Spinal Cord Motion in Degenerative Cervical Myelopathy: The Level of the Stenotic Segment and Gender Cause Altered Pathodynamics
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Imaging Protocol
2.3. MRI Data Processing
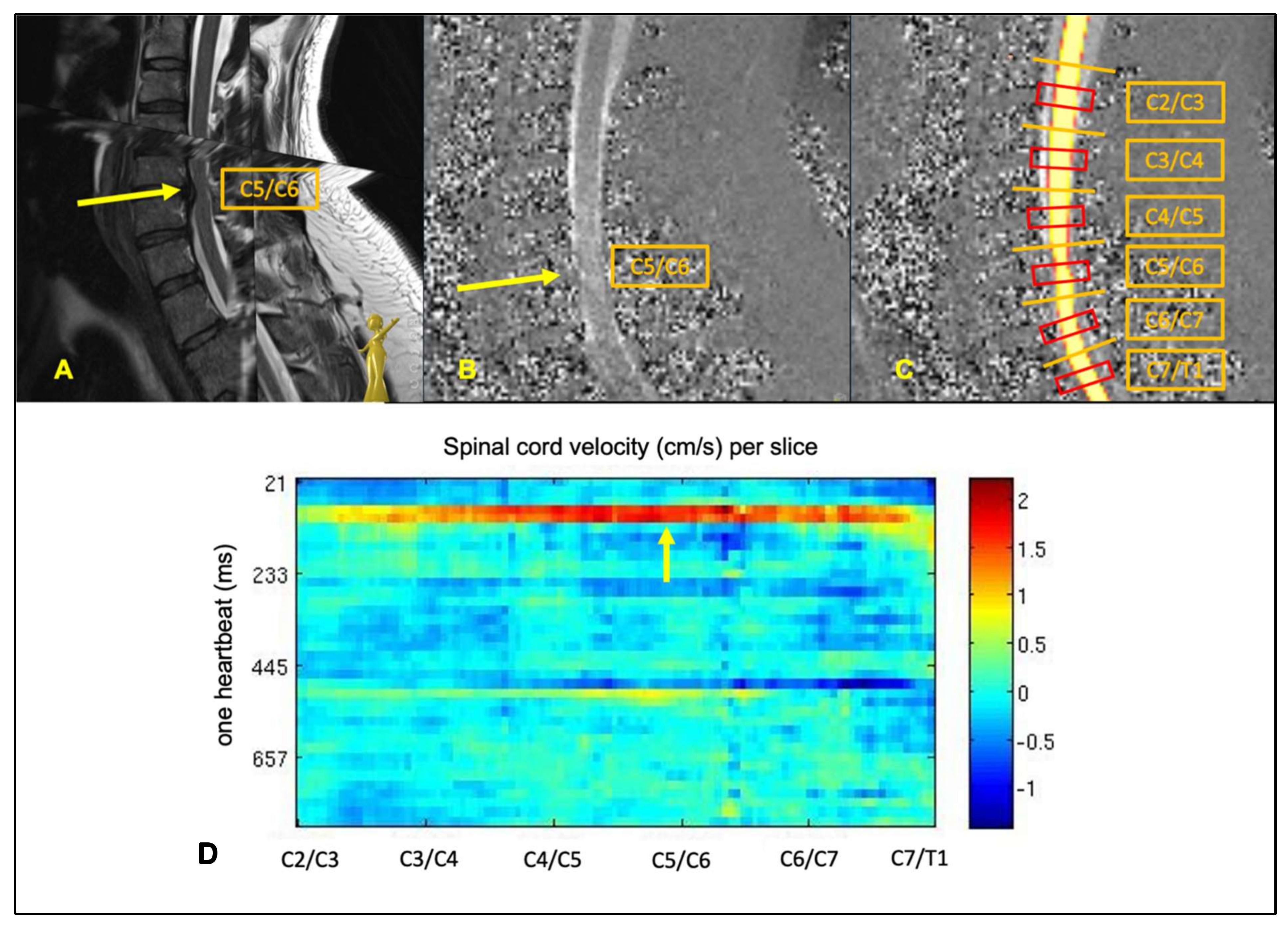
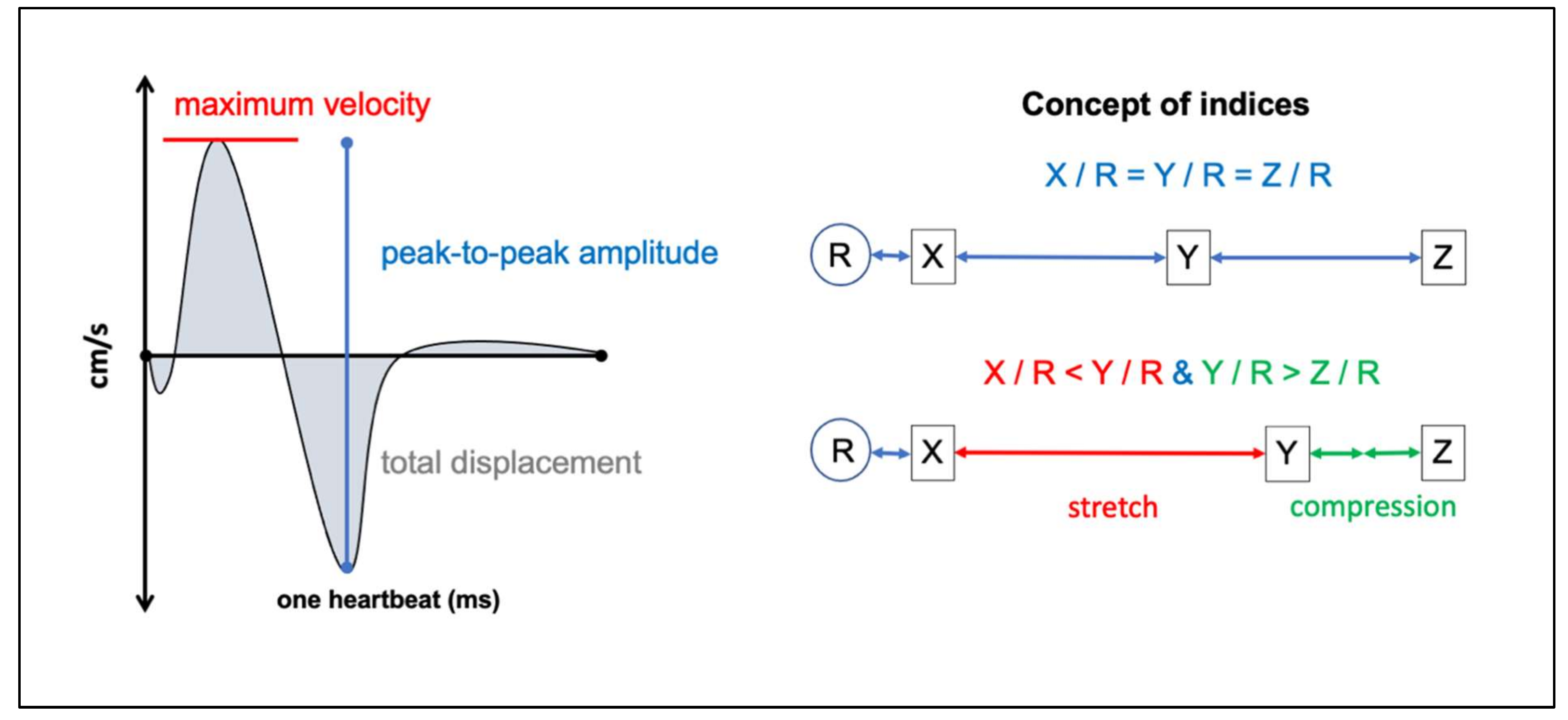
2.4. PC-MRI Parameters
2.5. Anatomical MRI-Parameters
2.6. Data Validity
2.7. Statistics
3. Results
3.1. Study Population
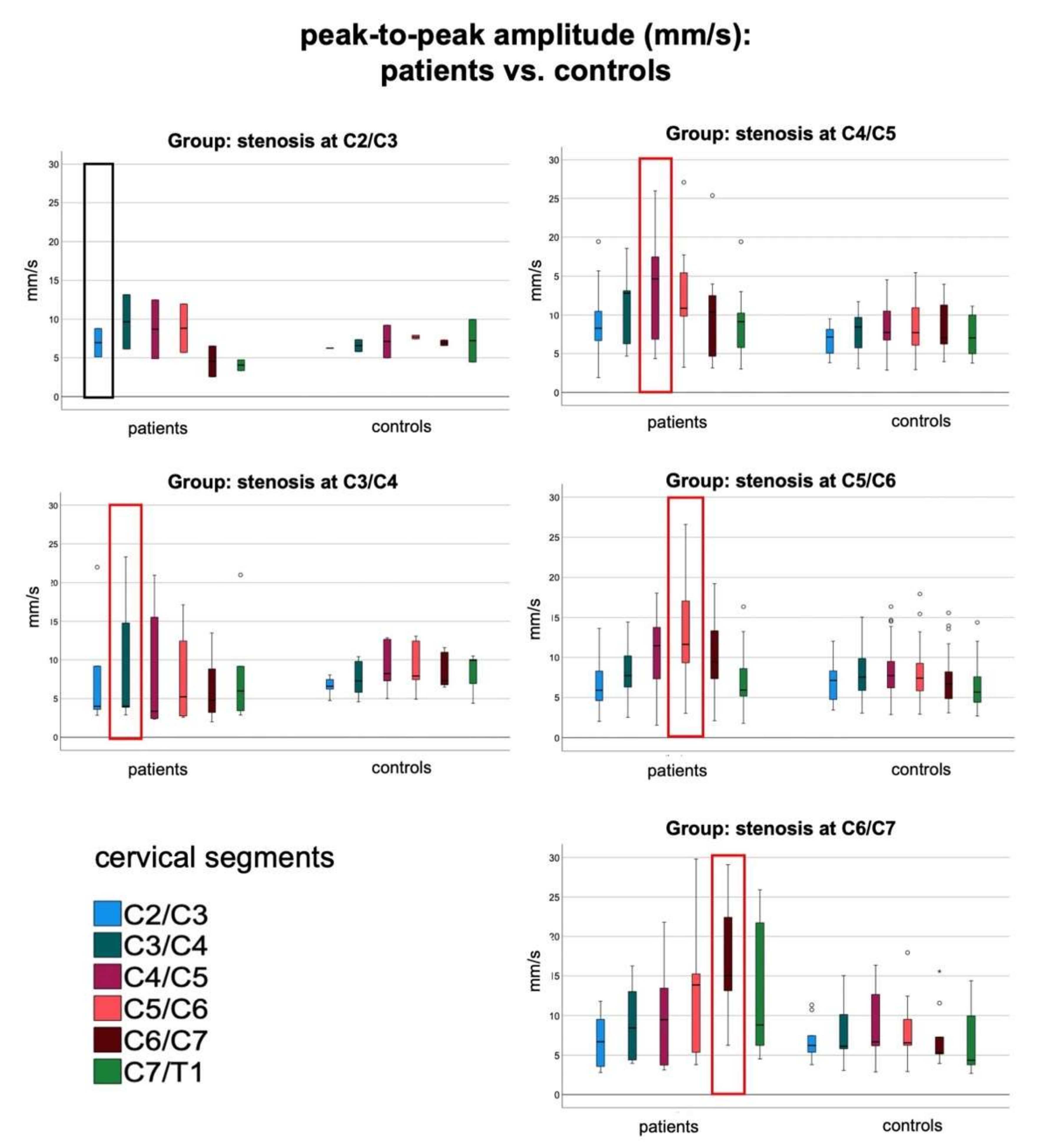
3.2. Focal Increase of Spinal Cord Motion within All Groups of Patients
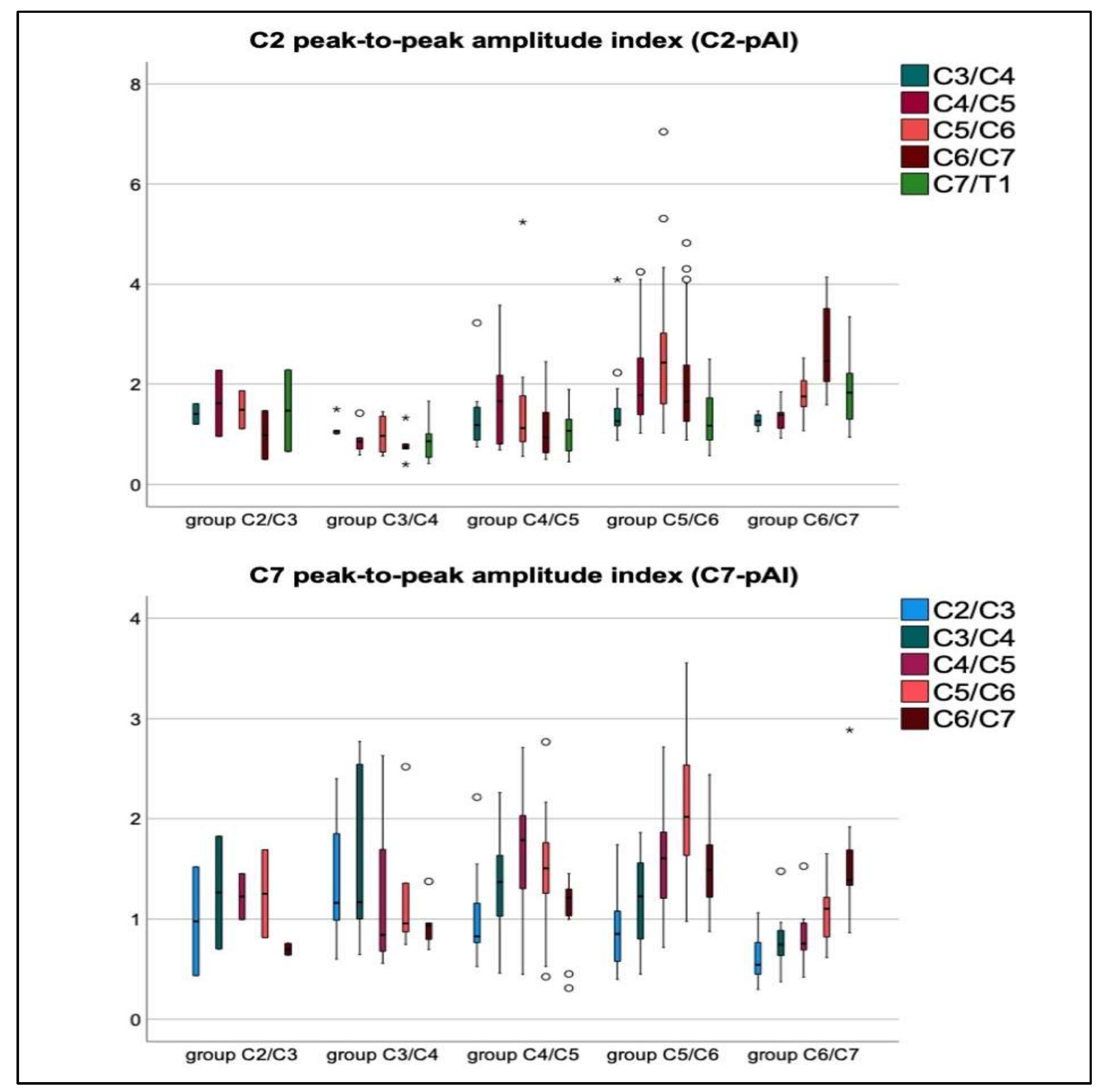
3.3. Mechanical Stretching and Compression of Interjacent Spinal Cord Tissue
3.4. Relations of Severity of Stenosis (aMCC/aSCOR), Age, Gender, and mJOA Score to Increased Spinal Cord Motion at Stenosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aMCC | adapted maximum canal compromise |
| aSCOR | adapted spinal cord occupation ratio |
| CNN | convolutional neural network |
| CSA | cross-sectional area |
| CSF | cerebrospinal fluid |
| DCM | degenerative cervical myelopathy |
| ICC | intra-class correlation coefficient |
| MRI | magnetic resonance imaging |
| pAI | peak-to-peak-amplitude index |
| PC-MRI | phase-contrast MRI |
| ptp | peak-to-peak |
| SPACE | sampling perfection with application-optimized contrasts using different flip angle evolution |
References
- Kalsi-Ryan, S.; Karadimas, S.K.; Fehlings, M.G. Cervical spondylotic myelopathy: The clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist 2013, 19, 409–421. [Google Scholar] [CrossRef]
- Badhiwala, J.H.; Ahuja, C.S.; Akbar, M.A.; Witiw, C.D.; Nassiri, F.; Furlan, J.C.; Curt, A.; Wilson, J.R.; Fehlings, M.G. Degenerative cervical myelopathy—Update and future directions. Nat. Rev. Neurol. 2020, 16, 108–124. [Google Scholar] [CrossRef]
- Davies, B.; Mowforth, O.D.; Smith, E.K.; Kotter, M.R. Degenerative cervical myelopathy. BMJ 2018, 360, k186. [Google Scholar] [CrossRef] [Green Version]
- Nouri, A.; Tetreault, L.; Singh, A.; Karadimas, S.K.; Fehlings, M. Degenerative Cervical Myelopathy. Spine 2015, 40, E675–E693. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Tetreault, L.A.; Riew, K.D.; Middleton, J.W.; Aarabi, B.; Arnold, P.M.; Brodke, D.S.; Burns, A.; Carette, S.; Chen, R.; et al. A Clinical Practice Guideline for the Management of Patients with Degenerative Cervical Myelopathy: Recommendations for Patients with Mild, Moderate, and Severe Disease and Nonmyelopathic Patients with Evidence of Cord Compression. Glob. Spine J. 2017, 7, 70S–83S. [Google Scholar] [CrossRef] [Green Version]
- Nouri, A.; Gondar, R.; Cheng, J.S.; Kotter, M.R.; Tessitore, E. Degenerative Cervical Myelopathy and the Aging Spine: Introduction to the Special Issue. J. Clin. Med. 2020, 9, 2535. [Google Scholar] [CrossRef] [PubMed]
- Witiw, C.D.; Mathieu, F.; Nouri, A.; Fehlings, M.G. Clinico-Radiographic Discordance: An Evidence-Based Commentary on the Management of Degenerative Cervical Spinal Cord Compression in the Absence of Symptoms or with Only Mild Symptoms of Myelopathy. Glob. Spine J. 2017, 8, 527–534. [Google Scholar] [CrossRef] [Green Version]
- Vavasour, I.M.; Meyers, S.M.; Macmillan, E.L.; Mädler, B.; Li, D.K.; Rauscher, A.; Vertinsky, T.; Venu, V.; Mackay, A.L.; Curt, A. Increased spinal cord movements in cervical spondylotic myelopathy. Spine J. 2014, 14, 2344–2354. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.S.; Nejo, T.; Yoshida, S.; Oya, S.; Matsui, T. Increased flow signal in compressed segments of the spinal cord in patients with cervical spondylotic myelopathy. Spine 2014, 39, 2136–2142. [Google Scholar] [CrossRef] [PubMed]
- Wolf, K.; Hupp, M.; Friedl, S.; Sutter, R.; Klarhöfer, M.; Grabher, P.; Freund, P.; Curt, A. In cervical spondylotic myelopathy spinal cord motion is focally increased at the level of stenosis: A controlled cross-sectional study. Spinal Cord 2018, 56, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Hupp, M.; Vallotton, K.; Brockmann, C.; Huwyler, S.; Rosner, J.; Sutter, R.; Klarhoefer, M.; Freund, P.; Farshad, M.; Curt, A. Segmental differences of cervical spinal cord motion: Advancing from confounders to a diagnostic tool. Sci. Rep. 2019, 9, 7415. [Google Scholar] [CrossRef]
- Wolf, K.; Reisert, M.; Beltrán, S.F.; Klingler, J.-H.; Hubbe, U.; Krafft, A.J.; Egger, K.; Hohenhaus, M. Focal cervical spinal stenosis causes mechanical strain on the entire cervical spinal cord tissue—A prospective controlled, matched-pair analysis based on phase-contrast MRI. NeuroImage Clin. 2021, 30, 102580. [Google Scholar] [CrossRef] [PubMed]
- Hupp, M.; Pfender, N.; Vallotton, K.; Rosner, J.; Friedl, S.; Zipser, C.M.; Sutter, R.; Klarhöfer, M.; Spirig, J.M.; Betz, M.; et al. The Restless Spinal Cord in Degenerative Cervical Myelopathy. Am. J. Neuroradiol. 2021, 42, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Wolf, K.; Krafft, A.J.; Egger, K.; Klingler, J.-H.; Hubbe, U.; Reisert, M.; Hohenhaus, M. Assessment of spinal cord motion as a new diagnostic MRI-parameter in cervical spinal canal stenosis: Study protocol on a prospective longitudinal trial. J. Orthop. Surg. Res. 2019, 14, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kato, S.; Oshima, Y.; Oka, H.; Chikuda, H.; Takeshita, Y.; Miyoshi, K.; Kawamura, N.; Masuda, K.; Kunogi, J.; Okazaki, R.; et al. Comparison of the Japanese Orthopaedic Association (JOA) Score and Modified JOA (mJOA) Score for the Assessment of Cervical Myelopathy: A Multicenter Observational Study. PLoS ONE 2015, 10, e0123022, Erratum in 2015, 10, e0128392. [Google Scholar] [CrossRef] [PubMed]
- Nora-Imaging. Available online: http://www.nora-imaging.org (accessed on 1 July 2021).
- Zhao, B.; Zhang, X.; Li, Z.; Hu, X. A multi-scale strategy for deep semantic segmentation with convolutional neural networks. Neurocomputing 2019, 365, 273–284. [Google Scholar] [CrossRef]
- Nouri, A.; Martin, A.R.; Mikulis, D.; Fehlings, M.G. Magnetic resonance imaging assessment of degenerative cervical myelopathy: A review of structural changes and measurement techniques. Neurosurg. Focus 2016, 40, E5. [Google Scholar] [CrossRef]
- Nouri, A.; Montejo, J.; Sun, X.; Virojanapa, J.; Kolb, L.E.; Abbed, K.M.; Cheng, J.S. Cervical Cord-Canal Mismatch: A New Method for Identifying Predisposition to Spinal Cord Injury. World Neurosurg. 2017, 108, 112–117. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. Cracking the code: Providing insight into the fundamentals of research and evidence-based practice a guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Cook, R.D. Detection of Influential Observation in Linear Regression. Technometrics 1977, 19, 15. [Google Scholar] [CrossRef]
- Huber, P.J. Robust Statistics; John Wiley: New York, NY, USA, 1981; ISBN 978-0-471-72524-4. [Google Scholar]
- Northover, J.R.; Wild, J.B.; Braybrooke, J.; Blanco, J. The epidemiology of cervical spondylotic myelopathy. Skelet. Radiol. 2012, 41, 1543–1546. [Google Scholar] [CrossRef] [PubMed]
- Figley, C.; Stroman, P. Investigation of human cervical and upper thoracic spinal cord motion: Implications for imaging spinal cord structure and function. Magn. Reson. Med. 2007, 58, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Sakurai, K.; Kashiwagi, N.; Fujita, N.; Hirabuki, N.; Inaba, F.; Harada, K.; Nakamura, H. Transition of the craniocaudal velocity of the spinal cord: From cervical segment to lumbar enlargement. Invest. Radiol. 1998, 33, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Mikulis, D.J.; Wood, M.L.; Zerdoner, O.A.; Poncelet, B.P. Oscillatory motion of the normal cervical spinal cord. Radiology 1994, 192, 117–121. [Google Scholar] [CrossRef]
- Winklhofer, S.; Schoth, F.; Stolzmann, P.; Krings, T.; Mull, M.; Wiesmann, M.; Stracke, C.P. Spinal Cord Motion: Influence of Respiration and Cardiac Cycle. RoFo 2014, 186, 1016–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuzaki, H.; Wakabayashi, K.; Ishihara, K.; Ishikawa, H.; Kawabata, H.; Onomura, T. The origin and significance of spinal cord pulsation. Spinal Cord 1996, 34, 422–426. [Google Scholar] [CrossRef] [Green Version]
- Khan, O.; Badhiwala, J.H.; Akbar, M.A.; Fehlings, M.G. Prediction of Worse Functional Status After Surgery for Degenerative Cervical Myelopathy: A Machine Learning Approach. Neurosurgery 2020, 88, 584–591. [Google Scholar] [CrossRef]
- Kirshblum, S.C.; Waring, W.; Biering-Sorensen, F.; Burns, S.P.; Johansen, M.; Schmidt-Read, M.; Donovan, W.; Graves, D.E.; Jha, A.; Jones, L.; et al. Reference for the 2011 revision of the International Standards for Neuro-logical Classification of Spinal Cord Injury. J. Spinal Cord Med. 2011, 34, 547–554. [Google Scholar] [CrossRef] [Green Version]
- Holly, L.T.; Matz, P.G.; Anderson, P.A.; Groff, M.W.; Heary, R.F.; Kaiser, M.G.; Mummaneni, P.V.; Ryken, T.C.; Choudhri, T.F.; Vresilovic, E.J.; et al. Clinical prognostic indicators of surgical outcome in cervical spondylotic myelopathy. J. Neurosurg. Spine 2009, 11, 112–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalsi-Ryan, S.; Riehm, L.E.; Tetreault, L.; Martin, A.R.; Teoderascu, F.; Massicotte, E.; Curt, A.; Verrier, M.C.; Velstra, I.-M.; Fehlings, M.G. Characteristics of Upper Limb Impairment Related to Degenerative Cervical Myelopathy: Development of a Sensitive Hand Assessment (Graded Redefined Assessment of Strength, Sensibility, and Prehension Version Myelopathy). Neurosurgery 2019, 86, E292–E299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Level of Stenosis | C2/C3 | C3/C4 | C4/C5 | C5/C6 | C6/C7 | |
|---|---|---|---|---|---|---|
| Patients | n | 2 | 6 | 14 | 33 | 10 |
| Male (%) | 1 (50) | 6 (100) | 9 (64.3) | 14 (42.4) | 6 (60) | |
| age (years) (mean ± SD) | 57 ± 8 | 64 ± 10 | 65± 9 | 53 ± 12 | 54 ± 12 | |
| mJOA (mean ± SD) | 18 | 14.50 ± 3.2 | 15.85 ± 2.2 | 16.47 ± 1.8 * | 15.4 ± 2.1 | |
| mJOA 18 (%) | 1 (50) | 1 (16.7) | 5 (35.7) | 10 (31.3) | 1 (10) | |
| mJOA 15–17 (%) | 1 (50) | 3 (50) | 7 (50) | 14 (43.8) | 5 (50) | |
| mJOA < 15 (%) | 2 (33.3) | 2 (14.4) | 7 (21.9) | 4 (40) | ||
| Surgical treatment (%) | 0 | 3 (50) | 8 (57.1) | 18 (54.5) | 6 (60) | |
| aMCC (mean ± SD) | 2.24 ± 0.3 | 1.96 ± 0.7 | 2.97 ± 1.2 ** | 2.28 ± 0.9 | 2.20 ± 0.6 | |
| aSCOR % (mean ± SD) | 60 ± 20 | 74 ± 18 | 84 ± 9 | 83 ± 14 | 79 ± 10 | |
| Controls age- & gender- matched pairs | n | 2 | 6 | 14 | 33 | 10 |
| Male (%) | 1 (50) | 6 (100) | 9 (64.3) | 13 (39.4) | 6 (60) | |
| age (years, mean ± SD) | 58 ± 8 | 64 ± 8 | 66 ± 9 | 54 ± 12 | 55 ± 12 | |
| p | 0.909 | 0.937 | 0.874 | 0.934 | 0.796 | |
| aMCC (mean ± SD) | 0.95 ± 0.1 | 1.12 ± 0.1 | 1.13 ± 0.1 | 1.21 ± 0.1 | 1.15 ± 0.1 | |
| p | 0.026 | 0.022 | <0.001 | <0.001 | <0.001 | |
| aSCOR % (mean ± SD) | 30 ± 2 | 36 ± 5 | 37 ± 5 | 41 ± 7 | 37 ± 8 | |
| p | 0.158 | 0.001 | <0.001 | <0.001 | <0.001 |
| C4/C5 | C5/C6 | C6/C7 | ||
|---|---|---|---|---|
| Age (years) (mean ± SD) | men | 65 ± 8 | 53 ± 13 | 51 ±9 |
| women | 66 ± 11 | 54 ± 11 | 59 ± 16 | |
| p | 0.819 | 0.875 | 0.345 | |
| mJOA (mean ± SD) | men | 15.9 ± 1.9 | 16.5 ± 1.7 | 15.5 ± 2.1 |
| women | 17.0 ± 0.8 | 16.3 ± 1.9 | 15.3 ± 2.4 | |
| p | 0.289 | 0.756 | 0.864 | |
| HB (ms) (mean ± SD) | men | 944 ± 109 | 926 ± 138 | 857 ± 172 |
| women | 962 ± 168 | 910 ± 134 | 934 ± 229 | |
| p | 0.827 | 0.732 | 0.761 | |
| aMCC at stenosis (mean ± SD) | men | 2.8 ± 1.1 | 2.6 ± 1.2 | 2.5 ± 0.6 |
| women | 3.3 ± 1.3 | 2.0 ± 0.7 | 1.8 ± 0.3 | |
| p | 0.468 | 0.084 | 0.065 | |
| aSCOR % at stenosis (mean ± SD) | men | 83.0 ± 9 | 82.9 ± 11 | 82.6 ± 12 |
| women | 85.0 ± 10 | 82.7 ± 16 | 73.3 ± 5 | |
| p | 0.72 | 0.968 | 0.182 | |
| Max. velocity (cm/s) at stenosis (mean ± SD) | men | 1.00 ± 0.5 | 1.09 ± 0.6 | 0.94 ± 0.6 |
| women | 0.59 ± 0.4 | 0.73 ± 0.4 | 1.49 ± 0.4 | |
| p | 0.03 | 0.03 | 0.13 | |
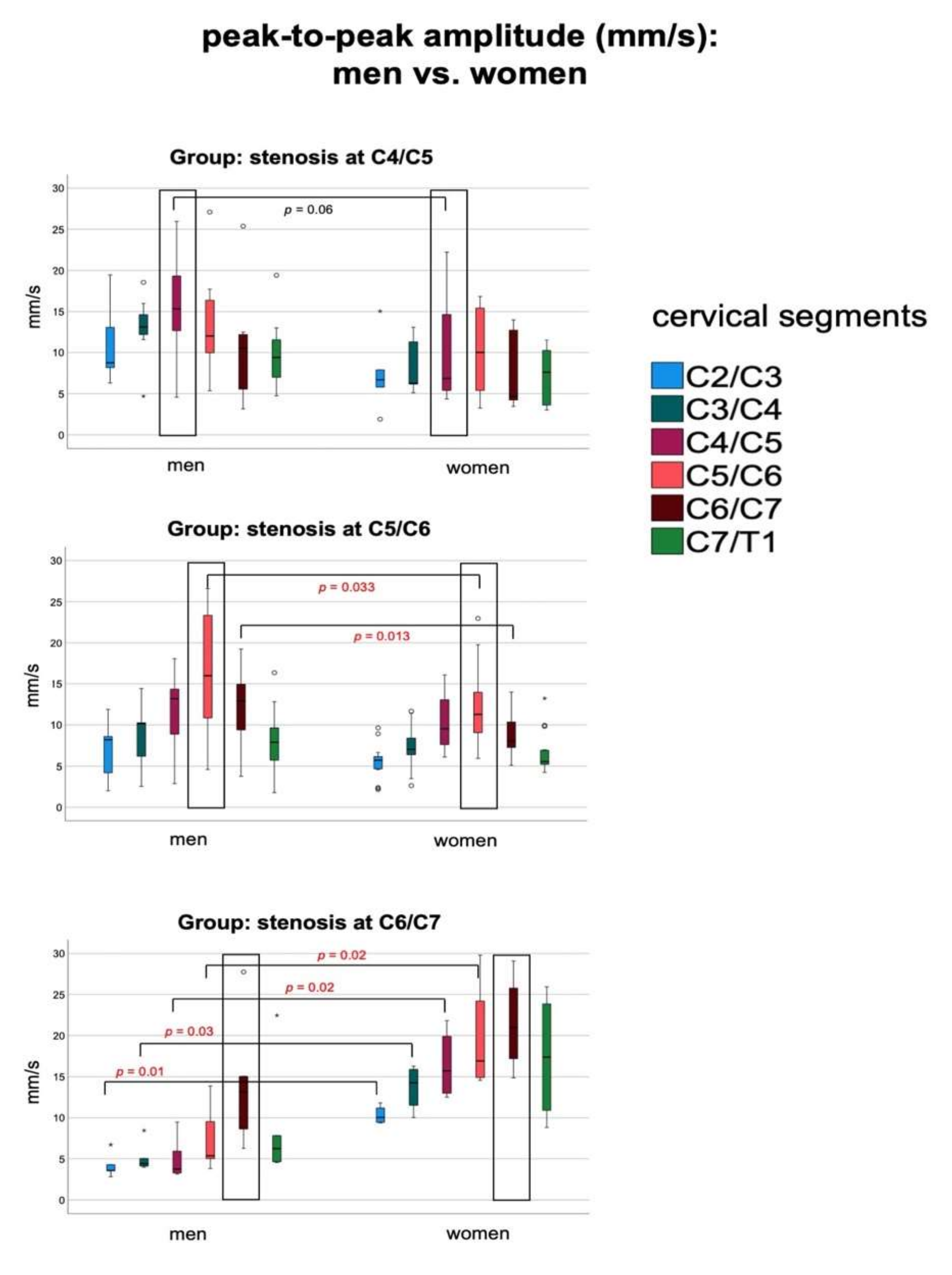
| ptp-amplitude (mm/s) at stenosis (mean ± SD) | men | 15.5 ± 5.9 | 16.3 ± 7.1 | 15.2 ± 7.9 |
| women | 10.7 ± 7.6 | 11.4 ± 5.1 | 21.5 ± 5.9 | |
| p | 0.064 | 0.028 | 0.211 | |
| Total displacement (mm) at stenosis (mean ± SD) | men | 1.92 ± 0.9 | 1.92 ± 0.9 | 1.7 ± 0.6 |
| women | 1.83 ± 1.5 | 1.49 ± 0.9 | 2.4 ± 0.9 | |
| p | 0.893 | 0.255 | 0.151 | |
| C2-pAI at stenosis (mean ± SD) | men | 1.56 ± 0.7 | 2.76 ± 1.1 | 3.23 ± 0.9 |
| women | 1.79 ± 1.3 | 2.45 ± 1.5 | 2.05 ± 0.4 | |
| p | 0.682 | 0.523 | 0.046 | |
| C7-pAI at stenosis (mean ± SD) | men | 1.79 ± 0.8 | 2.21 ± 0.7 | 1.71 ± 0.6 |
| women | 1.54 ± 0.6 | 1.96 ± 0.6 | 1.35 ± 0.4 | |
| p | 0.570 | 0.31 | 0.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolf, K.; Reisert, M.; Beltrán, S.F.; Klingler, J.-H.; Hubbe, U.; Krafft, A.J.; Kremers, N.; Egger, K.; Hohenhaus, M. Spinal Cord Motion in Degenerative Cervical Myelopathy: The Level of the Stenotic Segment and Gender Cause Altered Pathodynamics. J. Clin. Med. 2021, 10, 3788. https://doi.org/10.3390/jcm10173788
Wolf K, Reisert M, Beltrán SF, Klingler J-H, Hubbe U, Krafft AJ, Kremers N, Egger K, Hohenhaus M. Spinal Cord Motion in Degenerative Cervical Myelopathy: The Level of the Stenotic Segment and Gender Cause Altered Pathodynamics. Journal of Clinical Medicine. 2021; 10(17):3788. https://doi.org/10.3390/jcm10173788
Chicago/Turabian StyleWolf, Katharina, Marco Reisert, Saúl Felipe Beltrán, Jan-Helge Klingler, Ulrich Hubbe, Axel J. Krafft, Nico Kremers, Karl Egger, and Marc Hohenhaus. 2021. "Spinal Cord Motion in Degenerative Cervical Myelopathy: The Level of the Stenotic Segment and Gender Cause Altered Pathodynamics" Journal of Clinical Medicine 10, no. 17: 3788. https://doi.org/10.3390/jcm10173788
APA StyleWolf, K., Reisert, M., Beltrán, S. F., Klingler, J.-H., Hubbe, U., Krafft, A. J., Kremers, N., Egger, K., & Hohenhaus, M. (2021). Spinal Cord Motion in Degenerative Cervical Myelopathy: The Level of the Stenotic Segment and Gender Cause Altered Pathodynamics. Journal of Clinical Medicine, 10(17), 3788. https://doi.org/10.3390/jcm10173788






