Surgical Aortic Mitral Curtain Replacement: Systematic Review and Metanalysis of Early and Long-Term Results
Abstract
1. Introduction
2. Materials and Methods
2.1. Research
2.2. Data Extraction
2.3. Statistical Analysis and End-Points
3. Results
3.1. Meta-Analysis
3.1.1. Early Mortality
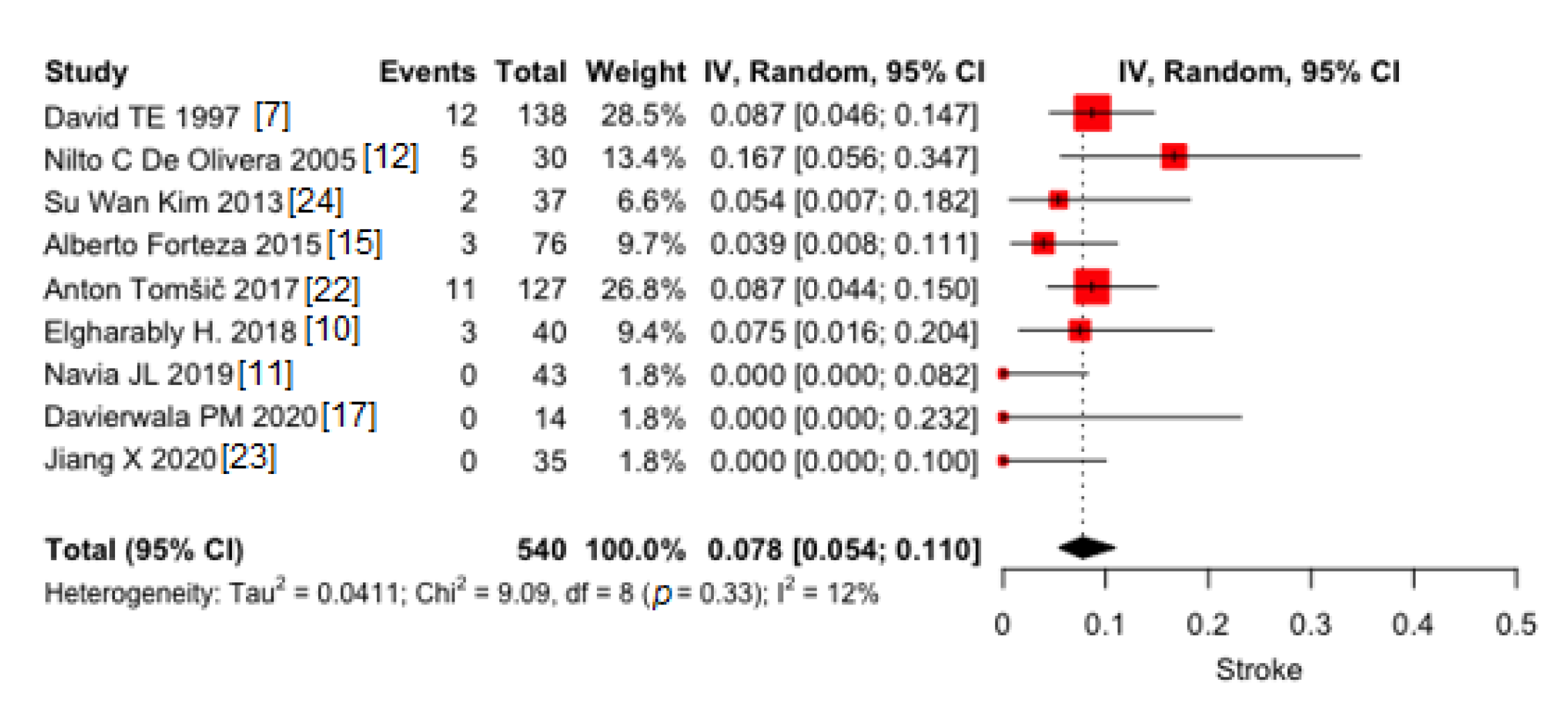
3.1.2. Stroke
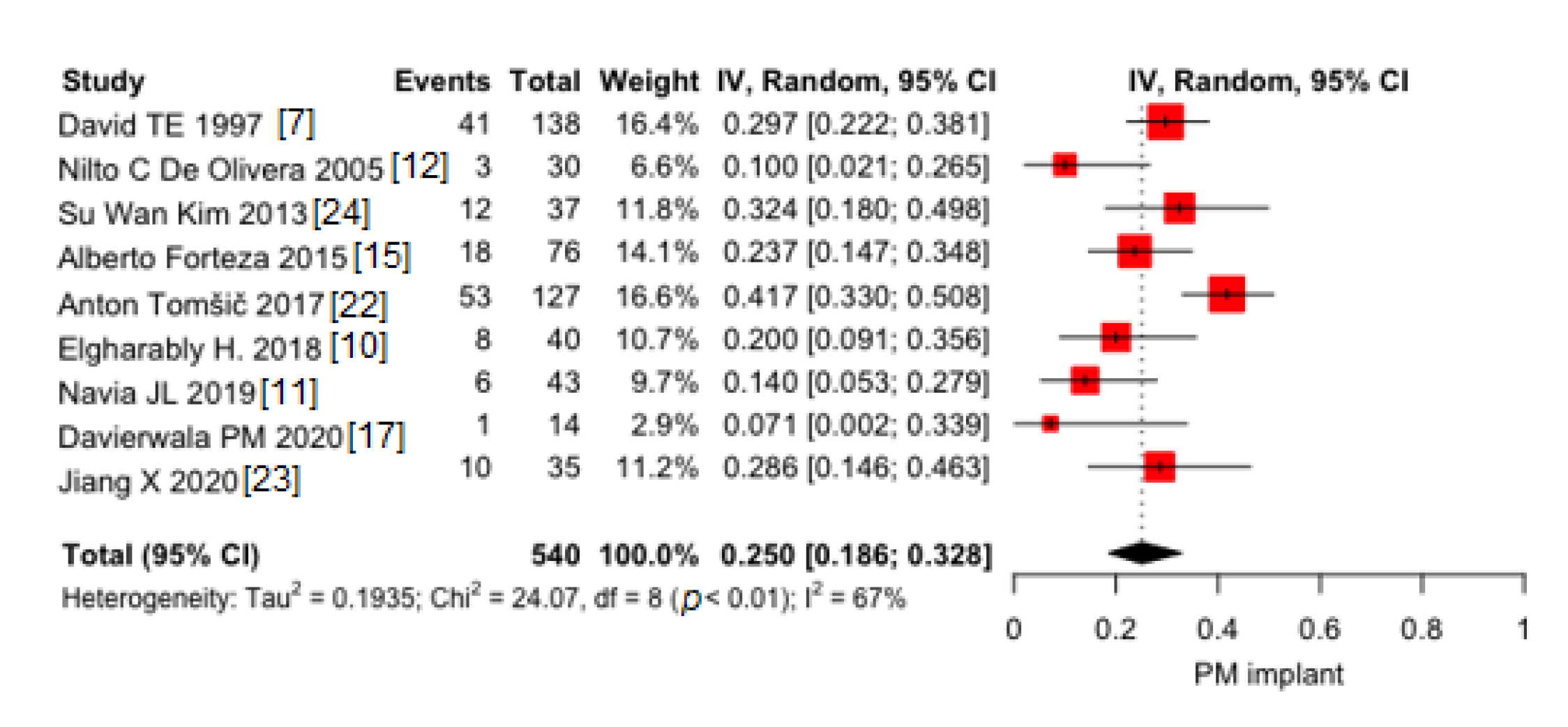
3.1.3. PM Implant
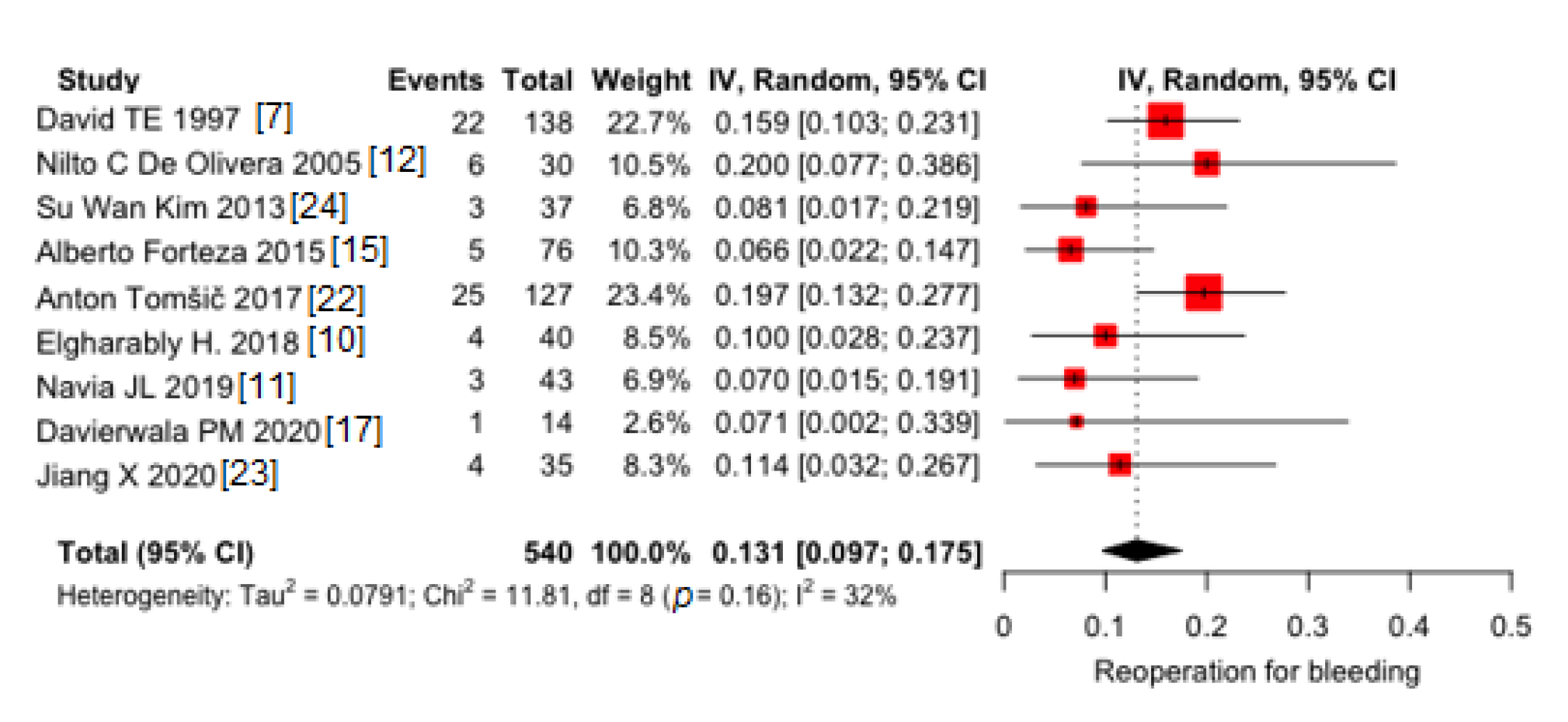
3.1.4. Redo for Bleeding
3.1.5. Modifier Variables
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lima, B.; Chamogeorgakis, T.; AMCHannaford, J.C.; Rafael, A.; Gonzalez-Stawinski, G.V.; Lima, M.B. How to Do It: The Commando Operation for Reconstruction of the Fibrous Skeleton with Double Valve Replacement. Heart Surg. Forum 2016, 19, E308–E310. [Google Scholar] [CrossRef]
- Pettersson, G.B.; Hussain, S.T.; Ramankutty, R.M.; Lytle, B.W.; Blackstone, E.H. Reconstruction of fibrous skeleton: Technique, pitfalls and results. Multimed. Man. Cardiothorac. Surg. 2014, 2014. [Google Scholar] [CrossRef]
- Misfeld, M.; Davierwala, P.M.; Borger, M.A.; Bakhtiary, F. The “UFO” procedure. Ann. Cardiothorac. Surg. 2019, 8, 691–698. [Google Scholar] [CrossRef]
- Manouguian, S. Erweiterungsplastik des hypoplastischen Aortenklappenringes: Beschreibung einer neuen Operationsmethode. Eine experimentelle Studie. Thoraxchir. Vask. Chir. 1976, 24, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Vojacek, J.; Zacek, P.; Ondrasek, J. Multiple valve endocarditis: A Hemi-Commando procedure. Ann. Cardiothorac. Surg. 2019, 8, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Navia, J.L.; Al-Ruzzeh, S.; Gordon, S.; Fraser, T.; Agüero, O.; Rodríguez, L. The incorporated aortomitral homograft: A new surgical option for double valve endocarditis. J. Thorac. Cardiovasc. Surg. 2010, 139, 1077–1081. [Google Scholar] [CrossRef]
- David, T.E.; Kuo, J.; Armstrong, S. Aortic and mitral valve replacement with reconstruction of the intervalvular fibrous body. J. Thorac. Cardiovasc. Surg. 1997, 114, 766–772. [Google Scholar] [CrossRef]
- Davierwala, P.M.; Binner, C.; Subramanian, S.; Luehr, M.; Pfannmueller, B.; Etz, C.; Dohmen, P.; Misfeld, M.; Borger, M.A.; Mohr, F.W. Double valve replacement and reconstruction of the intervalvular fibrous body in patients with active infective endocarditis. Eur. J. Cardio-Thorac. Surg. 2014, 45, 146–152. [Google Scholar] [CrossRef] [PubMed]
- d’Udekem, Y.; David, T.E.; Feindel, C.M.; Armstrong, S.; Sun, Z. Long-term results of surgery for active infective endocarditis1. Eur. J. Cardio-Thorac. Surg. 1997, 11, 46–52. [Google Scholar] [CrossRef]
- Elgharably, H.; Hakim, A.H.; Unai, S.; Hussain, S.T.; Shrestha, N.K.; Gordon, S.; Rodrıguez, L.; Gillinov, A.M.; Svensson, L.G.; Navia, J.L. The incorporated aortomitral homograft for double-valve endocarditis: The “hemi-Commando” procedure. Early and mid-term outcomes. Eur. J. Cardio-Thorac. Surg. 2018, 53, 1055–1061. [Google Scholar] [CrossRef]
- Navia, J.L.; Elgharably, H.; Hakim, A.H.; Witten, J.C.; Haupt, M.J.; Germano, E.; Houghtaling, P.L.; Bakaeen, F.G.; Pettersson, G.B.; Lytle, B.W.; et al. Long-term Outcomes of Surgery for Invasive Valvular Endocarditis Involving the Aortomitral Fibrosa. Ann. Thorac. Surg. 2019, 108, 1314–1323. [Google Scholar] [CrossRef]
- De Oliveira, N.C.; David, T.E.; Armstrong, S.; Ivanov, J. Aortic and mitral valve replacement with reconstruction of the intervalvular fibrous body: An analysis of clinical outcomes. J. Thorac. Cardiovasc. Surg. 2005, 129, 286–290. [Google Scholar] [CrossRef]
- Hassan, M.; Windsor, J.; Ricci, M. En bloc aortic and mitral valve replacement and left ventricular outflow tract enlargement using a combined transaortic and trans-septal atrial approach. Interact. Cardiovasc. Thorac. Surg. 2015, 21, 792–795. [Google Scholar] [CrossRef][Green Version]
- David, T.E.; Feindel, C.M.; Ropchan, G.V. Reconstruction of the left ventricle with autologous pericardium. J. Thorac. Cardiovasc. Surg. 1987, 94, 710–714. [Google Scholar] [CrossRef]
- Forteza, A.; Centeno, J.; Ospina, V.; Lunar, I.G.; Sanchez, V.; Pérez, E.; López, M.J.; Cortina, J. Outcomes in aortic and mitral valve replacement with intervalvular fibrous body reconstruction. Ann. Thorac. Surg. 2015, 99, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.prisma-statement.org/ (accessed on 2 July 2021).
- Davierwala, P.M.; Marin-Cuartas, M.; Misfeld, M.; Deo, S.V.; Lehmann, S.; Garbade, J.; Holzhey, D.M.; Borger, M.A.; Bakhtiary, F. Five-year outcomes following complex reconstructive surgery for infective endocarditis involving the intervalvular fibrous body. Eur. J. Cardiothorac. Surg. 2020, 58, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Warton, D.I.; Hui, F.K.C. The arcsine is asinine: The analysis of proportions in ecology. Ecology 2011, 92, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Ekong, P.S.; Sanderson, M.W.; Cernicchiaro, N. Prevalence and concentration of Escherichia coli O157 in different seasons and cattle types processed in North America: A systematic review and meta-analysis of published research. Prev. Vet. Med. 2015, 121, 74–85. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R; Version 1.1.463 © 2021-201; RStudio, PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 2 July 2021).
- Barendregt, J.J.; Doi, S.A.; Lee, Y.Y.; Norman, R.E.; Vos, T. Meta-analysis of prevalence. J. Epidemiol. Community Health 2013, 67, 974–978. [Google Scholar] [CrossRef]
- Tomšič, A.; Schneider, A.W.; Palmen, M.; van Brakel, T.J.; Versteegh, M.I.M.; Klautz, R.J.M. Extensive infective endocarditis of the aortic root and the aortic-mitral continuity: A mitral valve sparing approach. Eur. J. Cardio-Thorac. Surg. 2017, 51, 1100–1107. [Google Scholar] [CrossRef][Green Version]
- Jiang, X.; Liu, J.; Khan, F.; Tang, R.; Zhang, Y.; Gu, T. Aortic and mitral valve surgery for infective endocarditis with reconstruction of the intervalvular fibrous body: An analysis of clinical outcomes. J. Thorac. Dis. 2020, 12, 1427–1436. [Google Scholar] [CrossRef]
- Kim, S.W.; Park, P.W.; Kim, W.S.; Sung, K.; Lee, Y.T.; Jun, T.-G.; Jeong, D.S. Long-term results of aortomitral fibrous body reconstruction with double-valve replacement. Ann. Thorac. Surg. 2013, 95, 635–641. [Google Scholar] [CrossRef]
- Carpentier, A.F.; Pellerin, M.; Fuzellier, J.F.; Relland, J.Y. Extensive calcification of the mitral valve anulus: Pathology and surgical management. J. Thorac. Cardiovasc. Surg. 1996, 111, 718–729; discussion 729–730. [Google Scholar] [CrossRef]
- Leone, S.; Ravasio, V.; Durante-Mangoni, E.; Crapis, M.; Carosi, G.; Scotton, P.G.; Barzaghi, N.; Falcone, M.; Chinello, P.; Pasticci, M.B.; et al. Epidemiology, characteristics, and outcome of infective endocarditis in Italy: The Italian Study on Endocarditis. Infection 2012, 40, 527–535. [Google Scholar] [CrossRef]
- Olmos, C.; Vilacosta, I.; Fernández, C.; López, J.; Sarriá, C.; Ferrera, C.; Revilla, A.; Silva, J.; Vivas, D.; González, I.; et al. Contemporary epidemiology and prognosis of septic shock in infective endocarditis. Eur. Heart J. 2013, 34, 1999–2006. [Google Scholar] [CrossRef]
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miró, J.M.; Fowler, V.G., Jr.; Bayer, A.S.; Karchmer, A.W.; et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: The International Collaboration on Endocarditis—Prospective Cohort Study. Arch. Intern. Med. 2009, 169, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, M.; Rahouma, M.; Di Mauro, M.; Yanagawa, B.; Abouarab, A.; Demetres, M.; Di Franco, A.; Arisha, M.J.; Ibrahim, D.A.; Baudo, M.; et al. Early Versus Delayed Stroke After Cardiac Surgery: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2019, 8, e012447. [Google Scholar] [CrossRef] [PubMed]
- Nissinen, J.; Biancari, F.; Wistbacka, J.-O.; Peltola, T.; Loponen, P.; Tarkiainen, P.; Virkkilä, M.; Tarkka, M. Safe time limits of aortic cross-clamping and cardiopulmonary bypass in adult cardiac surgery. Perfusion 2009, 24, 297–305. [Google Scholar] [CrossRef]
- Biancari, F.; Kinnunen, E.-M.; Kiviniemi, T.; Tauriainen, T.; Anttila, V.; Airaksinen, J.K.; Brascia, D.; Vasques, F. Meta-analysis of the Sources of Bleeding after Adult Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2018, 32, 1618–1624. [Google Scholar] [CrossRef]
- Steyers, C.M., 3rd; Khera, R.; Bhave, P. Pacemaker Dependency after Cardiac Surgery: A Systematic Review of Current Evidence. PLoS ONE 2015, 10, e0140340. [Google Scholar] [CrossRef]
- Cetinkaya, A.; Poggenpohl, J.; Bramlage, K.; Hein, S.; Doss, M.; Bramlage, P.; Schönburg, M.; Richter, M. Long-term outcome after mitral valve replacement using biological versus mechanical valves. J. Cardiothorac. Surg. 2019, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Helmers, M.R.; Shin, M.; Iyengar, A.; Arguelles, G.R.; Mays, J.; Han, J.J.; Patrick, W.; Altshuler, P.; Hargrove, W.C.; Atluri, P. Permanent pacemaker implantation following mitral valve surgery: A retrospective cohort study of risk factors and long-term outcomes. Eur. J. Cardio-Thorac. Surg. 2021, ezab091. [Google Scholar] [CrossRef] [PubMed]
- Heiro, M.; Helenius, H.; Hurme, S.; Savunen, T.; Metsärinne, K.; Engblom, E.; Nikoskelainen, J.; Kotilainen, P. Long-term outcome of infective endocarditis: A study on patients surviving over one year after the initial episode treated in a Finnish teaching hospital during 25 years. BMC Infect. Dis. 2008, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Mokhles, M.M.; Ciampichetti, I.; Head, S.J.; Takkenberg, J.J.M.; Ad, J.J. Survival of Surgically Treated Infective Endocarditis: A Comparison with the General Dutch Population. Ann. Thorac. Surg. 2011, 91, 1407–1412. [Google Scholar] [CrossRef]
- Manne, M.B.; Shrestha, N.K.; Lytle, B.W.; Nowicki, E.R.; Blackstone, E.; Gordon, S.M.; Pettersson, G.; Fraser, T.G. Outcomes after surgical treatment of native and prosthetic valve infective endocarditis. Ann. Thorac. Surg. 2012, 93, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Croon, S.I.; Angkasuwan, A.; van Straten, A.H.; Khamooshian, A.; Elenbaas, T.W.; Soliman-Hamad, M.A. Surgical treatment and long-term outcome of aortic valve endocarditis with periannular abscess. Neth. Heart J. 2020. [Google Scholar] [CrossRef]
- Greco, R.; Muretti, M.; Djordjevic, J.; Jin, X.Y.; Hill, E.; Renna, M.; Petrou, M. Surgical Complexity and Outcome of Patients Undergoing Re-do Aortic Valve Surgery. Open Heart 2020, 7, e001209. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Tsai, W.-C.; Li, Y.-H.; Tsai, Y.-S.; Yu, Y.-T.; Chang, K.-C.; Luo, C.-Y.; Lin, T.-W. Non-Infectious, Non-Inflammatory Late Dehiscence of Prosthetic Aortic Valve: A “Rocking” Catastrophe. Heart Lung Circ. 2018, 27, e64–e65. [Google Scholar] [CrossRef]
- Kim, J.B.; Ejiofor, J.I.; Yammine, M.; Camuso, J.M.; Walsh, C.W.; Ando, M.; Melnitchouk, S.I.; Rawn, J.D.; Leacche, M.; MacGillivray, T.E.; et al. Are homografts superior to conventional prosthetic valves in the setting of infective endocarditis involving the aortic valve? Thorac. Cardiovasc. Surg. 2016, 151, 1239–1248.e2. [Google Scholar] [CrossRef]
- Nappi, F.; Singh, S.S.A.; Lusini, M.; Nenna, A.; Gambardella, I.; Chello, M. The use of allogenic and autologous tissue to treat aortic valve endocarditis. Ann. Transl. Med. 2019, 7, 491. [Google Scholar] [CrossRef] [PubMed]
- Acar, C. Monobloc or separate aortic and mitral homografts? J. Thorac. Cardiovasc. Surg. 2006, 132, 442–443. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Olivito, S.; Lalande, S.; Nappi, F.; Hammoudi, N.; D’Alessandro, C.; Fouret, P.; Acar, C. Structural deterioration of the cryopreserved mitral homograft valve. J. Thorac. Cardiovasc. Surg. 2012, 144, 313–320.e1. [Google Scholar] [CrossRef]
- Nappi, F.; Nenna, A.; Petitti, T.; Spadaccio, C.; Gambardella, I.; Lusini, M.; Chello, M.; Acar, C. Long-term outcome of cryopreserved allograft for aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2018, 156, 1357–1365.e6. [Google Scholar] [CrossRef] [PubMed]
- Bekkers, J.A.; Klieverik, L.M.; Raap, G.B.; Takkenberg, J.; Bogers, A.J. Re-operations for aortic allograft root failure: Experience from a 21-year single-center prospective follow-up study. Eur. J. Cardio-Thorac. Surg. 2011, 40, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Alagna, L.; Park, L.P.; Nicholson, B.P.; Strahilevitz, J.; Morris, A.; Wray, D.; Gordon, D.; Delahaye, F.; Edathodu, J.; Miró, J.M.; et al. Repeat endocarditis: Analysis of risk factors based on the International Collaboration on Endocarditis—Prospective Cohort Study. Clin. Microbiol. Infect. 2014, 20, 566–575. [Google Scholar] [CrossRef] [PubMed]
- David, T.E.; Regesta, T.; Gavra, G.; Armstrong, S.; Maganti, M.D. Surgical treatment of paravalvular abscess: Long-term results. Eur. J. Cardiothorac. Surg. 2007, 31, 43–48. [Google Scholar] [CrossRef] [PubMed]

| First Author | Tot Patients and FUP | Survival | Re-Operation | Recurrent IE |
|---|---|---|---|---|
| David T.E. [7] | 43 patients Mean follow-up 38 ± 29 months | 7 intrahospital deaths (16%) 6 late deaths Actuarial survival 6 years 56 ± 6% | 2 (early IE) | 2 patients early IE 1 patient late IE |
| Nilto C. De Oliveira [12] | 76 patients Mean of 47± 47 months | 8 operative deaths (10%) 18 late deaths (24%) Overall survival 5 years 71 ± 6% 10 years 50% ± 9% | 12 patients Freedom from any re-operation 5 years 83 ± 5% 10 years 73 ± 7% | 7 patients Freedom from IE 5 years 85% ± 5% 10 years 85% ± 5% |
| Su Wan Kim [24] | 30 patients Mean follow-up 50.6 ± 43.5 months | 2 hospital deaths (6.7%) 5 late deaths Survival rate in IE patients 1 year 80.8% 5 year 74.6% (5 late deaths) Survival rate in non-IE patients 1 year 87.5% 5 years 87.5% p-value 0.76 | 3 patients (2 IE, 1 heart transplant) Freedom from REDO in IE patients 1 year 95.5% 5 year 84.8% | 1 patient Freedom from IE in IE patients at 1 year 94.7% 5 years 94.7% |
| Alberto Forteza [15] | 40 patients Mean follow-up was 53 ± 8 months | Hospital mortality rate 22.2% (5 patients) Survival rate group A 1 year 65.4% 5 year 57.7% 10 year 50% Group B 1 year 92.9% 5 years 85.7% 10 years 78.6% | 5 patients Freedom from re-operation group A 1 year 92.3% 5 year 84.6% 10 year 76.0% Group B 1 year 90.9% 5 years 90.9% 10 years 90.9% | 4 patients |
| Anton Tomšič [22] | 35 patients Median follow-up 29.8 months | 8 early deaths (23%) 5 follow-up deaths Overall survival 1 year: 69.4% 2 years: 65.3% 5 years: 65.3% | 7 patients Freedom from REDO 1 year: 84.6% 2 years: 78.9% 5 years: 51.2% | 2 patients |
| Elgharably H. [10] | 37 patients Mean follow-up 581 ± 729 days | 3 hospital deaths (8%) 2 post-discharge deaths (5%) Overall survival 1 year: 91% 3 years: 82% | 1 for aortic pseudoaneurysm (3%) | 1 patient (3%) |
| Navia J.L. [11] | 138 patients Mean follow-up 41 ± 5.9 months | 28 hospital deaths (20%); 21 Commando (24%), 7 Hemi-Commando (13%) Overall survival 1 year 67% 5 year 48% 10 year 37% Commando survival 1 year 60% 5 year 40% 10 year 35% Hemi-Commando survival 1 year 80% 5 year 64% 10 year 57% No significant difference | Commando: 19 (38%) Hemi-Commando: 4 (12%) Freedom from REDO Commando 1 year 83% 5 year 63% 8 year 50% Freedom from REDO Hemi-Commando 1 year 87% 5 year 74% 8 year 55% | Commando: 13 (26%); Hemi-Commando: 4 (12%) Freedom from recurrent IE Commando 1 year 87% 5 years 77% 8 years 70% Freedom from recurrent IE Hemi-Commando 1 year 95% 5 years 82% 8 years 41% No significant difference |
| Davierwala PM [17] | 127 patients Mean follow-up 477.5 ± 799.2 months | Early deaths (30 days): 36 (28.3%); 90 days 47 (37%) Late deaths (follow-up > 90 days): 13 (10.2%) Survival rates 3 years 45.3 ± 5.1 5 years 41.8 ± 5.8%, | 9 patients (7 IE, 2 miscellaneous) Freedom from REDO 5 years 85.1 ± 5.7% | 7 patients |
| Jiang X [23] | 14 patients Mean follow-up 18.9 ± 12.2 months | Early deaths (60 days): 1 No deaths at follow-up | No re-intervention | No IE |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giambuzzi, I.; Bonalumi, G.; Di Mauro, M.; Roberto, M.; Corona, S.; Alamanni, F.; Zanobini, M. Surgical Aortic Mitral Curtain Replacement: Systematic Review and Metanalysis of Early and Long-Term Results. J. Clin. Med. 2021, 10, 3163. https://doi.org/10.3390/jcm10143163
Giambuzzi I, Bonalumi G, Di Mauro M, Roberto M, Corona S, Alamanni F, Zanobini M. Surgical Aortic Mitral Curtain Replacement: Systematic Review and Metanalysis of Early and Long-Term Results. Journal of Clinical Medicine. 2021; 10(14):3163. https://doi.org/10.3390/jcm10143163
Chicago/Turabian StyleGiambuzzi, Ilaria, Giorgia Bonalumi, Michele Di Mauro, Maurizio Roberto, Silvia Corona, Francesco Alamanni, and Marco Zanobini. 2021. "Surgical Aortic Mitral Curtain Replacement: Systematic Review and Metanalysis of Early and Long-Term Results" Journal of Clinical Medicine 10, no. 14: 3163. https://doi.org/10.3390/jcm10143163
APA StyleGiambuzzi, I., Bonalumi, G., Di Mauro, M., Roberto, M., Corona, S., Alamanni, F., & Zanobini, M. (2021). Surgical Aortic Mitral Curtain Replacement: Systematic Review and Metanalysis of Early and Long-Term Results. Journal of Clinical Medicine, 10(14), 3163. https://doi.org/10.3390/jcm10143163







