A Rare Case of Patiromer Induced Hypercalcemia
Abstract
:1. Introduction
2. Case Presentation
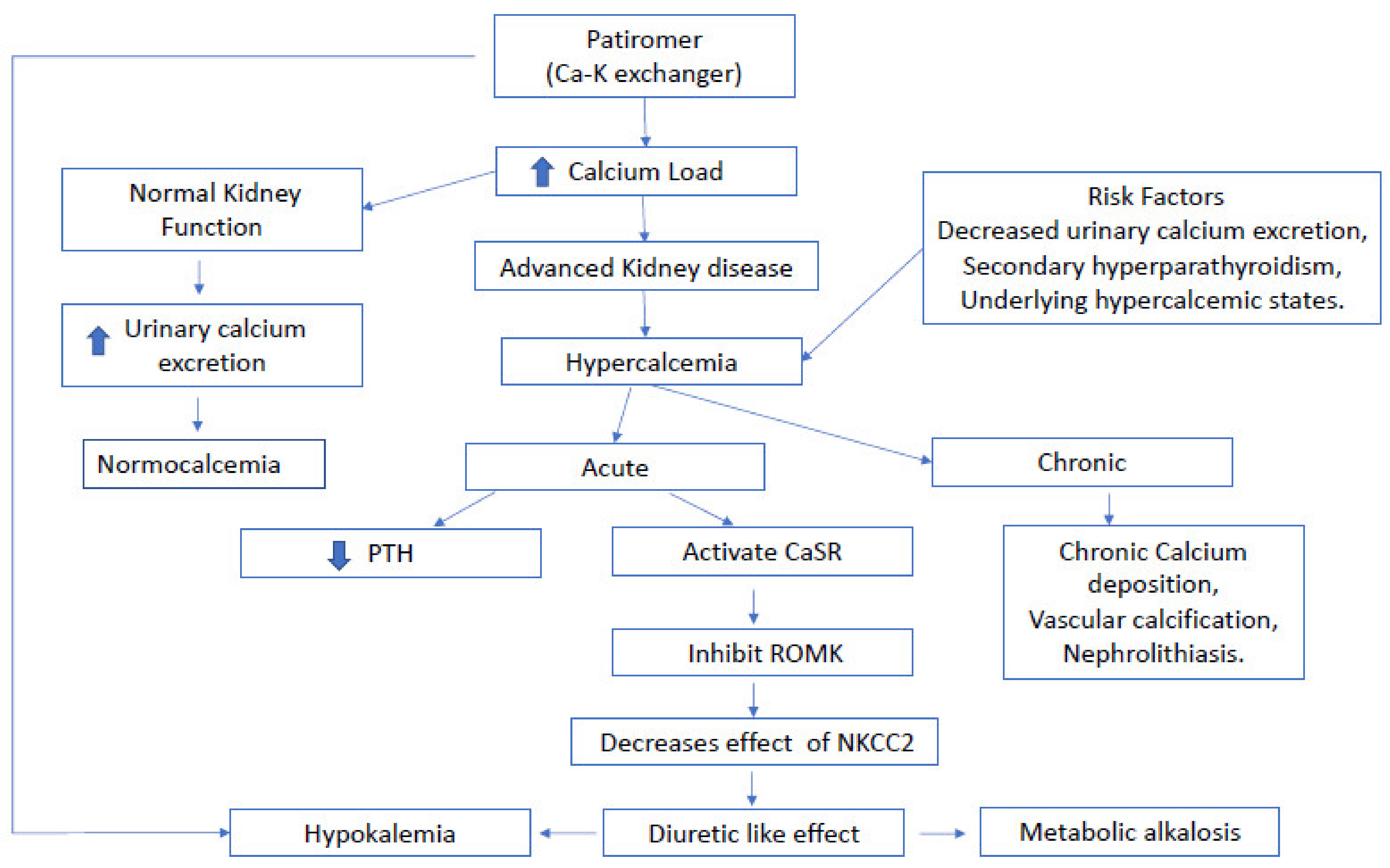
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Montford, J.R.; Linas, S. How Dangerous Is Hyperkalemia? J. Am. Soc. Nephrol. 2017, 28, 3155–3165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunlap, R.H.; Martinez, R. Total colectomy for colon perforation after kayexalate administration: A case report and literature review of a rare complication. J. Surg. Case Rep. 2016, 2016, rjw167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakris, G.L.; Pitt, B.; Weir, M.R.; Freeman, M.W.; Mayo, M.R.; Garza, D.; Stasiv, Y.; Zawadzki, R.; Berman, L.; Bushinsky, D.A.; et al. Effect of Patiromer on Serum Potassium Level in Patients With Hyperkalemia and Diabetic Kidney Disease: The AMETHYST-DN Randomized Clinical Trial. JAMA 2015, 314, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Weir, M.R.; Bakris, G.L.; Bushinsky, D.A.; Mayo, M.R.; Garza, D.; Stasiv, Y.; Wittes, J.; Christ-Schmidt, H.; Berman, L.; Pitt, B. Patiromer in Patients with Kidney Disease and Hyperkalemia Receiving RAAS Inhibitors. N. Engl. J. Med. 2014, 372, 211–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitt, B.; Anker, S.D.; Bushinsky, D.A.; Kitzman, D.W.; Zannad, F.; Huang, I.Z. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur. Heart J. 2011, 32, 820–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amdur, R.L.; Paul, R.; Barrows, E.D.; Kincaid, D.; Muralidharan, J.; Nobakht, E.; Centron-Vinales, P.; Siddiqi, M.; Patel, S.S.; Raj, D.S. The potassium regulator patiromer affects serum and stool electrolytes in patients receiving hemodialysis. Kidney Int. 2020, 98, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Bushinsky, D.A.; Rossignol, P.; Spiegel, D.M.; Benton, W.W.; Yuan, J.; Block, G.A.; Wilcox, C.S.; Agarwal, R. Patiromer Decreases Serum Potassium and Phosphate Levels in Patients on Hemodialysis. Am. J. Nephrol. 2016, 44, 404–410. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Veltassa-Accessdata.fda.gov. 2015. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/205739s000lbl.pdf (accessed on 19 August 2021).
- Bushinsky, D.A.; Williams, G.H.; Pitt, B.; Weir, M.R.; Freeman, M.W.; Garza, D.; Stasiv, Y.; Li, E.; Berman, L.; Bakris, G.L. Patiromer induces rapid and sustained potassium lowering in patients with chronic kidney disease and hyperkalemia. Kidney Int. 2015, 88, 1427–1433. [Google Scholar] [CrossRef] [Green Version]
- Weir, M.R.; Bushinsky, D.A.; Benton, W.W.; Woods, S.D.; Mayo, M.R.; Arthur, S.P.; Pitt, B.; Bakris, G.L. Effect of Patiromer on Hyperkalemia Recurrence in Older Chronic Kidney Disease Patients Taking RAAS Inhibitors. Am. J. Med. 2018, 131, 555–564.e553. [Google Scholar] [CrossRef] [Green Version]
- Wiederkehr, M.R.; Mehta, A.N.; Emmett, M. Case report: Patiromer-induced hypercalcemia. Clin. Nephrol. Case Stud. 2019, 7, 51–53. [Google Scholar] [CrossRef]
- Bhattarai, S.; Pupillo, S.; Dangol, G.M.S.; Sarac, E. Patiromer Acetate Induced Hypercalcemia: An Unreported Adverse Effect. Case Rep. Nephrol. 2019, 2019, 3507407. [Google Scholar] [CrossRef]
- Emmett, M.; Mehta, A. Patiromer—An Oral Calcium-Loaded Potassium Binder: Kalirrhea with Calciuresis. Clin. J. Am. Soc. Nephrol. 2016, 11, 1723–1725. [Google Scholar] [CrossRef] [Green Version]
- Bushinsky, D.A.; Spiegel, D.M.; Yuan, J.; Warren, S.; Fogli, J.; Pergola, P.E. Effects of the Potassium-Binding Polymer Patiromer on Markers of Mineral Metabolism. Clin. J. Am. Soc. Nephrol. 2019, 14, 103–110. [Google Scholar] [CrossRef]
- Bushinsky, D.A.; Spiegel, D.M.; Gross, C.; Benton, W.W.; Fogli, J.; Hill Gallant, K.M.; Du Mond, C.; Block, G.A.; Weir, M.R.; Pitt, B. Effect of Patiromer on Urinary Ion Excretion in Healthy Adults. Clin. J. Am. Soc. Nephrol. 2016, 11, 1769–1776. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, D.; Brown, E.M. Physiology and pathophysiology of the calcium-sensing receptor in the kidney. Am. J. Physiol. Renal Physiol. 2010, 298, F485–F499. [Google Scholar] [CrossRef] [Green Version]
- Sterns, R.H.; Grieff, M.; Bernstein, P.L. Treatment of hyperkalemia: Something old, something new. Kidney Int. 2016, 89, 546–554. [Google Scholar] [CrossRef] [Green Version]
| Laboratory Parameter | Normal Range | Before Patiromer Initiation | Laboratory Values while on Patiromer | Laboratory Values Immediately after Discontinuation | Five Days after Discontinuation |
|---|---|---|---|---|---|
| Sodium (mEq/L) | 136–145 | 138 | 141 | 141 | 141 |
| Potassium (mEq/L) | 3.4–5.1 | 5.7 | 2.5 | 2.5 | 4.1 |
| Carbon dioxide (mEq/L) | 22–29 | 24 | 34 | 32 | 32 |
| Chloride (mEq/L) | 96–105 | 105 | 97 | 98 | 103 |
| Calcium (mg/dL) | 8.6–10.2 | 9.3 | 12.6 | 11.2 | 7.7 |
| Corrected calcium (mg/dL) ^ | 8.6–10.2 | 9.5 | 12.8 | 11.4 | 8 |
| Albumin (g/dL) | 3.8–4.5 | 3.8 | 3.7 | 3.7 | 3.6 |
| Phosphorous (mg/dL) | 2.5–4.5 | 3.1 | 2.3 | 2.4 | 2.5 |
| Creatinine (mg/dL) | 0.5–1.1 | 2.7 | 2.9 | 2.8 | 2.3 |
| eGFR (ml/min/1.73m2) | 120 | 26 | * N/A | * N/A | 30.6 |
| Alkaline phosphatase (U/L) | 35–104 | 66 | 39 | 39 | 39 |
| PTH (pg/mL) | 15–72 | 338 | 21 | ||
| PTHrP (pm/L) | <4 | * N/A | 1.2 | ||
| 25-Hydroxyvitamin D (pg/mL) | 30–100 | 20 | 14 | ||
| 1,25-Hydroxyvitamin D (pg/mL) | 19.9–79.2 | 25 | 24 | ||
| Urinary calcium (mg/dL) | 0–15 | * N/A | 4.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanduri, S.R.; Suchow, K.J.; Velez, J.C.Q. A Rare Case of Patiromer Induced Hypercalcemia. J. Clin. Med. 2021, 10, 3756. https://doi.org/10.3390/jcm10163756
Kanduri SR, Suchow KJ, Velez JCQ. A Rare Case of Patiromer Induced Hypercalcemia. Journal of Clinical Medicine. 2021; 10(16):3756. https://doi.org/10.3390/jcm10163756
Chicago/Turabian StyleKanduri, Swetha Rani, Kathryn J. Suchow, and Juan Carlos Q. Velez. 2021. "A Rare Case of Patiromer Induced Hypercalcemia" Journal of Clinical Medicine 10, no. 16: 3756. https://doi.org/10.3390/jcm10163756
APA StyleKanduri, S. R., Suchow, K. J., & Velez, J. C. Q. (2021). A Rare Case of Patiromer Induced Hypercalcemia. Journal of Clinical Medicine, 10(16), 3756. https://doi.org/10.3390/jcm10163756






