
- Portes, E.S.; Oliveira, J.H.; MacCagnan, P.; Abucham, J. Changes in serum thyroid hormones levels and their mechanisms during long-term growth hormone (GH) replacement therapy in GH deficient children. Clin. Endocrinol. 2000, 53, 183–189. [Google Scholar] [CrossRef]
- Jorgensen, J.O.; Ovesen, P.; Juul, A.; Hansen, T.K.; Skakkebaek, N.E.; Christiansen, J.S. Impact of growth hormone administration on other hormonal axes. Horm. Res. 1999, 51 (Suppl. 3), 121–126. [Google Scholar] [CrossRef]
- Agha, A.; Walker, D.; Perry, L.; Drake, W.M.; Chew, S.L.; Jenkins, P.J.; Grossman, A.B.; Monson, J.P. Unmasking of central hypothyroidism following growth hormone replacement in adult hypopituitary patients. Clin. Endocrinol. 2007, 66, 72–77. [Google Scholar] [CrossRef]
- Smyczynska, J.; Hilczer, M.; Stawerska, R.; Lewinski, A. Thyroid function in children with growth hormone (GH) deficiency during the initial phase of GH replacement therapy-clinical implications. Thyroid. Res. 2010, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Butler, M.G.; Smith, B.K.; Lee, J.; Gibson, C.; Schmoll, C.; Moore, W.V.; Donnelly, J.E. Effects of growth hormone treatment in adults with Prader-Willi syndrome. Growth Horm. IGF Res. 2013, 23, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Cettour-Rose, P.; Burger, A.G.; Meier, C.A.; Visser, T.J.; Rohner-Jeanrenaud, F. Central stimulatory effect of leptin on T3 production is mediated by brown adipose tissue type II deiodinase. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E980–E987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinehr, T. Obesity and thyroid function. Mol. Cell Endocrinol. 2010, 316, 165–171. [Google Scholar] [CrossRef]
- Tauber, M.; Coupaye, M.; Diene, G.; Molinas, C.; Valette, M.; Beauloye, V. Prader-Willi syndrome: A model for understanding the ghrelin system. J. Neuroendocrinol. 2019, 31, e12728. [Google Scholar] [CrossRef]
- Cummings, D.E.; Clement, K.; Purnell, J.Q.; Vaisse, C.; Foster, K.E.; Frayo, R.S.; Schwartz, M.W.; Basdevant, A.; Weigle, D.S. Elevated plasma ghrelin levels in Prader Willi syndrome. Nat. Med. 2002, 8, 643–644. [Google Scholar] [CrossRef]
- Kordi, F.; Khazali, H. The effect of ghrelin and estradiol on mean concentration of thyroid hormones. Int. J. Endocrinol. Metab. 2015, 13, e17988. [Google Scholar] [CrossRef] [Green Version]

| Total | |
|---|---|
| n = 122 | |
| Age in years, median (IQR) | 29 (21–39) |
| BMI in kg/m2, median (IQR) | 29 (26–36) |
| Male gender, n (%) | 58 (48%) |
| Genetic subtype | |
| Deletion, n (%) | 66 (54%) |
| mUPD, n (%) a | 43 (35%) |
| ICD, n (%) | 3 (2%) |
| Unknown, n (%) | 10 (8%) |
| Growth hormone treatment | |
| Only during childhood, n (%) | 12 (10%) |
| Only during adulthood, n (%) | 3 (2%) |
| Both, n (%) | 44 (36%) |
| Never, n (%) | 63 (52%) |
| Current growth hormone treatment, n (%) | 43 (35%) |
| Use of hydrocortisone | |
| Daily, n (%) | 4 (3%) |
| During physical or psychological stress, n (%) | 49 (40%) |
| Use of estrogen replacement therapy or oral contraceptives before screening, n (%) | 34/64 females (53%) |
| Use of testosterone replacement therapy before screening, n (%) | 24/58 males (41%) |
| Use of thyroid hormone replacement therapy before screening, n (%) | 19 (16%) |
| Living situation | |
| With family, n (%) | 31 (25%) |
| In a specialized PWS group home, n (%) | 24 (20%) |
| In a non-specialized facility, n (%) | 67 (55%) |
| Education level | |
| Secondary vocational education, n (%) | 6 (5%) |
| Pre-vocational secondary education, n (%) | 3 (2%) |
| Special education, n (%) | 87 (71%) |
| No education, n (%) | 5 (4%) |
| Unknown, n (%) | 21 (17%) |
| Missing | Total n = 122 | Males n = 58 | Females n = 64 | p-Value | Deletion n = 66 | mUPD n = 43 | p-Value | |
|---|---|---|---|---|---|---|---|---|
| n of males, n of females | 0 | 58, 64 | 58, 0 | 0, 64 | NA | 39, 37 | 21, 22 | NA |
| Hypothyroidism, n (%) | 0 | 21 (17%) | 6 (10%) | 15 (23%) | 0.051 | 12 (18%) | 7 (16%) | 0.8 |
| Subclinical hypothyroidism, n (%) | 0 | 3 (2%) | 0 (0%) | 3 (5%) | NA | 1 (2%) | 2 (5%) | NA |
| Hyperthyroidism, n (%) | 0 | 1 (1%) | 0 (0%) | 1 (2%) | NA | 0 (0%) | 0 (0%) | NA |
| n of males, n of females with normal thyroid function (n = 97) | 0 | 52, 45 | 52, 0 | 0, 45 | NA | 26, 27 | 19, 15 | NA |
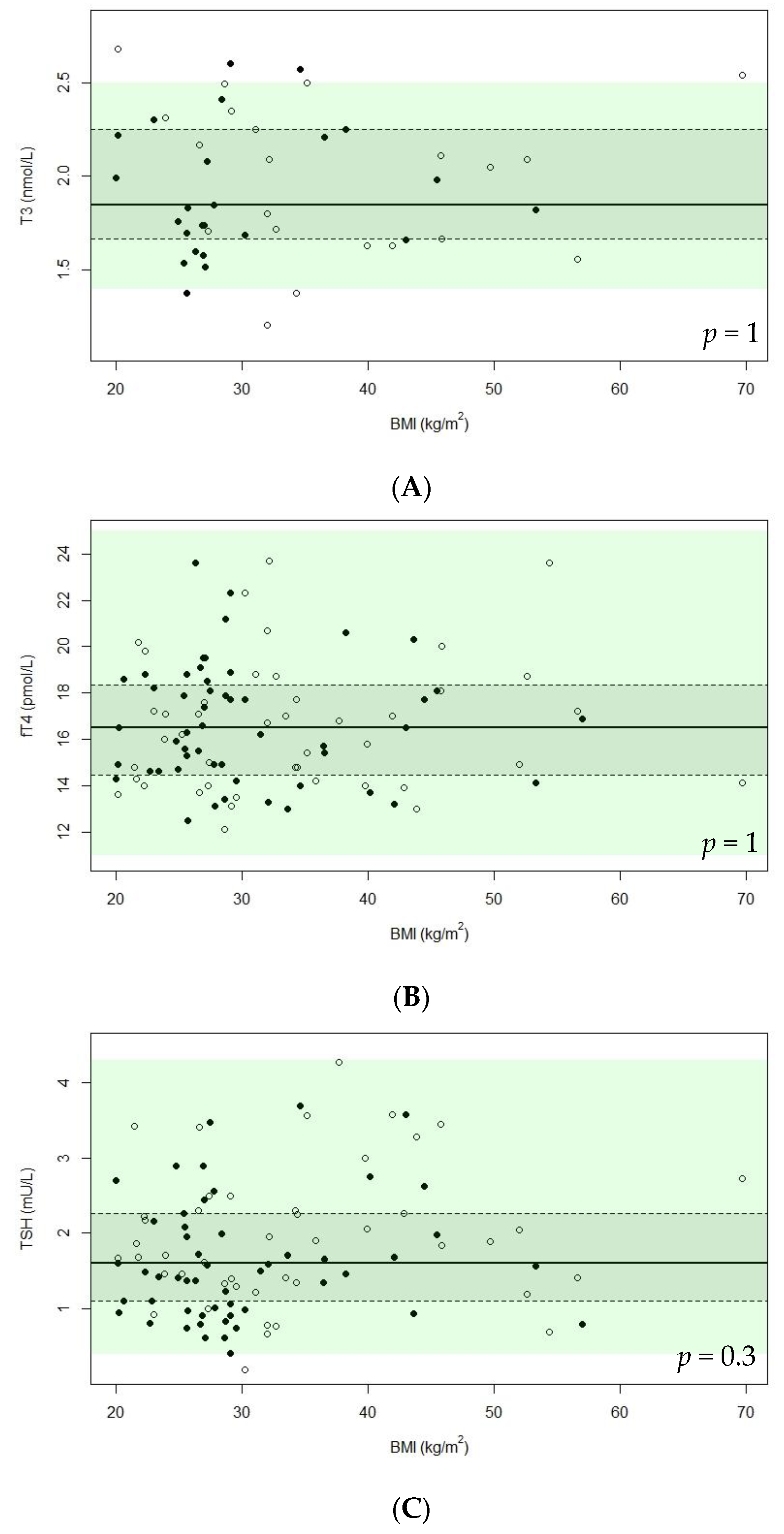
| fT4 (pmol/L), median (IQR) (n = 97) | 2 | 16.5 (14.3–18.5) | 16.5 (14.6–18.5) | 16.5 (14.1–18.6) | 0.7 | 16.2 (14.1–17.7) | 17.1 (14.8–18.9) | 0.2 |
| T3 (nmol/L), median (IQR) (n = 97) | 52 | 1.9 (1.7–2.3) | 1.8 (1.7–2.2) | 2.1 (1.7–2.3) | 0.5 | 2.0 (1.7–2.3) | 1.7 (1.5–2.3) | 0.2 |
| TSH (mU/L), median (IQR) (n = 97) | 0 | 1.6 (1.1–2.3) | 1.5 (1.0–2.1) | 1.9 (1.3–2.4) | 0.06 | 1.7 (1.4–2.3) | 1.5 (1.0–2.3) | 0.06 |
| Age < 25 years n = 47 | Age 25–30 years n = 22 | Age > 30 years n = 53 | p-Value | BMI < 25 kg/m2 n = 25 | BMI 25–30 kg/m2 n = 45 | BMI > 30 kg/m2 n = 52 | p-Value | |
|---|---|---|---|---|---|---|---|---|
| n of males, n of females | 21, 26 | 9, 13 | 28, 25 | NA | 12, 13 | 29, 16 | 17, 35 | NA |
| Hypothyroidism, n (%) | 10 (21%) | 6 (27%) | 5 (9%) | 0.4 | 5 (20%) | 8 (18%) | 8 (15%) | 0.6 |
| Subclinical hypothyroidism, n (%) | 1 (2%) | 1 (5%) | 1 (2%) | NA | 0 (0%) | 1 (2%) | 2 (4%) | NA |
| Hyperthyroidism, n (%) | 0 (0%) | 0 (0%) | 1 (2%) | NA | 0 (0%) | 1 (2%) | 0 (0%) | NA |
| n of males, n of females with normal thyroid function (n = 97) | 18, 18 | 8, 7 | 26, 20 | NA | 11, 9 | 25, 10 | 16, 26 | NA |
| fT4 (pmol/L), median (IQR) (n = 97) | 16.5 (14.9–18.1) | 15.4 (14.0–19.1) | 16.7 (14.1–18.9) | 0.2 | 15.9 (14.6–18.2) | 16.6 (14.2–18.8) | 16.7 (14.2–18.4) | 1 |
| T3 (nmol/L), median (IQR) (n = 97) | 2.1 (1.8–2.2) | 2.2 (1.8–2.5) | 1.7 (1.6–1.9) | 0.003 | 2.3 (1.9–2.4) | 1.7 (1.6–2.3) | 1.9 (1.7–2.2) | 1 |
| TSH (mU/L), median (IQR) (n = 97) | 1.6 (1.2–2.2) | 1.7 (1.4–2.7) | 1.5 (0.9–2.3) | 0.6 | 1.6 (1.2–2.2) | 1.4 (0.9–2.3) | 1.8 (1.3–2.7) | 0.3 |
| Current GH Treatment n = 43 | No Current GH Treatment n = 79 | p-Value | Psychotropic Drugs n = 49 | No Psychotropic Drugs n = 73 | p-Value | |
|---|---|---|---|---|---|---|
| n of males, n of females | 19, 24 | 39, 40 | NA | 25, 24 | 33, 40 | NA |
| Hypothyroidism, n (%) | 8 (19%) | 13 (16%) | 0.8 | 10 (20%) | 11 (15%) | 0.5 |
| Subclinical hypothyroidism, n (%) | 2 (5%) | 1 (1%) | NA | 1 (2%) | 2 (3%) | NA |
| Hyperthyroidism, n (%) | 0 (0%) | 1 (1%) | NA | 0 (0%) | 1 (1%) | NA |
| n of males, n of females with normal thyroid function (n = 97) | 17, 16 | 35, 29 | NA | 21, 17 | 31, 28 | NA |
| fT4 (pmol/L), median (IQR) (n = 97) | 16.0 (14.3–18.0) | 16.9 (14.5–18.8) | 0.3 | 16.6 (14.7–18.7) | 16.3 (14.1–18.1) | 0.8 |
| T3 (nmol/L), median (IQR) (n = 97) | 2.1 (1.8–2.4) | 1.8 (1.6–2.2) | 0.03 | 1.7 (1.6–2.0) | 2.1 (1.7–2.3) | 0.02 |
| TSH (mU/L), median (IQR) (n = 97) | 1.6 (1.2–2.0) | 1.6 (1.0–2.3) | 0.8 | 1.5 (0.9–2.5) | 1.7 (1.2–2.2) | 0.7 |
| Article | n | Country | Age Range (years) | Genotype (Deletion, mUPD, ICD, Translocation) | Gender | Mean BMI (kg/m2) | Current GH Treatment | |
|---|---|---|---|---|---|---|---|---|
| Children | Tauber et al. (2000) [33] | 28 | France | - | 36%, 14%, 0%, 0% (25% NA, 18% no genetic analysis, 7% no genetic abnormality) | 12 M, 16 F | - | 50% |
| Festen et al. (2007) [25] | 75 | The Netherlands | Median (IQR): 4.7 (2.7–7.6) | 35%, 32%, 9%, 1% (23% NA) | 39 M, 36 F | Median (IQR): 18 (16–20) | 0% | |
| Vaiani et al. (2010) [34] | 18 | Argentina | 0–2 | 61%, 28%, 0%, 0% (11% NA) | 11 M, 7 F | - | - | |
| Wong et al. (2010) [35] | 20 | USA | Mean ± SD: 4.0 ± 0.8 | - | 12 M, 8 F | 20 | 0% | |
| Diene et al. (2010) [36] | 127 | France | 0–18 a | 63%, 25%, 2%, 1% (1% other, 8% NA) a | 77 M, 65 F a | Median BMI Z-score +1.3 a | 87% a | |
| Sharkia et al. (2013) [37] | 31 b TRH-ST: 21 Neonates: 23 | Canada | TRH-ST: 0–18 Neonates: 0 | TRH-ST: 62%, 29%, 0%, 0% (10% NA) Neonates: 43%, 48%, 0%, 0% (9% NA) | TRH-ST: 7 M, 14 F Neonates: 9 M, 14 F | TRH-ST: mean BMI Z-score +1.2 Neonates: NA | TRH-ST: 86% Neonates: 0% | |
| Kim et al. (2014) [38] | 14 | Korea | 0–3 c | 93%, 7%, 0%, 0% c | 16 M, 14 F c | BMI-SDS: 0.66 | 0% | |
| Iughetti et al. (2019) [31] | 243 | Italy | 0–18 | 57%, 34%, 0%, 1% (8% NA) d | 233 M, 106 F d | 20 | 27% | |
| Oto et al. (2020) [39] | 51 | Japan | 0–7 | 61%, 39%, 0%, 0% | 29 M, 22 F | - | - | |
| Lu et al. (2020) [40] | 48 | China | 0–15 | 77%, 23%, 0%, 0% | 32 M, 16 F | Mean BMI Z-score: 0.8 | 0% | |
| Konishi et al. (2020) [41] | 43 | Japan | 0–3 | 60%, 40%, 0%, 0% | 17 M, 26 F | Median BMI-SDS: -1.47 | 0% | |
| Dağdeviren Çakır et al. (2021) [42] | 52 | Turkey | 0–15 | 69%, 12%, 2%, 0% (17% NA) | 26 M, 26 F | 20 | 40% | |
| Children and Adults | Höybye et al. (2002) [43] | 13 e | Sweden | 17–37 | - | 7 M, 6 F | 35 | - |
| Butler et al. (2007) [32] | 47 | USA | 10–44 | 55%, 45%, 0%, 0% | 21 M, 26 F | 34 | 0% | |
| Miller et al. (2008) [44] | 27 | USA | 0–39 | 74%, 26%, 0%, 0% | 17 M, 10 F | Obesity: 74% | 0% | |
| Mogul et al. (2008) [45] | 38 | USA | 17–49 | - | 13 M, 25 F | 35 | 0% f | |
| Farholt et al. (2011) [46] | 65 | Denmark | 0–48 | 65%, 20%, 3%, 0% (12% NA) | 33 M, 32 F | Median BMI-SDS: 0.92 | 62% | |
| Laurier et al. (2015) [47] | 154 | France | 16–54 | 66%, 16%, 2%, 2% (15% NA) | 68 M, 86 F | 42 | 14% | |
| Coupaye et al. (2016) [48] g | 73 | France | 16–58 | 64%, 36%, 0%, 0% | 35 M, 38 F | Deletion: 41, mUPD: 35 | 15% | |
| Proffitt et al. (2019) [49] | 2029 | USA | 0–84 | 42%, 19%, 2%, 0% (37% NA) | 934 M, 1000 F | Living: 29, deceased: 52 | Living: 51%, deceased: 22% | |
| Pemmasani et al. (2021) [50] | 480 | USA | Mean ± SD: 27 ± 19 | - | 242 M, 238 F | Obesity: 41% | - | |
| Adults | Van Nieuwpoort et al. (2011) [51,52] h | 15 | The Netherlands | 19–42 | 93%, 7%, 0%, 0% | 4 M, 11 F | Median: 28 | 0% |
| Sinnema et al. (2011) [52] h | 102 | The Netherlands | 18–66 | 54%, 43%, 3%, 0% | 49 M, 53 F | 32 | 5% | |
| Grugni et al. (2013) [53] i | 108 | Italy | 18–43 | 68%, 25%, 0%, 2% (6% NA) | 47 M, 61 F | Median in non-obese: 26, median in obese: 45 | - | |
| Iughetti et al. (2019) [31] i | 96 | Italy | 19–50 | 57%, 34%, 0%, 1% (8% NA) d | 233 M, 106 F d | 43 | - | |
| Radetti et al. (2020) [54] i | 120 | Italy | 18–59 | 71%, 28%, 0%, 0% (2% NA) | 69 M, 51 F | 37 | 20% |
| Article | Total Overt Hypothyroidism (Central, Primary Hypothyroidism) (%) | Subclinical Hypothyroidism (%) | TSH (mU/L) | Free T4 (pmol/L) | |
|---|---|---|---|---|---|
| Children | Tauber et al. (2000) [33] | 32 | - | - | Mean ± SD: 8.1 ± 1.1 pg/mL Mean: 10 nmol/L |
| Festen et al. (2007) [25] | 6 a | - | Median (IQR): 2.0 (1.6–2.7) mU/L | Median (IQR): 16.2 (14.3–17.8) pmol/L | |
| Vaiani et al. (2010) [34] | 72 b | - | Median (range): TAD: 1.4 (0.8–5.7) mU/L NTAD: 2.9 (1.4–5.3) mU/L | Median (range): TAD: 9.1 (2.6–11.8) pmol/L NTAD: 12.9 (12.1–21.0) pmol/L | |
| Wong et al. (2010) [35] | 0 | - | - | - | |
| Diene et al. (2010) [36] | 24 | - | - | - | |
| Sharkia et al. (2013) [37] | TRH-ST: 5 c Neonates: 0 e | - | Mean ± SD (range) TRH-ST: 1.9 ± 1.0 (0.8–4.2) mU/L d Neonates: 3.1 ± 2.3 (0.4–10.0) mU/L e | Mean ± SD (range) TRH-ST: 10.4 ± 1.1 (8.2–13.5) pmol/L d | |
| Kim et al. (2014) [38] | - | - | Mean ± SD: 3.2 ± 2.1 mU/L | Mean ± SD: 1.1 ± 0.2 ng/dL Mean: 14 pmol/L | |
| Iughetti et al. (2019) [31] | 11 (8, 2) f | 5 | Mean ± SD (median): 2.7 ± 2.1 (2.2) mU/L | Mean ± SD (median): 10.6 ± 2.2 (10.3) pg/mL Mean: 13.6 pmol/L, median: 13.3 pmol/L | |
| Oto et al. (2020) [39] | TRH-ST: 4 c | - | Median (IQR): 2.3 (1.2–3.6) mU/L | Median (IQR): 1.18 (1.02–1.24) ng/dL Median (IQR): 15 (13 – 16) pmol/L | |
| Lu et al. (2020) [40] | - | - | - | ≤2 years old: mean ± SD: 0.7 ± 0.2 ng/dL >2 years old: mean ± SD: 0.9 ± 0.2 ng/dL ≤2 years old: mean: 9 pmol/L >2 years old: mean: 12 pmol/L | |
| Konishi et al. (2020) [41] | 30 | - | Median (IQR): 2.4 (1.8–3.4) mU/L | Median (IQR): 11.6 (9.9–14.0) pmol/L | |
| Dağdeviren Çakır et al. (2021) [42] | 33 (31, 2) | - | - | - | |
| Children and Adults | Höybye et al. (2002) [43] | 0 | - | - | - |
| Butler et al. (2007) [32] | 2 (2, 0) | - | Mean ± SD: 2.2 ± 1.3 mU/L | Mean ± SD: 1.1 ± 0.2 ng/dL (n = 43) Mean: 14 pmol/L | |
| Miller et al. (2008) [44] | NA (19, NA) g | - | - | - | |
| Mogul et al. (2008) [45] | 0 | - | Mean ± SD (range): 1.5 ± 0.2 (0.01–7.7) mU/L | Mean ± SD (range): 1.1 ± 0.04 (0.6–1.7) ng/dL Mean (range): 14 (8–22) pmol/L | |
| Farholt et al. (2011) [46] | 5 | - | - | - | |
| Laurier et al. (2015) [47] | 26 | - | - | - | |
| Coupaye et al. (2016) [48] | 26 h | - | - | Mean ± SD: Deletion: 14.0 ± 2.0 pmol/L UPD: 15.1 ± 2.7 pmol/L | |
| Proffitt et al. (2019) [49] | 9 | - | - | - | |
| Pemmasani et al. (2021) [50] | 16 | - | - | - | |
| Adults | Van Nieuwpoort et al. (2011) [51] | 13 i | - | Median (IQR): 2.3 (1.85) mU/L | Median (IQR): 15.4 (1.9) pmol/L |
| Sinnema et al. (2011) [52] | 9 | - | - | - | |
| Grugni et al. (2013) [53] | 5 | - | - | - | |
| Iughetti et al. (2019) [31] | 6 (4, 2) | 1 | Mean ± SD (median): 2.2 ± 1.4 (2.0) mU/L | Mean ± SD (median): 11.4 ± 2.0 (11.2) pg/mL Mean: 15 pmol/L, median: 14 pmol/L | |
| Radetti et al. (2020) [54] | 10 | - | - | - |
| Article | Total T4 (nmol/L) | Free T3 (pmol/L) | Total T3 (nmol/L) | Reverse T3 (nmol/L) | |
|---|---|---|---|---|---|
| Children | Tauber et al. (2000) [33] | - | - | Mean ± SD: 118 ± 3.1 ng/dL Mean: 1.8 pmol/L | - |
| Festen et al. (2007) [25] | Median (IQR): 98.0 (85.3–113) nmol/L | - | Median (IQR): 2.6 (2.3–3.0) nmol/L | Median (IQR): 0.3 (0.3–0.4) nmol/L | |
| Vaiani et al. (2010) [34] | Median (range): TAD: 88.8 (57.9–109) nmol/L NTAD: 112 (86.2–126) nmol/L | - | Median (range): TAD: 2.5 (1.4–3.2) nmol/L NTAD: 2.4 (2.3–3.0) nmol/L | - | |
| Wong et al. (2010) [35] | - | - | - | - | |
| Diene et al. (2010) [36] | - | - | - | - | |
| Sharkia et al. (2013) [37] | - - | Mean ± SD (range) TRH-ST: 6.1 ± 1.0 (4.8–8.4) pmol/L a | - | - | |
| Kim et al. (2014) [38] | - | - | - | - | |
| Iughetti et al. (2019) [31] | - | Mean ± SD (median): 3.7 ± 1.0 (3.6) pg/mL Mean: 5.7 pmol/L, median: 5.5 pmol/L | - | - | |
| Oto et al. (2020) [39] | - | Median (IQR): 4.0 (3.5–4.4) pg/mL Median (IQR): 6.2 (5.4–6.8) pmol/L | - | - | |
| Lu et al. (2020) [40] | ≤2 years old: mean ± SD: 7.5 ± 1.7 µg/dL >2 years old: mean ± SD: 9.0 ± 2.5 µg/dL ≤2 years old: mean: 96.5 nmol/L >2 years old: mean: 116 nmol/L | - | - | - | |
| Konishi et al. (2020) [41] | - | Median (IQR): 4.8 (4.1–5.6) pmol/L | - | - | |
| Dağdeviren Çakır et al. (2021) [42] | - | - | - | - | |
| Children and Adults | Höybye et al. (2002) [43] | - | - | - | - |
| Butler et al. (2007) [32] | Mean ± SD: 8.1 ± 2.0 μg/dL (n = 38) Mean: 104 nmol/L | - | Mean ± SD: 137 ± 38 ng/dL(n = 41) Mean: 2.1 nmol/L | - | |
| Miller et al. (2008) [44] | - | - | - | - | |
| Mogul et al. (2008) [45] | Mean ± SD (range): 8.6 ± 0.3 (4.2–14.4) μg/dL Mean (range): 111 (54–185) nmol/L | - | Mean ± SD (range): 131 ± 8 (46–251) ng/dL Mean (range): 2.0 (0.7–3.9) nmol/L | - | |
| Farholt et al. (2011) [46] | - | - | - | - | |
| Laurier et al. (2015) [47] | - | - | - | - | |
| Coupaye et al. (2016) [48] | - | - | - | - | |
| Proffitt et al. (2019) [49] | - | - | - | - | |
| Pemmasani et al. (2021) [50] | - | - | - | - | |
| Adults | Van Nieuwpoort et al. (2011) [51] | - | Median (IQR): 5.1 (0.8) pmol/L | - | - |
| Sinnema et al. (2011) [52] | - | - | - | - | |
| Grugni et al. (2013) [53] | - | - | - | - | |
| Iughetti et al. (2019) [31] | - | Mean ± SD (median): 3.1 ± 0.7 (3.0) pg/mL Mean: 4.7 pmol/L, median: 4.6 pmol/L | - | - | |
| Radetti et al. (2020) [54] | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellikaan, K.; Snijders, F.; Rosenberg, A.G.W.; Davidse, K.; van den Berg, S.A.A.; Visser, W.E.; van der Lely, A.J.; de Graaff, L.C.G. Thyroid Function in Adults with Prader–Willi Syndrome; a Cohort Study and Literature Review. J. Clin. Med. 2021, 10, 3804. https://doi.org/10.3390/jcm10173804
Pellikaan K, Snijders F, Rosenberg AGW, Davidse K, van den Berg SAA, Visser WE, van der Lely AJ, de Graaff LCG. Thyroid Function in Adults with Prader–Willi Syndrome; a Cohort Study and Literature Review. Journal of Clinical Medicine. 2021; 10(17):3804. https://doi.org/10.3390/jcm10173804
Chicago/Turabian StylePellikaan, Karlijn, Fleur Snijders, Anna G. W. Rosenberg, Kirsten Davidse, Sjoerd A. A. van den Berg, W. Edward Visser, Aart J. van der Lely, and Laura C. G. de Graaff. 2021. "Thyroid Function in Adults with Prader–Willi Syndrome; a Cohort Study and Literature Review" Journal of Clinical Medicine 10, no. 17: 3804. https://doi.org/10.3390/jcm10173804
APA StylePellikaan, K., Snijders, F., Rosenberg, A. G. W., Davidse, K., van den Berg, S. A. A., Visser, W. E., van der Lely, A. J., & de Graaff, L. C. G. (2021). Thyroid Function in Adults with Prader–Willi Syndrome; a Cohort Study and Literature Review. Journal of Clinical Medicine, 10(17), 3804. https://doi.org/10.3390/jcm10173804







