One-Year Outcomes after Surgical versus Transcatheter Aortic Valve Replacement with Newer Generation Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
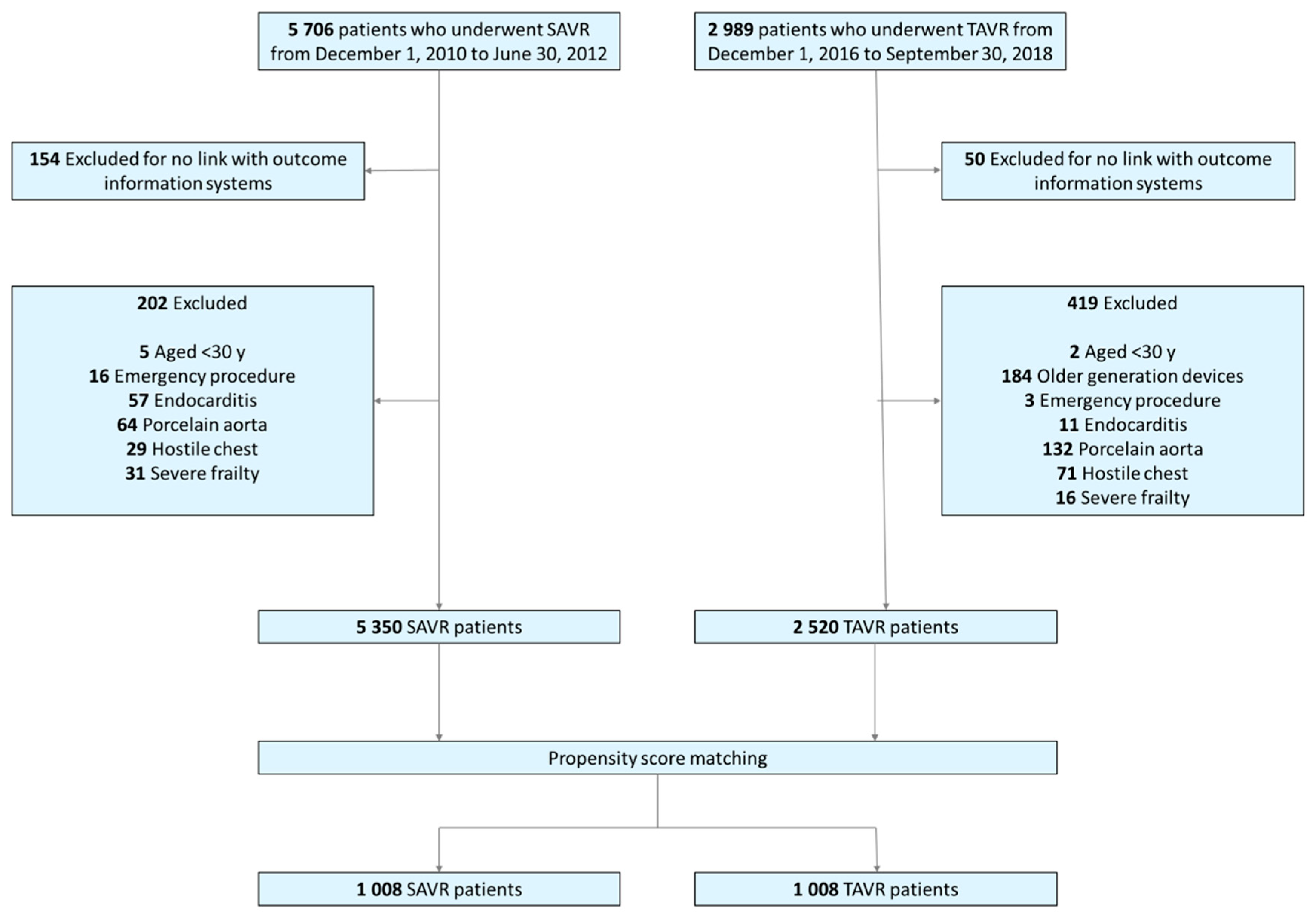
2.2. Study Population
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Study Population Characteristics
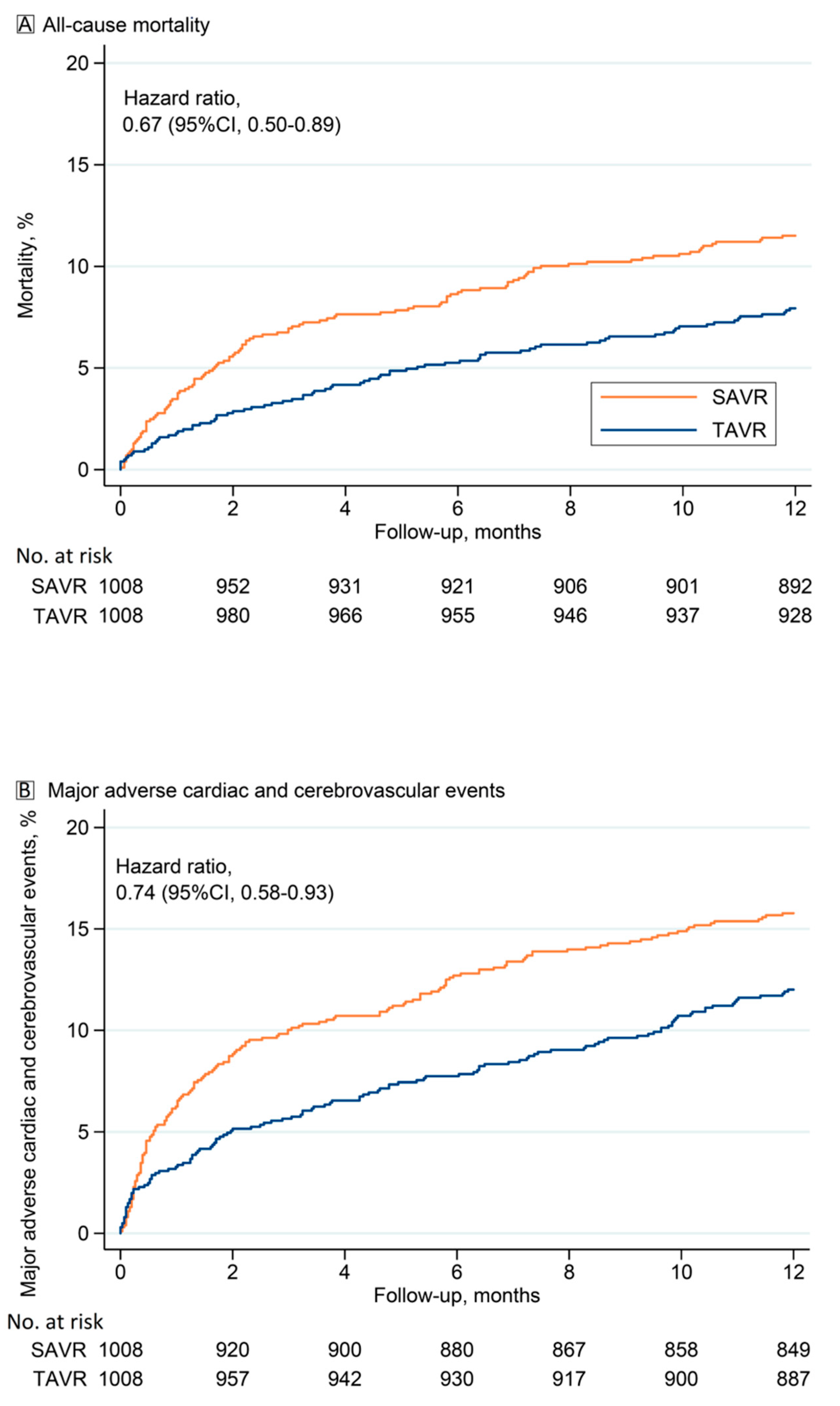
3.2. Outcomes
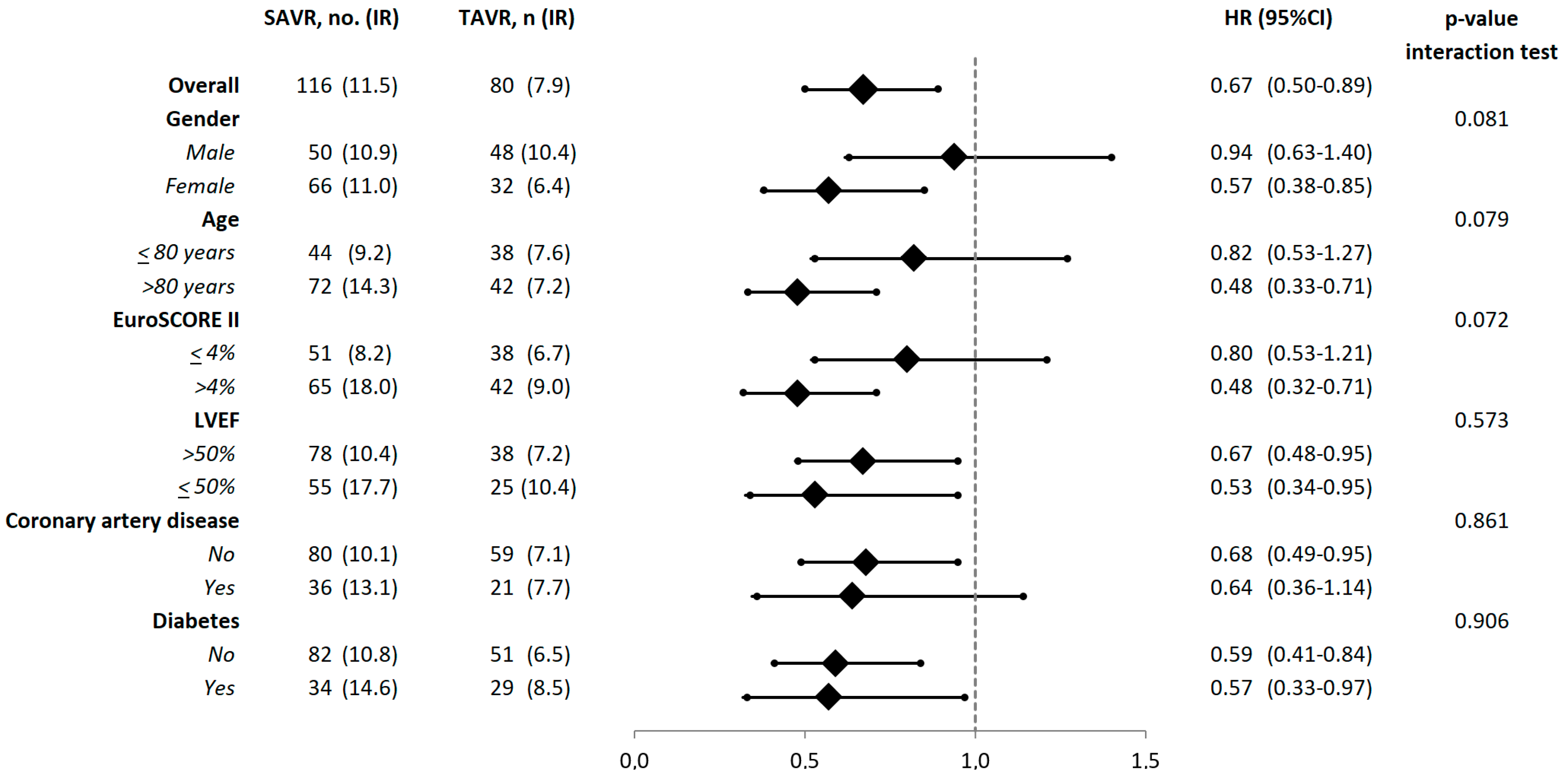
3.3. Additional Analyses
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. OBSERVANT II Investigators
- IFC—Italian Federation of Cardiology
- GISE—Italian Society of Interventional Cardiology
- ITACTA—Italian Association of Cardiothoracic Anesthesia
- National
- Italian Regional Authorities
- A.O.U. Città della Salute e della Scienza di Torino (TO)—Mauro Rinaldi, Stefano Salizzoni.
- A.O. S. Croce e Carle (CN)—Giuseppe Musumeci, Giorgio Baralis.
- A.O. SS. Antonio e Biagio e Cesare Arrigo (AL)—Gianfranco Pistis, Maurizio Reale.
- I.R.C.C.S Policlinico San Donato (San Donato Milanese—MI)—Francesco Bedogni, Giovanni Bianchi.
- I.R.C.C.S Multimedica (Sesto San Giovanni—MI)—Flavio Airoldi, Iassen Michev.
- Fondazione I.R.C.C.S. Policlinico San Matteo (PV)—Maurizio Ferrario, Umberto Canosi.
- ASST Lecco—Ospedale “A. Manzoni” (LC)—Luigi Piatti, Gianluca Tiberti.
- ASST degli Spedali Civili—Presidio Ospedaliero di Brescia (BS)—Federica Ettori (retired), Salvatore Curello, Marianna Adamo.
- I.R.C.C.S Ospedale San Raffaele (MI)—Antonio Colombo, Matteo Montorfano, Marco Ancona.
- ASST Monza & Brianza—Ospedale S. Gerardo (MB)—Virgilio Colombo, Ivan Calchera.
- Fondazione Poliambulanza (BS)—Ornella Leonzi, Diego Maffeo.
- ASST Papa Giovanni XXIII (BG)—Orazio Valsecchi, Federica Roncali, Angelina Vassileva.
- Policlinico di Monza (MB)—Filippo Scalise, Giovanni Sorropago.
- A.O. di Padova—Centro Gallucci (PD)—Giuseppe Tarantini, Alessandro Schiavo.
- Hesperia Hospital (MO)—Giuseppe D’Anniballe, Davide Gabbieri.
- A.O.U. di Parma (PR)—Luigi Vignali, Michela Bollettino.
- A.O.U. Careggi (FI)—Carlo Di Mario, Francesco Meucci.
- A.O.U. Senese—Ospedale Santa Maria alle Scotte (SI)—Carlo Pierli (retired), Massimo Fineschi, Alessandro Iadanza.
- Fondazione Toscana Gabriele Monasterio—Ospedale del Cuore "G. Pasquinucci" (MS)—Sergio Berti, Giuseppa Lo Surdo.
- Ospedale San Filippo Neri (RM)—Giulio Speciale, Andrea Bisciglia.
- Fondazione Policlinico Universitario Agostino Gemelli IRCCS—Università Cattolica del Sacro Cuore (RM)—Carlo Trani, Diana Verdirosi.
- A.O. San Camillo Forlanini (RM)—Roberto Violini, Laura Zappavigna.
- A.O. San Giuseppe Moscati (AV)—Emilio Di Lorenzo, Michele Capasso.
- A.O.U. Federico II (NA)—Giovanni Esposito, Fabio Magliulo.
- A.O.U. OO.RR. San Giovanni di Dio e Ruggi d’Aragona (SA)—Pietro Giudice, Tiziana Attisano.
- A.O.U.C. Policlinico di Bari (BA)—Alessandro Santo Bortone, Emanuela De Cillis.
- A.O.U. Policlinico-Vittorio Emanuele, Università di Catania (CT)—Corrado Tamburino, Marco Barbanti.
- Centro Cuore Morgagni—Pedara (CT)—Sebastiano Immè, Martina Patanè.
References
- Leon, M.B.; Mack, M.J.; Hahn, R.T.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Alu, M.C.; Madhavan, M.V.; Chau, K.H.; Russo, M.; et al. PARTNER 3 Investigators. Outcomes 2 years after transcatheter aortic valve replacement in patients at low surgical risk. J. Am. Coll. Cardiol. 2021, 77, 1149–1161. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, M.P.O.; Eskola, M.; Jalava, M.P.; Husso, A.; Laakso, T.; Niemelä, M.; Ahvenvaara, T.; Tauriainen, T.; Maaranen, P.; Kinnunen, E.M.; et al. Comparison of outcomes after transcatheter aortic valve replacement vs. surgical aortic valve replacement among patients with aortic stenosis at low operative risk. JAMA Netw. Open. 2019, 2, e195742. [Google Scholar] [CrossRef] [Green Version]
- Forrest, J.K.; Ramlawi, B.; Deeb, G.M.; Zahr, F.; Song, H.K.; Kleiman, N.S.; Chetcuti, S.J.; Michelena, H.I.; Mangi, A.A.; Skiles, J.A.; et al. Transcatheter aortic valve replacement in low-risk patients with bicuspid aortic valve stenosis. JAMA Cardiol. 2021, 6, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Brennan, J.M.; Thomas, L.; Cohen, D.J.; Shahian, D.; Wang, A.; Mack, M.J.; Holmes, D.R.; Edwards, F.H.; Frankel, N.Z.; Baron, S.J.; et al. Transcatheter versus surgical aortic valve replacement: Propensity-matched comparison. J. Am. Coll. Cardiol. 2017, 70, 439–450. [Google Scholar] [CrossRef]
- Fischlein, T.; Folliguet, T.; Meuris, B.; Shrestha, M.L.; Roselli, E.E.; McGlothlin, A.; Kappert, U.; Pfeiffer, S.; Corbi, P.; Lorusso, R. Sutureless versus conventional bioprostheses for aortic valve replacement in severe symptomatic aortic valve stenosis. J. Thorac. Cardiovasc. Surg. 2021, 161, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Di Eusanio, M.; Alfonsi, J.; Berretta, P.; Zahedi, H.; Pierri, M.D.; Cefarelli, M. Ultra fast-track trans-axillary mini-aortic valve replacement. Ann. Cardiothorac. Surg. 2020, 9, 427–428. [Google Scholar] [CrossRef]
- Tamburino, C.; Barbanti, M.; D’Errigo, P.; Ranucci, M.; Onorati, F.; Covello, R.D.; Santini, F.; Rosato, S.; Santoro, G.; Fusco, D.; et al. 1-year outcomes after transfemoral transcatheter or surgical aortic valve replacement: Results from the Italian OBSERVANT study. J. Am. Coll. Cardiol. 2015, 66, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Seccareccia, F.; Tarantini, G.; Bedogni, F.; Berti, S.; Santoro, G.; Tamburino, C.; Ussia, G.P.; Barbanti, M.; Baiocchi, M.; Ranucci, M.; et al. OBSERVANT II: OBservational Study of Effectiveness of transcatheter aortic valve implantation with new-generation deVices for severe Aortic steNosis Treatment. Study protocol. G. Italiano Cardiol. 2017, 18 (Suppl. 1), 14S–26S. [Google Scholar]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [Green Version]
- Rockwood, K.; Stadnyk, K.; MacKnight, C.; McDowell, I.; Hébert, R.; Hogan, D.B. A brief clinical instrument to classify frailty in elderly people. Lancet 1999, 353, 205–206. [Google Scholar] [CrossRef]
- Kappetein, A.P.; Head, S.J.; Généreux, P.; Piazza, N.; van Mieghem, N.M.; Blackstone, E.H.; Brott, T.G.; Cohen, D.J.; Cutlip, D.E.; van Es, G.A.; et al. Valve Academic Research Consortium-2. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document. EuroIntervention 2012, 8, 782–795. [Google Scholar] [CrossRef] [Green Version]
- Winter, M.P.; Bartko, P.; Hofer, F.; Zbiral, M.; Burger, A.; Ghanim, B.; Kastner, J.; Lang, I.M.; Mascherbauer, J.; Hengstenberg, C.; et al. Evolution of outcome and complications in TAVR: A meta-analysis of observational and randomized studies. Sci. Rep. 2020, 10, 15568. [Google Scholar] [CrossRef] [PubMed]
- Mohananey, D.; Jobanputra, Y.; Kumar, A.; Krishnaswamy, A.; Mick, S.; White, J.M.; Kapadia, S.R. Clinical and echocardiographic outcomes following permanent pacemaker implantation after transcatheter aortic valve replacement: Meta-analysis and meta-regression. Circ. Cardiovasc. Interv. 2017, 10, e005046. [Google Scholar] [CrossRef] [PubMed]
- Biancari, F.; Pykäri, J.; Savontaus, M.; Laine, M.; Husso, A.; Virtanen, M.; Maaranen, P.; Niemelä, M.; Mäkikallio, T.; Tauriainen, T.; et al. Early and late pacemaker implantation after transcatheter and surgical aortic valve replacement. Catheter. Cardiovasc. Interv. 2021, 97, E560–E568. [Google Scholar] [CrossRef]
- Ando, T.; Briasoulis, A.; Telila, T.; Afonso, L.; Grines, C.L.; Takagi, H. Does mild paravalvular regurgitation post transcatheter aortic valve implantation affect survival? A meta-analysis. Catheter. Cardiovasc. Interv. 2018, 91, 135–147. [Google Scholar] [CrossRef]
- Saad, A.M.; Kassis, N.; Isogai, T.; Gad, M.M.; Ahuja, K.R.; Abdelfattah, O.; Shekhar, S.; Farwati, M.; Yun, J.J.; Krishnaswamy, A.; et al. Trends in outcomes of transcatheter and surgical aortic valve replacement in the United States (2012–2017). Am. J. Cardiol. 2021, 141, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Gaede, L.; Blumenstein, J.; Husser, O.; Liebetrau, C.; Dörr, O.; Grothusen, C.; Eckel, C.; Al-Terki, H.; Kim, W.K.; Nef, H.; et al. Aortic valve replacement in Germany in 2019. Clin. Res. Cardiol. 2021, 110, 460–465. [Google Scholar] [CrossRef]
- Kirmani, B.H.; Jones, S.G.; Malaisrie, S.G.; Chung, D.A.; Williams, R.J. Limited versus full sternotomy for aortic valve replacement. Cochrane Database Syst. Rev. 2017, 4, CD011793. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.; Fujita, B.; Frerker, C.; Bauer, T.; Beckmann, A.; Bekeredjian, R.; Bleiziffer, S.; Möllmann, H.; Walther, T.; Hamm, C.; et al. Transcatheter versus rapid-deployment aortic valve replacement: A propensity-matched analysis from the German aortic valve registry. JACC. Cardiovasc. Interv. 2020, 13, 2642–2654. [Google Scholar] [CrossRef]
- Lemor, A.; Villablanca, P.; Hernandez, G.; Dyal, M.; Jain, T.; Frisoli, T.M.; Wang, D.D.; Eng, M.H.; O’Neill, W. Comparison of outcomes of transcatheter versus surgical aortic valve replacement in patients ≥ 80 years of age. Am. J. Cardiol. 2019, 123, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Panoulas, V.F.; Francis, D.P.; Ruparelia, N.; Malik, I.S.; Chukwuemeka, A.; Sen, S.; Anderson, J.; Nihoyannopoulos, P.; Sutaria, N.; Hannan, E.L.; et al. Female-specific survival advantage from transcatheter aortic valve implantation over surgical aortic valve replacement: Meta-analysis of the gender subgroups of randomised controlled trials including 3758 patients. Int. J. Cardiol. 2018, 250, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Dagan, M.; Yeung, T.; Stehli, J.; Stub, D.; Walton, A.S.; Duffy, S.J. Transcatheter versus surgical aortic valve replacement: An updated systematic review and meta-analysis with a focus on outcomes by sex. Heart Lung Circ. 2021, 30, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Nashef, S.A.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardiothorac. Surg. 2012, 41, 734–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Unmatched Patients | Propensity Score Matched Patients | |||||
|---|---|---|---|---|---|---|
| Variables | SAVR | TAVR | p-Value | SAVR | TAVR | p-Value |
| n = 5350 | n = 2520 | (n = 1008) | (n = 1008) | |||
| Age (years) | 73.2 (9.3) | 82.1 (6.1) | <0.001 | 79.3 (5.5) | 79.5 (6.7) | 0.357 |
| Female | 2488 (46.5) | 1408 (55.9) | <0.001 | 567 (56.3) | 557 (55.3) | 0.651 |
| EuroSCORE II (%) | 3.1 (3.9) | 7.0 (6.9) | <0.001 | 4.5 (5.7) | 4.7 (4.0) | 0.419 |
| Body mass index (kg/m2) | 27.2 (4.4) | 26.4 (4.8) | <0.001 | 26.9 (4.7) | 27.0 (4.8) | 0.529 |
| Hemoglobin (g/dL) a | 12.6 (1.7) | 11.8 (1.7) | <0.001 | 12.3 (1.6) | 12.0 (1.7) | <0.001 |
| eGFR classes | <0.001 | 0.987 | ||||
| >60 mL/min/1.73 m2 | 3539 (68.0) | 1143 (45.4) | 563 (55.9) | 572 (56.7) | ||
| 60–30 mL/min/1.73 m2 | 1441 (27.7) | 1121 (44.5) | 381 (37.8) | 366 (36.3) | ||
| <30 mL/min/1.73 m2 | 227 (4.4) | 253 (10.1) | 64 (6.3) | 70 (6.9) | ||
| Dialysis | 65 (1.2) | 60 (2.4) | <0.001 | 20 (2.0) | 18 (1.8) | 0.739 |
| GSS classes | <0.001 | 0.895 | ||||
| 0 | 4455 (83.3) | 1433 (56.9) | 688 (68.3) | 680 (67.5) | ||
| 1 | 635 (11.9) | 625 (24.8) | 203 (20.1) | 200 (19.8) | ||
| 2 | 260 (4.9) | 461 (18.3) | 117 (11.6) | 128 (12.7) | ||
| Extracardiac arteriopathy | 750 (14.3) | 453 (18.2) | <0.001 | 159 (15.8) | 171 (17.0) | 0.476 |
| Pulmonary disease | 516 (9.6) | 389 (15.5) | <0.001 | 151 (15.0) | 132 (13.1) | 0.225 |
| Diabetes | 1246 (23.3) | 694 (27.7) | <0.001 | 264 (26.2) | 276 (27.4) | 0.541 |
| Neurological or motoric dysfunction | 123 (2.3) | 51 (2.0) | 0.379 | 23 (2.3) | 31 (3.1) | 0.267 |
| Liver chirrosis | 96 (1.9) | 40 (1.6) | 0.434 | 29 (2.9) | 27 (2.7) | 0.789 |
| Pulmonary hypertension | 261 (5.2) | 124 (4.9) | 0.594 | 56 (5.6) | 55 (5.5) | 0.923 |
| Active malignancy | 57 (1.1) | 99 (4.0) | <0.001 | 28 (2.8) | 30 (3.0) | 0.789 |
| Oxygen therapy | 49 (0.9) | 81 (3.2) | <0.001 | 20 (2.0) | 23 (2.3) | 0.639 |
| Prior aortoiliac revascularization | 101 (2.0) | 87 (3.5) | <0.001 | 28 (2.8) | 31 (3.1) | 0.696 |
| Prior cardiac surgery | 223 (4.2) | 382 (15.2) | <0.001 | 66 (6.5) | 76 (7.5) | 0.369 |
| Prior CABG | 71 (1.3) | 243 (9.6) | <0.001 | 33 (3.3) | 42 (4.2) | 0.272 |
| Prior PCI | 409 (8.0) | 353 (14.0) | <0.001 | 111 (11.0) | 117 (11.6) | 0.674 |
| Prior myocardial infarction | <0.001 | 0.329 | ||||
| No | 4746 (90.4) | 2168 (86.1) | 906 (89.9) | 885 (87.8) | ||
| Within 90 days from the procedure | 183 (3.5) | 51 (2.0) | 21 (2.1) | 25 (2.5) | ||
| More than 90 days from the procedure | 323 (6.2) | 299 (11.9) | 81 (8.0) | 98 (9.7) | ||
| Critical preoperative state | 84 (1.6) | 51 (2.0) | 0.153 | 19 (1.9) | 18 (1.8) | 0.869 |
| NYHA classes | <0.001 | 0.952 | ||||
| 1 | 855 (16.1) | 27 (1.1) | 21 (2.1) | 24 (2.4) | ||
| 2 | 2437 (45.8) | 654 (26.1) | 383 (38.0) | 388 (38.5) | ||
| 3 | 1705 (32.0) | 1687 (67.5) | 551 (54.7) | 542 (53.8) | ||
| 4 | 325 (6.1) | 133 (5.3) | 53 (5.3) | 54 (5.4) | ||
| CCS class IV | 245 (4.7) | 107 (4.3) | 0.408 | 39 (3.9) | 42 (4.2) | 0.732 |
| No. of diseased coronary arteries | <0.001 | 0.238 | ||||
| 0 | 3572 (66.8) | 1841 (74.4) | 765 (75.9) | 756 (75.0) | ||
| 1 | 803 (15.0) | 353 (14.3) | 135 (13.4) | 131 (13.0) | ||
| 2 | 530 (9.9) | 134 (5.4) | 65 (6.4) | 58 (5.8) | ||
| 3 | 445 (8.3) | 147 (5.9) | 43 (4.3) | 63 (6.3) | ||
| Left ventricular ejection fraction (%) | 56.5 (9.8) | 53.7 (11.3) | <0.001 | 55.1 (10.4) | 55.0 (11.0) | 0.832 |
| Left ventricular ejection fraction classes | <0.001 | 0.887 | ||||
| >50% | 4130 (83.0) | 1842 (73.2) | 788 (78.2) | 779 (77.3) | ||
| 30–50% | 770 (15.5) | 589 (23.4) | 196 (19.4) | 204 (20.2) | ||
| <30% | 74 (1.5) | 87 (3.5) | 24 (2.4) | 25 (2.5) | ||
| Mitral valve regurgitation | <0.001 | 0.980 | ||||
| None/trace | 2528 (47.3) | 367 (14.7) | 251 (24.9) | 248 (24.6) | ||
| Mild | 2196 (41.0) | 1329 (53.2) | 539 (53.5) | 534 (53.0) | ||
| Moderate | 555 (10.4) | 690 (27.6) | 189 (18.8) | 196 (19.4) | ||
| Severe | 71 (1.3) | 113 (4.5) | 29 (2.9) | 30 (3.0) | ||
| Aortic valve area (cm2) a | 0.7 (0.2) | 0.7 (0.2) | <0.001 | 0.7 (0.2) | 0.7 (0.2) | 0.002 |
| Aortic annulus diameter (mm) a | 21.7 (2.3) | 22.5 (2.4) | <0.001 | 21.5 (2.3) | 22.4 (2.3) | <0.001 |
| Mean transvalvular gradient (mmHg) a | 50.7 (15.1) | 47.0 (15.1) | <0.001 | 51.6 (14.5) | 47.8 (14.2) | <0.001 |
| Peak transvalvular gradient (mmHg) a | 81.7 (22.8) | 75.3 (23.0) | <0.001 | 82.9 (21.8) | 76.3 (22.2) | <0.001 |
| Concomitant coronary revascularization | 1407 (27.3) | 165 (6.6) | <0.001 | 117 (11.6) | 109 (10.8) | 0.566 |
| Outcomes | SAVR (n = 1008) | TAVR (n = 1008) | p-Value |
|---|---|---|---|
| 30-day death | 35 (3.5) | 18 (1.8) | 0.020 |
| Stroke | 23 (2.3) | 8 (0.8) | 0.005 |
| Conversion to cardiac surgery | 0 | 3 (0.3) | - |
| Major complication at LV apex | 0 | 1 (0.1 | - |
| Major vascular injury | 1 (0.1) | 22 (2.2) | <0.001 |
| Acute kidney injury | 81 (8.2) | 6 (0.6) | <0.001 |
| Postop. change in eGFR (mL/min/1.73 m2) | −12.54 (18.0) | 1.9 (15.5) | <0.001 |
| Prosthesis migration | 0 | 13 (1.3) | - |
| Permanent pacemaker implantation | 33 (3.3) | 139 (13.8) | <0.001 |
| Cardiogenic shock | 51 (5.1) | 14 (1.4) | <0.001 |
| Infectious complication | 64 (6.5) | 38 (3.8) | 0.006 |
| Type of infection | 0.006 | ||
| Surgical site infection | 20 (2.0) | 4 (0.4) | |
| Organ/system infection | 34 (3.5) | 26 (2.6) | |
| Sepsis | 10 (1.0) | 7 (0.7) | |
| Red blood cell transfusion | 580 (58.9) | 156 (15.5) | <0.001 |
| No. of transfused RBC units | 1.8 (2.9) | 0.3 (1.0) | <0.001 |
| Cardiac tamponade | <0.001 | ||
| Surgical treatment | 35 (3.5) | 6 (0.6) | |
| Percutaneous treatment | 2 (0.2) | 7 (0.7) | |
| Mean transvalvular gradient (mmHg) | 13.5 (6.3) | 8.9 (4.8) | <0.001 |
| Peak transvalvular gradient (mmHg) | 24.7 (10.7) | 16.3 (7.9) | <0.001 |
| Paravalvular regurgitation | <0.001 | ||
| None/trace | 840 (87.7) | 559 (58.4) | |
| Mild | 98 (10.3) | 340 (35.5) | |
| Moderate | 15 (1.6) | 54 (5.6) | |
| Severe | 5 (0.5) | 5 (0.5) | |
| Not reported | 50 | 50 |
| One-Year Outcomes | SAVR (n = 1008) | TAVR (n = 1008) | p-Value | HR/SHR, 95% CI * |
|---|---|---|---|---|
| Death | 11.5% | 7.9% | 0.006 | 0.67, 0.50–0.89 |
| MACCE | 15.8% | 12.0% | 0.011 | 0.74, 0.58–0.93 |
| Readmission due to heart failure | 15.9% | 10.8% | <0.001 | 0.66, 0.52–0.85 |
| Reoperation for aortic valve prosthesis complications | 0.3% | 0.4% | 0.705 | 1.33, 0.30–5.96 |
| Permanent pacemaker implantation | 6.4% | 16.2% | <0.001 | 2.77, 2.09–3.68 |
| Stroke | 5.1% | 3.2% | 0.033 | 0.62, 0.40–0.97 |
| Myocardial infarction | 1.8% | 1.6% | 0.728 | 0.89, 0.45–1.74 |
| Percutaneous coronary intervention | 0.2% | 1.3% | 0.004 | 6.54, 1.48–28.94 |
| Coronary artery bypass grafting | 0 | 0 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosato, S.; Biancari, F.; D’Errigo, P.; Barbanti, M.; Tarantini, G.; Bedogni, F.; Ranucci, M.; Costa, G.; Juvonen, T.; Ussia, G.P.; et al. One-Year Outcomes after Surgical versus Transcatheter Aortic Valve Replacement with Newer Generation Devices. J. Clin. Med. 2021, 10, 3703. https://doi.org/10.3390/jcm10163703
Rosato S, Biancari F, D’Errigo P, Barbanti M, Tarantini G, Bedogni F, Ranucci M, Costa G, Juvonen T, Ussia GP, et al. One-Year Outcomes after Surgical versus Transcatheter Aortic Valve Replacement with Newer Generation Devices. Journal of Clinical Medicine. 2021; 10(16):3703. https://doi.org/10.3390/jcm10163703
Chicago/Turabian StyleRosato, Stefano, Fausto Biancari, Paola D’Errigo, Marco Barbanti, Giuseppe Tarantini, Francesco Bedogni, Marco Ranucci, Giuliano Costa, Tatu Juvonen, Gian Paolo Ussia, and et al. 2021. "One-Year Outcomes after Surgical versus Transcatheter Aortic Valve Replacement with Newer Generation Devices" Journal of Clinical Medicine 10, no. 16: 3703. https://doi.org/10.3390/jcm10163703
APA StyleRosato, S., Biancari, F., D’Errigo, P., Barbanti, M., Tarantini, G., Bedogni, F., Ranucci, M., Costa, G., Juvonen, T., Ussia, G. P., Marcellusi, A., Baglio, G., Cicala, S. D., Badoni, G., Seccareccia, F., Tamburino, C., & on behalf of the OBSERVANT II Research Group. (2021). One-Year Outcomes after Surgical versus Transcatheter Aortic Valve Replacement with Newer Generation Devices. Journal of Clinical Medicine, 10(16), 3703. https://doi.org/10.3390/jcm10163703










