Diagnosis and Management of Aortic Valve Stenosis: The Role of Non-Invasive Imaging
Abstract
1. Introduction
2. Echocardiographic Diagnosis and Pitfalls
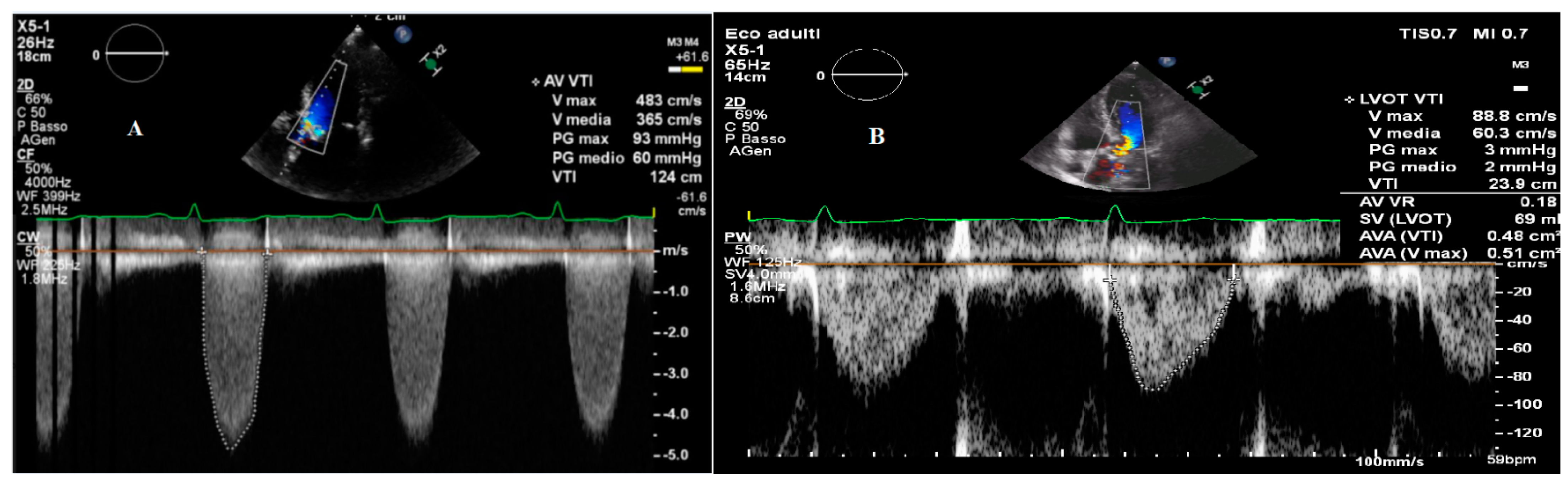
2.1. Transvalvular Pressure Gradients
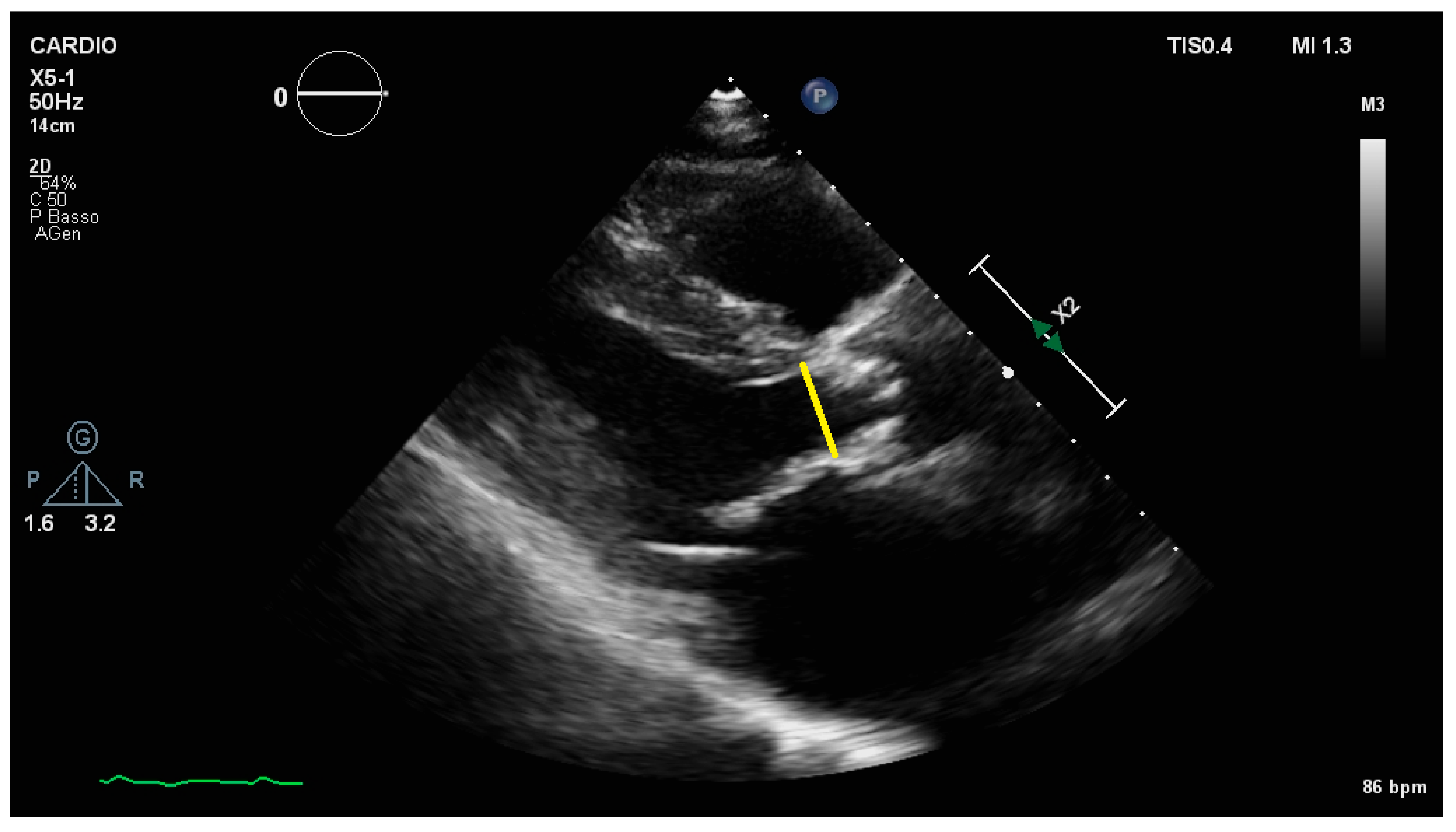
2.2. Aortic Valve Area
2.3. Exercise Testing
2.4. Strain
2.5. Extra-Valvular Cardiac Damage
3. Current Guidelines
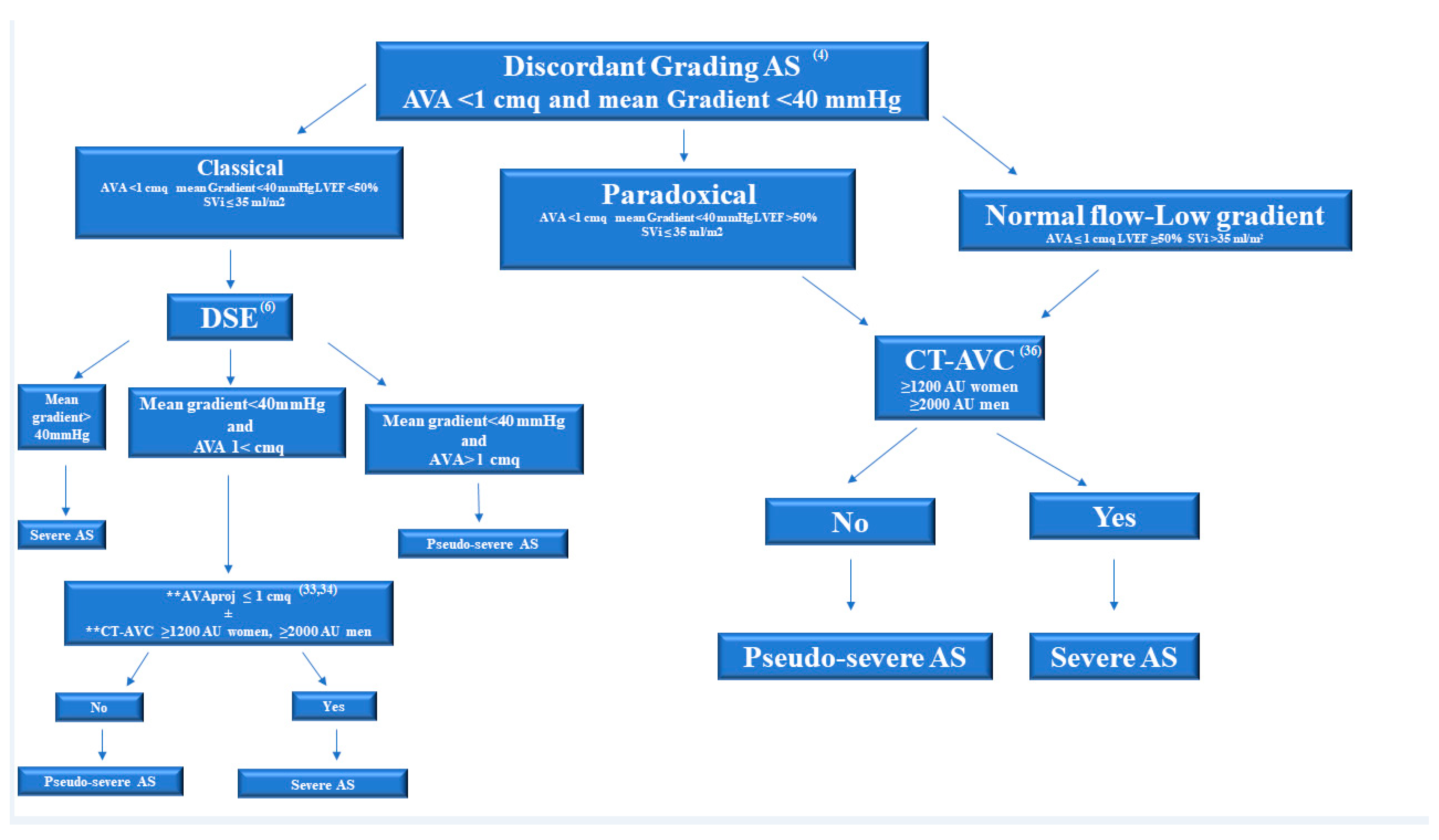
4. Multimodality Imaging for Discordant (Low-Gradient) AS
4.1. Dobutamine Stress Echocardiographic
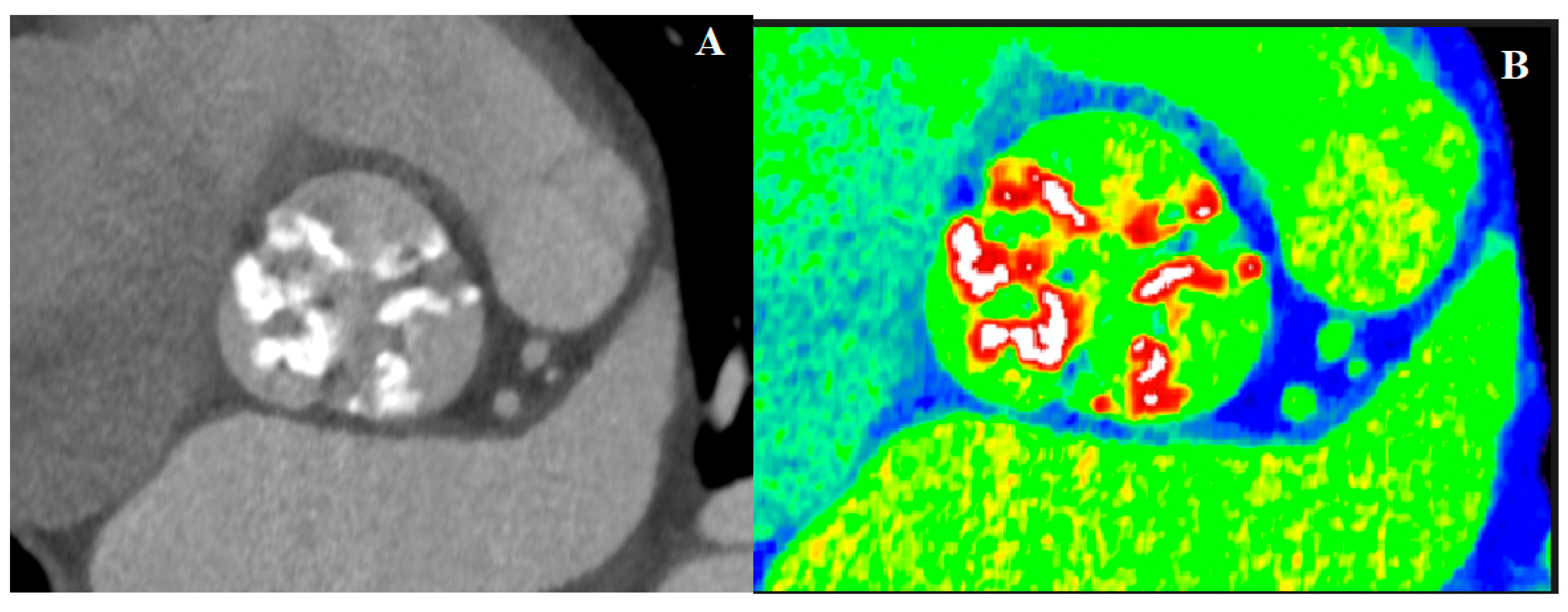
4.2. Computed Tomography in AS
4.3. Cardiovascular Magnetic Resonance
4.4. Positron Emission Tomography
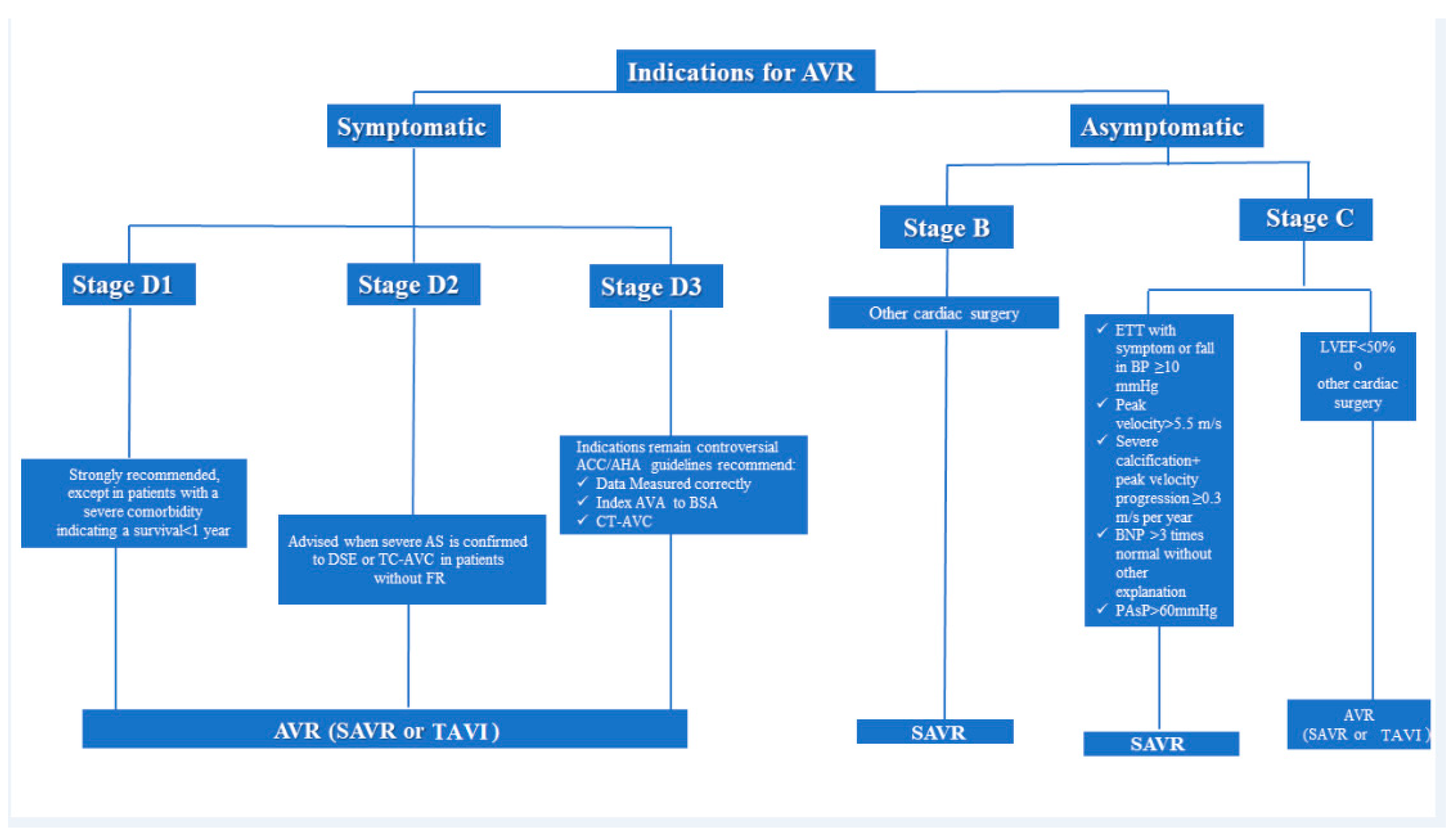
5. Indications for Intervention
Special Patient Populations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AS | Aortic stenosis |
| AU | Agatston Units |
| AVA | Aortic Valve Area |
| AVAi | Aortic Valve Area index to Body Surface Area |
| AVC | Aortic valve calcium scoring |
| AVR | Aortic Valve Replacement |
| BSA | Body Surface Area |
| CA | Cardiac amyloidosis |
| CABG | Coronary artery bypass graft surgery |
| CAD | Coronary Artery Disease |
| CMR | Cardiovascular magnetic resonance |
| CT | Computed tomography |
| DSE | Dobutamine stress echocardiography |
| 18F-NaF | Radiolabeled sodium fluoride |
| FR | Flow rate |
| GLS | Global Longitudinal Strain |
| LFLG | Low Flow-Low Gradient |
| LGE | Late gadolinium enhancement |
| LV | Left ventricular |
| LVEF | Left ventricular ejection fraction |
| LVOT | Left ventricular outflow tract |
| MR | Mitral Regurgitation |
| PET | Positron emission tomography |
| SAVR | Surgical aortic valve replacement |
| SV | Stroke Volume |
| TAVI | Transcatheter Aortic Valve Implantation |
| TEE | Transesophageal echocardiography |
| TPG | Transvalvular pressure gradient |
| TTE | Transthoracic Echocardiography |
| Vmax | Peak Aortic Jet Velocity |
| VTI | velocity-time integral |
References
- Dweck, M.R.; Chin, C.; Newby, D.E. Small valve area with low-gradient aortic stenosis: Beware the hard hearted. J. Am. Coll. Cardiol. 2013, 62, 2339–2340. [Google Scholar] [CrossRef]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Edvardsen, T.; Goldstein, S.; Lancellotti, P.; Lefevre, M.; Miller, F.; Otto, C.M. Recommendations on the Echocardiographic Assessment of Aortic Valve Stenosis: A Focused Update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2017, 30, 372–392. [Google Scholar] [CrossRef] [PubMed]
- Hachicha, Z.; Dumesnil, J.G.; Bogaty, P.; Pibarot, P. Paradoxical Low-Flow, Low-Gradient Severe Aortic Stenosis Despite Preserved Ejection Fraction Is Associated with Higher Afterload and Reduced Survival. Circulation 2007, 115, 2856–2864. [Google Scholar] [CrossRef] [PubMed]
- Minners, J.; Allgeier, M.; Gohlke-Baerwolf, C.; Kienzle, R.-P.; Neumann, F.-J.; Jander, N. Inconsistencies of echocardiographic criteria for the grading of aortic valve stenosis. Eur. Heart J. 2007, 29, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Clavel, M.A.; Messika-Zeitoun, D.; Pibarot, P.; Aggarwal, S.R.; Malouf, J.; Araoz, P.A.; Michelena, H.I.; Cueff, C.; Larose, E.; Capoulade, R.; et al. The complex nature of discordant severe calcified aortic valve disease grading: New insights from combined Doppler echocardiographic and computed tomographic study. J. Am. Coll. Cardiol. 2013, 62, 2329–2338. [Google Scholar] [CrossRef]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Munoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar]
- Hagendorff, A.; Knebel, F.; Helfen, A.; Knierim, J.; Sinning, C.; Stöbe, S.; Fehske, W.; Ewen, S. Expert consensus document on the assessment of the severity of aortic valve stenosis by echocardiography to provide diagnostic conclusiveness by standardized verifiable documentation. Clin. Res. Cardiol. 2019, 109, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-Q.; Hansen, K.L.; Bechsgaard, T.; Lönn, L.; Jensen, J.A.; Nielsen, M.B. Non-Invasive Assessment of Intravascular Pressure Gradients: A Review of Current and Proposed Novel Methods. Diagnostics 2018, 9, 5. [Google Scholar] [CrossRef]
- Dumesnil, J.G.; Pibarot, P.; Akins, C. New approaches to quantifying aortic stenosis severity. Curr. Cardiol. Rep. 2008, 10, 91–97. [Google Scholar] [CrossRef]
- Baumgartner, H.; Stefenelli, T.; Niederberger, J.; Schima, H.; Maurer, G. “Overestimation” of catheter gradients by doppler ultrasound in patients with aortic stenosis: A predictable manifestation of pressure recovery. J. Am. Coll. Cardiol. 1999, 33, 1655–1661. [Google Scholar] [CrossRef]
- Benfari, G.; Nistri, S.; Cerrito, L.F.; Maritan, L.; Tafciu, E.; Setti, M.; Bursi, F.; Tadiello, E.; De Manna, N.D.; Rossi, A.; et al. Usefulness of the Right Parasternal Echocardiographic View to Improve the Hemodynamic Assessment After Valve Replacement for Aortic Stenosis. Am. J. Cardiol. 2020, 142, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Saikrishnan, N.; Sawaya, F.J.; Lerakis, S.; Yoganathan, A.P. Response to letter regarding article, “accurate assessment of aortic stenosis: A review of diagnostic modalities and hemodynamics”. Circulation 2014, 130, e135. [Google Scholar] [CrossRef]
- Pibarot, P.; Clavel, M.-A. Left Ventricular Outflow Tract Geometry and Dynamics in Aortic Stenosis: Implications for the Echocardiographic Assessment of Aortic Valve Area. J. Am. Soc. Echocardiogr. 2015, 28, 1267–1269. [Google Scholar] [CrossRef] [PubMed]
- Leye, M.; Brochet, E.; Lepage, L.; Cueff, C.; Boutron, I.; Detaint, D.; Hyafil, F.; Iung, B.; Vahanian, A.; Messika-Zeitoun, D. Size-Adjusted Left Ventricular Outflow Tract Diameter Reference Values: A Safeguard for the Evaluation of the Severity of Aortic Stenosis. J. Am. Soc. Echocardiogr. 2009, 22, 445–451. [Google Scholar] [CrossRef]
- Vulesevic, B.; Kubota, N.; Burwash, I.G.; Cimadevilla, C.; Tubiana, S.; Duval, X.; Nguyen, V.; Arangalage, D.; Chan, K.L.; E Mulvihill, E.; et al. Size-adjusted aortic valve area: Refining the definition of severe aortic stenosis. Eur. Heart J. Cardiovasc. Imaging 2020. [Google Scholar] [CrossRef]
- Park, S.-J.; Dweck, M.R. Multimodality Imaging for the Assessment of Severe Aortic Stenosis. J. Cardiovasc. Imaging 2019, 27, 235–246. [Google Scholar] [CrossRef]
- Hahn, R.T.; Little, S.H.; Monaghan, M.J.; Kodali, S.K.; Williams, M.; Leon, M.B.; Gillam, L.D. Recommendations for comprehensive intraprocedural echocardiographic imaging during TAVI. JACC Cardiovasc. Imaging 2015, 8, 261–287. [Google Scholar] [CrossRef]
- Lancellotti, P.; Pellikka, P.A.; Budts, W.; Chaudhry, F.A.; Donal, E.; Dulgheru, R.; Edvardsen, T.; Garbi, M.; Ha, J.-W.; Kane, G.C.; et al. The clinical use of stress echocardiography in non-ischaemic heart disease: Recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1191–1229. [Google Scholar] [CrossRef]
- Lancellotti, P.; Magne, J.; Piérard, L.A. The role of stress testing in evaluation of asymptomatic patients with aortic stenosis. Curr. Opin. Cardiol. 2013, 28, 531–539. [Google Scholar] [CrossRef]
- Huded, C.P.; Masri, A.; Kusunose, K.; Goodman, A.L.; Grimm, R.A.; Gillinov, A.M.; Johnston, D.R.; Rodriguez, L.L.; Popovic, Z.B.; Svensson, L.G.; et al. Outcomes in Asymptomatic Severe Aortic Stenosis with Preserved Ejection Fraction Undergoing Rest and Treadmill Stress Echocardiography. J. Am. Heart Assoc. 2018, 7, e007880. [Google Scholar] [CrossRef]
- Postolache, A.; Nguyen, M.L.; Julien, T.; Sperlongano, S.; Chitroceanu, A.M.; Dulgheru, R.; Lancellotti, P. Exercise echocardiography in aortic stenosis with preserved ejection fraction. Anatol. J. Cardiol. 2020, 23, 312–317. [Google Scholar] [PubMed]
- Maréchaux, S.; Hachicha, Z.; Bellouin, A.; Dumesnil, J.G.; Meimoun, P.; Pasquet, A.; Bergeron, S.; Arsenault, M.; Le Tourneau, T.; Ennezat, P.V.; et al. Usefulness of exercise-stress echocardiography for risk stratification of true asymptomatic patients with aortic valve stenosis. Eur. Heart J. 2010, 31, 1390–1397. [Google Scholar] [CrossRef]
- Lancellotti, P.; Donal, E.; Magne, J.; O’Connor, K.; Moonen, M.L.; Cosyns, B.; Pierard, L.A. Impact of global left ventricular afterload on left ventricular function in asymptomatic severe aortic stenosis: A two-dimensional speckle-tracking study. Eur. J. Echocardiogr. 2010, 11, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Donal, E.; Magne, J.; Moonen, M.; O’Connor, K.; Daubert, J.-C.; Pierard, L.A. Risk stratification in asymptomatic moderate to severe aortic stenosis: The importance of the valvular, arterial and ventricular interplay. Heart 2010, 96, 1364–1371. [Google Scholar] [CrossRef]
- Zito, C.; Salvia, J.; Cusmà-Piccione, M.; Antonini-Canterin, F.; Lentini, S.; Oreto, G.; Di Bella, G.; Montericcio, V.; Carerj, S. Prognostic Significance of Valvuloarterial Impedance and Left Ventricular Longitudinal Function in Asymptomatic Severe Aortic Stenosis Involving Three-Cuspid Valves. Am. J. Cardiol. 2011, 108, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.; Blanke, P.; Bax, J.J.; Leipsic, J. Multimodality imaging in valvular heart disease: How to use state-of-the-art technology in daily practice. Eur. Heart J. 2020, 42, 1912–1925. [Google Scholar] [CrossRef]
- Galli, E.; Fournet, M.; Chabanne, C.; Leguerrier, A.; Mabo, P.; Lelong, B.; Flecher, E.; Donal, E. Prognostic value of left atrial reservoir function in patients with severe aortic stenosis: A 2D speckle-tracking echocardiographic study. Eur. Heart J. Cardiovasc. Imaging 2015, 17, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Pibarot, P.; Redfors, B.; Mack, M.J.; Makkar, R.R.; A Jaber, W.; Svensson, L.G.; Kapadia, S.; Tuzcu, E.M.; Thourani, V.H.; et al. Staging classification of aortic stenosis based on the extent of cardiac damage. Eur. Heart J. 2017, 38, 3351–3358. [Google Scholar] [CrossRef]
- Tastet, L.; Tribouilloy, C.; Maréchaux, S.; Vollema, E.M.; Delgado, V.; Salaun, E.; Shen, M.; Capoulade, R.; Clavel, M.-A.; Arsenault, M.; et al. Staging Cardiac Damage in Patients with Asymptomatic Aortic Valve Stenosis. J. Am. Coll. Cardiol. 2019, 74, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Rosa, V.; Ribeiro, H.B.; Sampaio, R.O.; Morais, T.C.; Rosa, M.E.; Pires, L.J.; Vieira, M.L.; Jr, W.M.; Rochitte, C.E.; de Santis, A.S.; et al. Myocardial Fibrosis in Classical Low-Flow, Low-Gradient Aortic Stenosis. Circ. Cardiovasc. Imaging 2019, 12, e008353. [Google Scholar] [CrossRef]
- Vamvakidou, A.; Jin, W.; Danylenko, O.; Pradhan, J.; Li, W.; West, C.; Khattar, R.; Senior, R. Impact of Pre-Intervention Transaortic Flow Rate Versus Stroke Volume Index on Mortality Across the Hemodynamic Spectrum of Severe Aortic Stenosis: Implications for a New Hemodynamic Classification of Aortic Stenosis. JACC Cardiovasc. Imaging 2019, 12, 205–206. [Google Scholar] [CrossRef] [PubMed]
- Clavel, M.-A.; Magne, J.; Pibarot, P. Low-gradient aortic stenosis. Eur. Heart J. 2016, 37, 2645–2657. [Google Scholar] [CrossRef]
- Magne, J.; Donal, E.; Davin, L.; O’Connor, K.; Szymanski, C.; Piérard, L.A.; Lancellotti, P. 194 Clinical outcome in asymptomatic severe aortic stenosis. Insights from the new proposed aortic stenosis grading classification. J. Am. Coll. Cardiol. 2012, 4, 61. [Google Scholar] [CrossRef][Green Version]
- Côté, N.; Simard, L.; Zenses, A.; Tastet, L.; Shen, M.; Clisson, M.; Clavel, M. Impact of Vascular Hemodynamics on Aortic Stenosis Evaluation: New Insights into the Pathophysiology of Normal Flow—Small Aortic Valve Area—Low Gradient Pattern. J. Am. Heart Assoc. 2017, 6, e006276. [Google Scholar] [CrossRef]
- Blais, C.; Burwash, I.G.; Mundigler, G.; Dumesnil, J.G.; Loho, N.; Rader, F.; Baumgartner, H.; Beanlands, R.S.; Chayer, B.; Kadem, L.; et al. Projected Valve Area at Normal Flow Rate Improves the Assessment of Stenosis Severity in Patients with Low-Flow, Low-Gradient Aortic Stenosis. Circulation 2006, 113, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Clavel, M.-A.; Burwash, I.G.; Mundigler, G.; Dumesnil, J.G.; Baumgartner, H.; Bergler-Klein, J.; Sénéchal, M.; Mathieu, P.; Couture, C.; Beanlands, R.; et al. Validation of Conventional and Simplified Methods to Calculate Projected Valve Area at Normal Flow Rate in Patients with Low Flow, Low Gradient Aortic Stenosis: The Multicenter TOPAS (True or Pseudo Severe Aortic Stenosis) Study. J. Am. Soc. Echocardiogr. 2010, 23, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Annabi, M.-S.; Touboul, E.; Dahou, A.; Burwash, I.G.; Bergler-Klein, J.; Enriquez-Sarano, M.; Orwat, S.; Baumgartner, H.; Mascherbauer, J.; Mundigler, G.; et al. Dobutamine Stress Echocardiography for Management of Low-Flow, Low-Gradient Aortic Stenosis. J. Am. Coll. Cardiol. 2018, 71, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Simard, L.; Cote, N.; Dagenais, F.; Mathieu, P.; Couture, C.; Trahan, S.; Bosse, Y.; Mohammadi, S.; Page, S.; Joubert, P.; et al. Sex-Related Discordance Between Aortic Valve Calcification and Hemodynamic Severity of Aortic Stenosis: Is Valvular Fibrosis the Explanation? Circ. Res. 2017, 120, 681–691. [Google Scholar] [CrossRef]
- Clavel, M.A.; Pibarot, P.; Messika-Zeitoun, D.; Capoulade, R.; Malouf, J.; Aggarval, S.; Araoz, P.A.; Michelena, H.I.; Cueff, C.; Larose, E.; et al. Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis: Results of an international registry study. J. Am. Coll. Cardiol. 2014, 64, 1202–1213. [Google Scholar] [CrossRef] [PubMed]
- Pawade, T.; Clavel, M.-A.; Tribouilloy, C.; Dreyfus, J.; Mathieu, T.; Tastet, L.; Renard, C.; Gun, M.; Jenkins, W.S.A.; Macron, L.; et al. Computed Tomography Aortic Valve Calcium Scoring in Patients with Aortic Stenosis. Circ. Cardiovasc. Imaging 2018, 11, e007146. [Google Scholar] [CrossRef]
- Shen, M.; Tastet, L.; Capoulade, R.; Larose, E.; Bedard, E.; Arsenault, M.; Chetaille, P.; Dumesnil, J.G.; Mathieu, P.; Clavel, M.A.; et al. Effect of age and aortic valve anatomy on calcification and haemodynamic severity of aortic stenosis. Heart 2016, 103, 32–39. [Google Scholar] [CrossRef]
- Woldendorp, K.; Bannon, P.G.; Grieve, S.M. Evaluation of aortic stenosis using cardiovascular magnetic resonance: A systematic review & meta-analysis. J. Cardiovasc. Magn. Reson. 2020, 22, 1–9. [Google Scholar] [CrossRef]
- Weininger, M.; Sagmeister, F.; Herrmann, S.; Lange, V.; Schoepf, U.J.; Beissert, M.; Voelker, W.; Koestler, H.; Hahn, D.; Weidemann, F.; et al. Hemodynamic assessment of severe aortic stenosis: MRI evaluation of dynamic changes of vena contracta. Investig. Radiol. 2011, 46, 1–10. [Google Scholar] [CrossRef]
- Azevedo, C.F.; Nigri, M.; Higuchi, M.D.L.; Pomerantzeff, P.M.; Spina, G.S.; Sampaio, R.O.; Tarasoutchi, F.; Grinberg, M.; Rochitte, C.E. Prognostic Significance of Myocardial Fibrosis Quantification by Histopathology and Magnetic Resonance Imaging in Patients with Severe Aortic Valve Disease. J. Am. Coll. Cardiol. 2010, 56, 278–287. [Google Scholar] [CrossRef]
- Magne, J.; Cosyns, B.; Popescu, B.A.; Carstensen, H.G.; Dahl, J.; Desai, M.Y.; Kearney, L.; Lancellotti, P.; Marwick, T.H.; Sato, K.; et al. Distribution and Prognostic Significance of Left Ventricular Global Longitudinal Strain in Asymptomatic Significant Aortic Stenosis: An Individual Participant Data Meta-Analysis. JACC Cardiovasc. Imaging 2019, 12, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Barone-Rochette, G.; Piérard, S.; Ravenstein, C.D.M.D.; Seldrum, S.; Melchior, J.; Maes, F.; Pouleur, A.-C.; Vancraeynest, D.; Pasquet, A.; Vanoverschelde, J.-L.; et al. Prognostic Significance of LGE by CMR in Aortic Stenosis Patients Undergoing Valve Replacement. J. Am. Coll. Cardiol. 2014, 64, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Flett, A.S.; Hayward, M.P.; Ashworth, M.T.; Hansen, M.S.; Taylor, A.M.; Elliott, P.M.; McGregor, C.; Moon, J.C. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: Preliminary validation in humans. Circulation 2010, 122, 138–144. [Google Scholar] [CrossRef]
- Lancellotti, P.; Vannan, M.A. Timing of Intervention in Aortic Stenosis. N. Engl. J. Med. 2020, 382, 191–193. [Google Scholar] [CrossRef]
- Bohbot, Y.; Renard, C.; Manrique, A.; Levy, F.; Maréchaux, S.; Gerber, B.L.; Tribouilloy, C. Usefulness of Cardiac Magnetic Resonance Imaging in Aortic Stenosis. Circ. Cardiovasc. Imaging 2020, 13, e010356. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-W.; Kim, S.M.; Park, S.-J.; Cho, E.J.; Kim, E.K.; Chang, S.-A.; Lee, S.-C.; Choe, Y.H.; Park, S.W. Assessment of reverse remodeling predicted by myocardial deformation on tissue tracking in patients with severe aortic stenosis: A cardiovascular magnetic resonance imaging study. J. Cardiovasc. Magn. Reson. 2017, 19, 80. [Google Scholar] [CrossRef]
- Dweck, M.R.; Khaw, H.J.; Sng, G.K.; Luo, E.L.; Baird, A.; Williams, M.C.; Makiello, P.; Mirsadraee, S.; Joshi, N.V.; van Beek, E.J.R.; et al. Aortic stenosis, atherosclerosis, and skeletal bone: Is there a common link with calcification and inflammation? Eur. Heart J. 2013, 34, 1567–1574. [Google Scholar] [CrossRef]
- Dweck, M.R.; Jones, C.; Joshi, N.V.; Fletcher, A.M.; Richardson, H.; White, A.; Marsden, M.; Pessotto, R.; Clark, J.C.; Wallace, W.A.; et al. Assessment of Valvular Calcification and Inflammation by Positron Emission Tomography in Patients with Aortic Stenosis. Circulation 2012, 125, 76–86. [Google Scholar] [CrossRef]
- Dweck, M.; Jenkins, W.S.; Vesey, A.T.; Pringle, M.A.; Chin, C.; Malley, T.S.; Cowie, W.J.; Tsampasian, V.; Richardson, H.; Fletcher, A.; et al. 18F-Sodium Fluoride Uptake Is a Marker of Active Calcification and Disease Progression in Patients with Aortic Stenosis. Circ. Cardiovasc. Imaging 2014, 7, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Lindman, B.R.; Dweck, M.R.; Lancellotti, P.; Genereux, P.; Pierard, L.A.; O’Gara, P.T.; Bonow, R.O. Management of Asymptomatic Severe Aortic Stenosis: Evolving Concepts in Timing of Valve Replacement. JACC Cardiovasc. Imaging 2020, 13, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Brennan, J.M.; Brindis, R.; Carroll, J.; Edwards, F.; Grover, F.; Shahian, D.; Tuzcu, E.M.; Peterson, E.D.; Rumsfeld, J.S.; et al. Outcomes Following Transcatheter Aortic Valve Replacement in the United States. JAMA 2013, 310, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
- Rapezzi, C.; Giannini, F.; Campo, G. Aortic stenosis, transcatheter aortic valve replacement and transthyretin cardiac amyloidosis: Are we progressively unraveling the tangle? Eur. J. Heart Fail. 2021, 23, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, C.; Scully, P.R.; Patel, K.P.; Kammerlander, A.A.; Koschutnik, M.; Dona, C.; Wollenweber, T.; Ahmed, N.; Thornton, G.D.; Kelion, A.D.; et al. Prevalence and Outcomes of Concomitant Aortic Stenosis and Cardiac Amyloidosis. J. Am. Coll. Cardiol. 2020, 77, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Ternacle, J. PPaCM. Aortic Stenosis and Cardiac Amyloidosis. JACC Case Rep. 2020, 2, 2210–2212. [Google Scholar] [CrossRef]
- Peix, A.; Padron, K. Imagine, believe, and achieve. J. Nucl. Cardiol. 2021, 28, 831–834. [Google Scholar] [CrossRef]
- Dorbala, S.; Ando, Y.; Bokhari, S.; Dispenzieri, A.; Falk, R.H.; Ferrari, V.A.; Fontana, M.; Gheysens, O.; Gillmore, J.D.; Glaudemans, A.W.J.M.; et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI Expert Consensus Recommendations for Multimodality Imaging in Cardiac Amyloidosis: Part 2 of 2—Diagnostic Criteria and Appropriate Utilization. Circ. Cardiovasc. Imaging 2021, 14, e000030. [Google Scholar] [CrossRef]
- Scully, P.R.; Patel, K.P.; A Treibel, T.; Thornton, G.D.; Hughes, R.K.; Chadalavada, S.; Katsoulis, M.; Hartman, N.; Fontana, M.; Pugliese, F.; et al. Prevalence and outcome of dual aortic stenosis and cardiac amyloid pathology in patients referred for transcatheter aortic valve implantation. Eur. Heart J. 2020, 41, 2759–2767. [Google Scholar] [CrossRef]

| Parameters | Mild AS | Moderate | Severe |
|---|---|---|---|
| Vmax (m/s) | 2.6–2.9 | 3.0–4.0 | ≥4.0 |
| Mean gradient (mmHg) | <20 | 20–40 | ≥40 |
| AVA (cm2) | >1.5 | 1.0–1.5 | <1 |
| AVAi (cm2 /m2) | >0.85 | 0.6–0.85 | <0.6 |
| 2017 ESC/EACTS Guidelines for the Management of Valvular Heart Disease | |||||
|---|---|---|---|---|---|
| High-Gradient AS | Low-Flow, Low-Gradient AS with Reduced LVEF | Low-Flow, Low-Gradient AS with Preserved LVEF | Normal-Flow, Low-Gradient AS with Preserved LVEF | ||
| AVA < 1 cm2, ΔP > 40 mmHg | AVA < 1 cm2, ΔP < 40 mmHg, LVEF < 50%, SVi ≤ 35 mL/m2 | AVA < 1 cm2, ΔP < 40 mmHg, LVEF ≥ 50%, SVi ≤ 35 mL/m2 | |||
| 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease | |||||
| Stage | Definition | Valve Anatomy | Valve Hemodynamics | Hemodynamic Consequences | Symptoms |
| A | At risk of AS | BAV or Aortic sclerosis | AVmax < 2 m/s | None | None |
| B | Progressive AS | Mild/moderate leaflet calcification or rheumatic valve changes | Mild AS: AVmax 2.0–2.9 m/s or mean Δ < 20 mmHg Moderate AS: AVmax 3.0–3.9 m/s or Δ 20–39 mmHg | Early left ventricular diastolic dysfunction may be present Normal LVEF | None |
| C1 | Asymptomatic severe AS | Severe leaflet calcification/fibrosis or congenital stenosis with severely reduced leaflet opening | AVmax ≥ 4 m/s or Δ ≥ 40 mmHg AVA typically is ≤1.0 cm2, but not required to define severe AS Very severe AS is an AVmax ≥ 5 m/s or Δ ≥ 60 mm Hg | Left ventricular diastolic dysfunction Mild left ventricular hypertrophy Normal LVEF | None |
| C2 | Asymptomatic severe AS with reduced LVEF | Severe leaflet calcification/fibrosis or congenital stenosis with severely reduced leaflet opening | AVmax ≥ 4 m/s or Δ ≥ 40 mmHg AVA typically is ≤1.0 cm2, but not required to define severe AS | LVEF < 50% | None |
| D1 | Symptomatic severe high- gradient AS | Severe leaflet calcification/fibrosis or congenital stenosis with severely reduced leaflet opening | AVmax ≥ 4 m/s or Δ ≥ 40 mmHg AVA typically is ≤1.0 cm2, but not required to define severe AS | Left ventricular diastolic dysfunction Left ventricular hypertrophy Pulmonary hypertension may be present | Exertional dyspnea, angina or pre-syncope or syncope, decreased exercise tolerance or HF |
| D2 | Symptomatic severe low-flow, low-gradient AS with reduced LVEF | Severe leaflet calcification/fibrosis with severely reduced leaflet motion | AVA ≤ 1.0 cm2 with resting AVmax < 4 m/s or ΔP < 40 mmHg Dobutamine stress echo shows AVA < 1.0 cm2 with AVmax ≥ 4 m/s at any flow rate | Left ventricular diastolic dysfunction Left ventricular hypertrophy LVEF < 50% | HF Angina Syncope or pre-syncope |
| D3 | Symptomatic severe low-gradient AS with normal LVEF or paradoxical low-flow severe AS | Severe leaflet calcification/fibrosis with severely reduced leaflet motion | AVA ≤ 1.0 cm2 with resting AVmax < 4 m/s or Δ < 40 mmHg AND SVi ≤ 35 mL/m2 measured when patient is normotensive (systolic blood pressure <140 mmHg) | Increased left ventricular relative wall thickness Small left ventricular chamber with low SV Restrictive diastolic filling Normal LVEF | HF Angina Syncope or pre-syncope |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santangelo, G.; Rossi, A.; Toriello, F.; Badano, L.P.; Messika Zeitoun, D.; Faggiano, P. Diagnosis and Management of Aortic Valve Stenosis: The Role of Non-Invasive Imaging. J. Clin. Med. 2021, 10, 3745. https://doi.org/10.3390/jcm10163745
Santangelo G, Rossi A, Toriello F, Badano LP, Messika Zeitoun D, Faggiano P. Diagnosis and Management of Aortic Valve Stenosis: The Role of Non-Invasive Imaging. Journal of Clinical Medicine. 2021; 10(16):3745. https://doi.org/10.3390/jcm10163745
Chicago/Turabian StyleSantangelo, Gloria, Andrea Rossi, Filippo Toriello, Luigi Paolo Badano, David Messika Zeitoun, and Pompilio Faggiano. 2021. "Diagnosis and Management of Aortic Valve Stenosis: The Role of Non-Invasive Imaging" Journal of Clinical Medicine 10, no. 16: 3745. https://doi.org/10.3390/jcm10163745
APA StyleSantangelo, G., Rossi, A., Toriello, F., Badano, L. P., Messika Zeitoun, D., & Faggiano, P. (2021). Diagnosis and Management of Aortic Valve Stenosis: The Role of Non-Invasive Imaging. Journal of Clinical Medicine, 10(16), 3745. https://doi.org/10.3390/jcm10163745







