Anti-Obesity Medication Use in Children and Adolescents with Prader–Willi Syndrome: Case Review and Literature Search
Abstract
1. Introduction
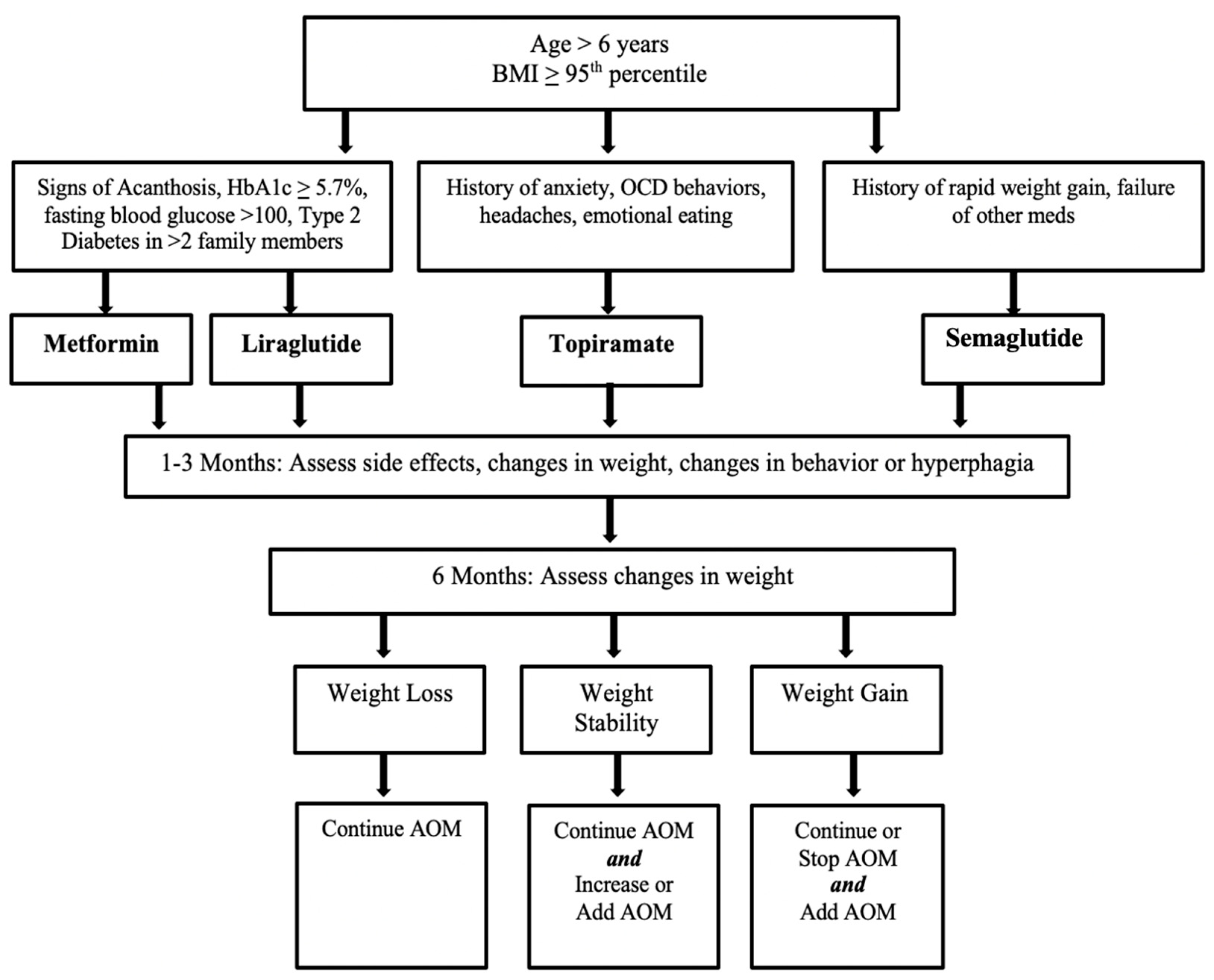
2. Materials and Methods
3. Results
3.1. Summary of Literature Review
3.1.1. Topiramate
3.1.2. Metformin
3.1.3. Phentermine
3.1.4. Glucagon-Like Peptide-1 (GLP-1) Receptor Agonist
3.1.5. Orlistat
3.1.6. Oxytocin
3.1.7. Naltrexone-Bupropion
3.1.8. Other Medications under Investigation
3.2. Case Series Results
3.2.1. Participants
3.2.2. Metformin
3.2.3. Topiramate
3.2.4. Glucagon-Like Peptide-1 Agonist
Liraglutide
Semaglutide
3.2.5. Combination Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cassidy, S.B.; Schwartz, S.; Miller, J.L.; Driscoll, D.J. Prader-Willi syndrome. Genet. Med. 2011, 14, 10–26. [Google Scholar] [CrossRef]
- Harris, R.M.; Stafford, D.E. Prader Willi syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 56–62. [Google Scholar] [CrossRef]
- Tan, Q.; Orsso, C.E.; Deehan, E.C.; Triador, L.; Field, C.; Tun, H.M.; Han, J.C.; Müller, T.D.; Haqq, A.M. Current and emerging therapies for managing hyperphagia and obesity in Prader-Willi syndrome: A narrative review. Obes. Rev. 2019, 21, e12992. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.L.; Linville, T.D.; Dykens, E.M. Effects of metformin in children and adolescents with Prader-Willi syndrome and early-onset morbid obesity: A pilot study. J. Pediatr. Endocrinol. Metab. 2013, 27, 23–29. [Google Scholar] [CrossRef][Green Version]
- Carias, K.V.; Wevrick, R. Preclinical Testing in Translational Animal Models of Prader-Willi Syndrome: Overview and Gap Analysis. Mol. Ther. Methods Clin. Dev. 2019, 13, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Kimonis, V.; Surampalli, A.; Wencel, M.; Gold, J.-A.; Cowen, N.M. A randomized pilot efficacy and safety trial of diazoxide choline controlled-release in patients with Prader-Willi syndrome. PLoS ONE 2019, 14, e0221615. [Google Scholar] [CrossRef] [PubMed]
- McCandless, S.E.; Yanovski, J.; Miller, J.; Fu, C.; Bird, L.; Salehi, P.; Chan, C.L.; Stafford, D.; Abuzzahab, M.J.; Viskochil, D.; et al. Effects of MetAP2 inhibition on hyperphagia and body weight in Prader–Willi syndrome: A randomized, double-blind, placebo-controlled trial. Diabetes Obes. Metab. 2017, 19, 1751–1761. [Google Scholar] [CrossRef] [PubMed]
- Bischof, J.M.; Van Der Ploeg, L.H.T.; Colmers, W.F.; Wevrick, R. Magel2-null mice are hyper-responsive to setmelanotide, a melanocortin 4 receptor agonist. Br. J. Pharmacol. 2016, 173, 2614–2621. [Google Scholar] [CrossRef]
- Shapira, N.A.; Lessig, M.C.; Lewis, M.H.; Goodman, W.K.; Driscoll, D.J. Effects of topiramate in adults with Prader-Willi syndrome. Am. J. Ment. Retard. 2004, 109, 301–309. [Google Scholar] [CrossRef]
- Shapira, N.A.; Lessig, M.C.; Murphy, T.K.; Driscoll, D.J.; Goodman, W.K. Topiramate attenuates self-injurious behaviour in Prader–Willi syndrome. Int. J. Neuropsychopharmacol. 2002, 5, 141–145. [Google Scholar] [CrossRef]
- Kramer, C.K.; Leitao, C.; Pinto, L.C.; Canani, L.H.; Azevedo, M.J.; Gross, J.L. Efficacy and safety of topiramate on weight loss: A meta-analysis of randomized controlled trials. Obes. Rev. 2011, 12, e338–e347. [Google Scholar] [CrossRef]
- Consoli, A.; Berthoumieu, S.; Raffin, M.; Thuilleaux, D.; Poitou, C.; Coupaye, M.; Pinto, G.; Lebbah, S.; Zahr, N.; Tauber, M.; et al. Effect of topiramate on eating behaviours in Prader-Willi syndrome: TOPRADER double-blind randomised placebo-controlled study. Transl. Psychiatry 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Smathers, S.A.; Wilson, J.G.; Nigro, M.A. Topiramate effectiveness in Prader-Willi syndrome. Pediatric Neurol. 2003, 28, 130–133. [Google Scholar] [CrossRef]
- East, N.; Maroney, M. Topiramate in the treatment of Prader-Willi syndrome: A case report. Ment. Health Clin. 2017, 7, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Thomas, I.; Gregg, B. Metformin; a review of its history and future: From lilac to longevity. Pediatr. Diabetes 2017, 18, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Malin, S.; Kashyap, S. Effects of metformin on weight loss. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Yerevanian, A.; Soukas, A.A. Metformin: Mechanisms in Human Obesity and Weight Loss. Curr. Obes. Rep. 2019, 8, 156–164. [Google Scholar] [CrossRef]
- Lentferink, Y.E.; Knibbe, C.A.J.; Van Der Vorst, M.M.J. Efficacy of Metformin Treatment with Respect to Weight Reduction in Children and Adults with Obesity: A Systematic Review. Drugs 2018, 78, 1887–1901. [Google Scholar] [CrossRef]
- Smith, S.M.; Meyer, M.E.; Trinkley, K. Phentermine/Topiramate for the Treatment of Obesity. Ann. Pharmacother. 2013, 47, 340–349. [Google Scholar] [CrossRef]
- Senda, M.; Ogawa, S.; Nako, K.; Okamura, M.; Sakamoto, T.; Ito, S. The glucagon-like peptide-1 analog liraglutide suppresses ghrelin and controls diabetes in a patient with Prader-Willi syndrome. Endocr. J. 2012, 59, 889–894. [Google Scholar] [CrossRef]
- Cyganek, K.; Koblik, T.; Kozek, E.; Wojcik, M.; Starzyk, J.; Malecki, M.T. Liraglutide therapy in Prader-Willi syndrome. Diabet. Med. 2011, 28, 755–756. [Google Scholar] [CrossRef] [PubMed]
- Fintini, D.; Grugni, G.; Brufani, C.; Bocchini, S.; Cappa, M.; Crinò, A. Use of GLP-1 Receptor Agonists in Prader-Willi Syndrome: Report of Six Cases. Diabetes Care 2014, 37, e76–e77. [Google Scholar] [CrossRef]
- Chao, A.M.; Wadden, T.A.; Berkowitz, R.I. The safety of pharmacologic treatment for pediatric obesity. Expert Opin. Drug Saf. 2018, 17, 379–385. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Lee, Y.J.; Kim, S.Y.; Cheon, C.K.; Lim, H.H. Successful rapid weight reduction and the use of liraglutide for morbid obesity in adolescent Prader-Willi syndrome. Ann. Pediatr. Endocrinol. Metab. 2020, 25, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Candler, T.; McGregor, D.; Narayan, K.; Moudiotis, C.; Burren, C. Improvement in glycaemic parameters using SGLT-2 inhibitor and GLP-1 agonist in combination in an adolescent with diabetes mellitus and Prader-Willi syndrome: A case report. J. Pediatr. Endocrinol. Metab. 2020, 33, 951–955. [Google Scholar] [CrossRef]
- Sano, H.; Kudo, E.; Yamazaki, T.; Ito, T.; Hatakeyama, K.; Kawamura, N. Efficacy of sodium-glucose cotransporter 2 inhibitor with glucagon-like peptide-1 receptor agonist for the glycemic control of a patient with Prader-Willi syndrome: A case report. Clin. Pediatr. Endocrinol. 2020, 29, 81–84. [Google Scholar] [CrossRef]
- Christou, G.; Katsiki, N.; Blundell, J.; Fruhbeck, G.; Kiortsis, D.N. Semaglutide as a promising antiobesity drug. Obes. Rev. 2019, 20, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, L.B.; Lau, J. The Discovery and Development of Liraglutide and Semaglutide. Front. Endocrinol. 2019, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Saunders, K.H.; Umashanker, D.; Igel, L.I.; Kumar, R.B.; Aronne, L.J. Obesity Pharmacotherapy. Med. Clin. N. Am. 2018, 102, 135–148. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, E.A.; Evans, C.V.; Burda, B.U.; Walsh, E.S.; Eder, M.; Lozano, P. Screening for Obesity and Intervention for Weight Management in Children and Adolescents. JAMA 2017, 317, 2427–2444. [Google Scholar] [CrossRef]
- Lawson, E.A. The effects of oxytocin on eating behaviour and metabolism in humans. Nat. Rev. Endocrinol. 2017, 13, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, D.; Betley, J.N.; Su, H.H.; Sternson, S.M. Deconstruction of a neural circuit for hunger. Nature 2012, 488, 172–177. [Google Scholar] [CrossRef]
- McCormack, S.E.; Blevins, J.; Lawson, E.A. Metabolic Effects of Oxytocin. Endocr. Rev. 2019, 41, 121–145. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.L.; Tamura, R.; Butler, M.G.; Kimonis, V.; Sulsona, C.; Gold, J.-A.; Driscoll, D.J. Oxytocin treatment in children with Prader-Willi syndrome: A double-blind, placebo-controlled, crossover study. Am. J. Med. Genet. Part A 2017, 173, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Einfeld, S.L.; Smith, E.; Mcgregor, I.; Steinbeck, K.; Taffe, J.; Rice, L.; Horstead, S.K.; Rogers, N.; Hodge, M.A.; Guastella, A.J. A double-blind randomized controlled trial of oxytocin nasal spray in Prader Willi syndrome. Am. J. Med. Genet. Part A 2014, 164, 2232–2239. [Google Scholar] [CrossRef] [PubMed]
- Kuppens, R.; Donze, S.; Hokken-Koelega, A. Promising effects of oxytocin on social and food-related behaviour in young children with Prader-Willi syndrome: A randomized, double-blind, controlled crossover trial. Clin. Endocrinol. 2016, 85, 979–987. [Google Scholar] [CrossRef]
- Damen, L.; Grootjen, L.N.; Juriaans, A.F.; Donze, S.H.; Huisman, T.M.; Visser, J.A.; Delhanty, P.J.; Hokken-Koelega, A.C. Oxytocin in young children with Prader-Willi syndrome: Results of a randomized, double-blind, placebo-controlled, crossover trial investigating 3 months of oxytocin. Clin. Endocrinol. 2020, 94, 774–785. [Google Scholar] [CrossRef]
- Hollander, E.; Levine, K.G.; Ferretti, C.J.; Freeman, K.; Doernberg, E.; Desilva, N.; Taylor, B.P. Intranasal oxytocin versus placebo for hyperphagia and repetitive behaviors in children with Prader-Willi Syndrome: A randomized controlled pilot trial. J. Psychiatr. Res. 2021, 137, 643–651. [Google Scholar] [CrossRef]
- Yanovski, S.Z.; Yanovski, J. Naltrexone Extended-Release Plus Bupropion Extended-Release for Treatment of Obesity. JAMA 2015, 313, 1213–1214. [Google Scholar] [CrossRef]
- Puri, M.R.; Sahl, R.; Ogden, S.; Malik, S. Prader–Willi Syndrome, Management of Impulsivity, and Hyperphagia in an Adolescent. J. Child Adolesc. Psychopharmacol. 2016, 26, 403–404. [Google Scholar] [CrossRef]
- Srivastava, G.; Apovian, C. Future Pharmacotherapy for Obesity: New Anti-obesity Drugs on the Horizon. Curr. Obes. Rep. 2018, 7, 147–161. [Google Scholar] [CrossRef]
- Astrup, A.; Madsbad, S.; Breum, L.; Jensen, T.J.; Kroustrup, J.P.; Larsen, T.M. Effect of tesofensine on bodyweight loss, body composition, and quality of life in obese patients: A randomised, double-blind, placebo-controlled trial. Lancet 2008, 372, 1906–1913. [Google Scholar] [CrossRef]
- Poitou, C.; Mosbah, H.; Clément, K. Mechanisms in endocrinology: Update on treatments for patients with genetic obesity. Eur. J. Endocrinol. 2020, 183, R149–R166. [Google Scholar] [CrossRef]
- Bischof, J.M.; Wevrick, R. Chronic diazoxide treatment decreases fat mass and improves endurance capacity in an obese mouse model of Prader-Willi syndrome. Mol. Genet. Metab. 2018, 123, 511–517. [Google Scholar] [CrossRef]
- Allas, S.; Caixàs, A.; Poitou, C.; Coupaye, M.; Thuilleaux, D.; Lorenzini, F.; Diene, G.; Crinò, A.; Illouz, F.; Grugni, G.; et al. AZP-531, an unacylated ghrelin analog, improves food-related behavior in patients with Prader-Willi syndrome: A randomized placebo-controlled trial. PLoS ONE 2018, 13, e0190849. [Google Scholar] [CrossRef] [PubMed]
- Tester, J.M.; Rosas, L.G.; Leung, C.W. Food Insecurity and Pediatric Obesity: A Double Whammy in the Era of COVID-19. Curr. Obes. Rep. 2020, 9, 442–450. [Google Scholar] [CrossRef]
- Kang, H.M.; Jeong, D.C.; Suh, B.-K.; Ahn, M.B. The Impact of the Coronavirus Disease-2019 Pandemic on Childhood Obesity and Vitamin D Status. J. Korean Med. Sci. 2021, 36, e21. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Barrea, L.; Faggiano, F.; Maiorino, M.I.; Parrillo, M.; Pugliese, G.; Ruggeri, R.M.; Scarano, E.; Savastano, S.; Colao, A.; et al. Obesity in Prader–Willi syndrome: Physiopathological mechanisms, nutritional and pharmacological approaches. J. Endocrinol. Investig. 2021, 44, 2057–2070. [Google Scholar] [CrossRef]
- Crinò, A.; Fintini, D.; Bocchini, S.; Grugni, G. Obesity management in Prader–Willi syndrome: Current perspectives. Diabetes, Metab. Syndr. Obesity Targets Ther. 2018, 11, 579–593. [Google Scholar] [CrossRef] [PubMed]
| Article | Date | AOMs | Study Design | Sample | Effectiveness | Adverse Events |
|---|---|---|---|---|---|---|
| Smathers et al. [13] | 2003 | Topiramate | Open Label study | 7 individuals Ages: 10–18 years 3 female, 4 male | All with weight loss or reduced weight gain All with improved mood, decreased aggression, less obsessive-compulsive behaviors | 3 patients had increased somnolence, all of whom improved with altered dosage or administration timing |
| East and Maroney [14] | 2018 | Topiramate | Case Report | 11-year-old male | Reduced aggression and “demand for food” following topiramate No impact on BMI | No side effects reported |
| Consoli et al. [12] | 2019 | Topiramate | Double-blind, randomized placebo-controlled study—TOPRADER study: 32 placebo 30 topiramate Duration: 8 weeks | 62 individuals Ages: 12–45 year 2 female, 30 male | Decreased BMI trend, but without statistical significance Dose-dependent improvement in hyperphagia behavior (Dykens Hyperphagia Questionnaire) | 4 patients with sedative effects. 2 patients with infectious episode (bronchiolitis, asthma, sinusitis). Both placebo and topiramate groups had several individuals with biological modifications in hepatic function (3 vs. 4), hyperammonemia (2 vs. 4) |
| Miller et al. [4] | 2014 | Metformin | Pilot Study | 21 individuals; 6 with early morbid obesity Ages: 7–17 years 11 female, 10 male | Improvement in “food-related distress,” anxiety, ability to be redirected away from food (Hyperphagia Questionnaire) 5 of 13 parents of children with PWS reported children feeling full (often for the first time) No significant weight loss in PWS | 7 out of 10 males with PWS reported worsening behavioral problems All of those who stopped metformin had subsequent weight gain |
| Cyganek et al. [21] | 2011 | Liraglutide (+Metformin) | Case Report | 17-year-old female with diabetes | HbA1c decreased 1.9% and body mass by 3.2 kg over 14 weeks | No hypoglycemia or other side effects |
| Kim et al. [24] | 2020 | Liraglutide | Case Report | 18-year-old female | Continued previous regimen of metformin, insulin detemir, growth hormone, estrogen Following hospital discharge, was able to maintain BMI with decreased HbA1c while on newly added liraglutide | No reported side effects |
| Candler et al [25] | 2020 | Liraglutide + Empagliflozin | Case Report | 13-year-old with diabetes | Decrease in HbA1c and glucose on combination of liraglutide + empagliflozin No decrease in glucose or HbA1c while on metformin + insulin or metformin + liraglutide | No reported side effects |
| Sano et al. [26] | 2020 | Liraglutide + Empagliflozin | Case Report | 19-year-old female with diabetes | With Liraglutide: HbA1c decreased 1.3% after 4 months; No significant change in body weight With addition of empagliflozin, had 7.4% weight loss and 2% decrease in HbA1c | No reported side effects |
| Einfeld et al. [35] | 2014 | Oxytocin | Randomized, double-blind, placebo-controlled, crossover trial: 8 weeks of oxytocin, 2-week washout, 8 weeks placebo | 30 individuals Ages: 12–30 years 10 female, 20 male | Oxytocin had little impact on any measure | Increase in temper outbursts with higher doses of oxytocin |
| Kuppens et al. [36] | 2016 | Oxytocin | Randomized, double-blind, placebo-controlled, crossover trial: Intranasal oxytocin vs. placebo Duration: 4 weeks | 25 indiviuduals Ages: 6–14 years 11 female, 14 male | No change in social behavior or hyperphagia in total group In children younger than 11 years, parents reported decreased sadness, anger, conflict, as well as improvement in food-related and social behaviors No significant change in BMI | In children older than 11 years, increased anger and sadness and decreased happiness in oxytocin group No adverse events or other reported side effects |
| Miller et al. [34] | 2017 | Oxytocin | double-blind, placebo-controlled crossover study: 5 days of intranasal oxytocin vs. 5 days placebo, followed by 4-week washout | 24 individuals Ages: 5–11 years 9 female, 15 male | Decrease in overall anxiety, self-injurious behavior Improvement in socialization, appetite No change in weight | Nasal irritation 4 with increased irritability, resolved |
| Damen et al. [37] | 2021 | Oxytocin | Randomized, double-blind, placebo-controlled, crossover trial: Twice daily intranasal oxytocin (dose range 16–40 IU/day) versus placebo Duration: 3 months | 26 individuals with PWS Ages: 3–11 years 13 female, 13 male | No significant change in social behavior or hyperphagia were found in total group Oxytocin had positive impact on social and eating behaviors in boys with PWS and children with PWS who had a deletion | No significant side effects |
| Hollander et al. [38] | 2021 | Oxytocin | Randomized, double-blind, placebo-controlled trial: 11 oxytocin, 12 placebo Duration: 8 weeks | 23 individuals Ages: 5–18 years 5 female, 18 male | Placebo was associated with improvement in hyperphagia and repetitive behaviors; oxytocin was not Oxytocin well-tolerated | Nocturia in individuals given oxytocin |
| Puri et al. [40] | 2016 | Naltrexone-Bupropion | Case Report | 13-year-old female | Improved eating habits and BMI Decreased aggression | No reported side effects |
| Case | Sex | Age (Year) | Genetic Mutation | Other Medical Conditions | Chronic Medications | Anti-Obesity Medication | zBMI Change (≥12 Weeks on AOM) | Side Effect Profile | Behavioral Change (Parental Report) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 12 | Uniparental Disomy | Anxiety OCD | Somatropin, coenzyme Q10 | Metformin 1000 mg twice daily | −0.35 * | No diarrhea, vomiting, abdominal pain | Improved food-seeking behaviors |
| 2 | F | 10 | Maternal Isodisomy | Asthma Seasonal allergies | Montelukast, fluticasone, cetirizine | Topiramate 100 mg nightly | −0.11 * | No nausea, abdominal pain, fatigue, brain fog | Continued hyperphagia |
| 3 | M | 10 | Deletion of 15q11.2–13 | Allergies Insomnia Psychosis | Aripiprazole, guanfacine, cetirizine, fluticasone | Metformin 1000 mg twice daily | 0.0 * | No side effects | Continued compulsive aggressive outbursts |
| 4 | M | 14 | Deletion of 15q11.2–13 | Hypogonadotropic hypogonadism | Depo-Testosterone | Topiramate 100 mg nightly | +0.05 * | No fatigue or brain fog | Improved behavior, worsened hyperphagia |
| 5 | M | 12 | Uniparental Disomy | Asthma Allergies OSA Hypogonadotropic hypogonadism | Fluticasone albuterol, loratadine | Topiramate 100 mg nightly | −0.02 | Increased sleepiness | |
| Above medications + tiotropium bromide, montelukast | Metformin 1000 mg twice daily | −0.03 * | No side effects | ||||||
| Above medications + Depo-Testosterone, symbicort | Semaglutide 1 mg weekly | −0.02 * | Nausea after semaglutide injections (resolved after 2 months) | ||||||
| 6 | M | 12 | Deletion of 15q11.2–13 | Asthma Allergies OSA Hypogonadotropic hypogonadism Anxiety | Flonase, albuterol, loratadine | Metformin 1000 mg twice daily | +0.02 | No nausea or vomiting | |
| Above medications + Depo-Testosterone, olanzapine, oxcarbazepine, sertraline | Semaglutide 1 mg weekly | +0.02 * | No side effects | Anti-psychotic medications and pandemic have worsened hyperphagia | |||||
| 7 | F | 17 | Deletion of 15q11.2–13 | Type 2 diabetes Hypogonadotropic hypogonadism Anxiety OCD | Basaglar, Humalog, Vivelle | Topiramate 100 mg nightly | −0.01 * | No side effects | Decreased appetite and impulse to eat |
| Above medications | Liraglutide 3 mg daily | −0.02 * | No side effects | ||||||
| 8 | M | 15 | Deletion of 15q11.2–13 | Hypogonadotropic hypogonadism Anxiety | Depo-Testosterone | Metformin 1000 mg twice daily | +0.02 | No side effects | Improved hyperphagia but continued weight gain |
| Above Medications | Topiramate 100 mg nightly | +0.01 | No side effects | Continued weight gain during pandemic | |||||
| 9 | F | 18 | Deletion of 15q11.2–13 | Hypertension hypogonadotropic Hypogonadism Type 2 Diabetes | Enalapril, estradiol, Lantus, progesterone | Liraglutide 3 mg daily | 0.0 * | No side effects | Hyperphagia worse during pandemic |
| 10 | M | 12 | Deletion of 15q11.2–13 | Growth hormone deficiency Asthma Hypogonadotropic hypogonadism | Somatotropin, Albuterol | Metformin 1000 mg twice daily | +0.02 | No abdominal pain or nausea | Increased activity |
| Above medications + Depo-Testosterone, | Liraglutide 3 mg daily | +0.02 * | No side effects |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goldman, V.E.; Naguib, M.N.; Vidmar, A.P. Anti-Obesity Medication Use in Children and Adolescents with Prader–Willi Syndrome: Case Review and Literature Search. J. Clin. Med. 2021, 10, 4540. https://doi.org/10.3390/jcm10194540
Goldman VE, Naguib MN, Vidmar AP. Anti-Obesity Medication Use in Children and Adolescents with Prader–Willi Syndrome: Case Review and Literature Search. Journal of Clinical Medicine. 2021; 10(19):4540. https://doi.org/10.3390/jcm10194540
Chicago/Turabian StyleGoldman, Victoria E., Monica N. Naguib, and Alaina P. Vidmar. 2021. "Anti-Obesity Medication Use in Children and Adolescents with Prader–Willi Syndrome: Case Review and Literature Search" Journal of Clinical Medicine 10, no. 19: 4540. https://doi.org/10.3390/jcm10194540
APA StyleGoldman, V. E., Naguib, M. N., & Vidmar, A. P. (2021). Anti-Obesity Medication Use in Children and Adolescents with Prader–Willi Syndrome: Case Review and Literature Search. Journal of Clinical Medicine, 10(19), 4540. https://doi.org/10.3390/jcm10194540






