Diagnosis of Cardiac Surgery-Associated Acute Kidney Injury
Abstract
:1. Introduction
2. Pathophysiology of CSA-AKI
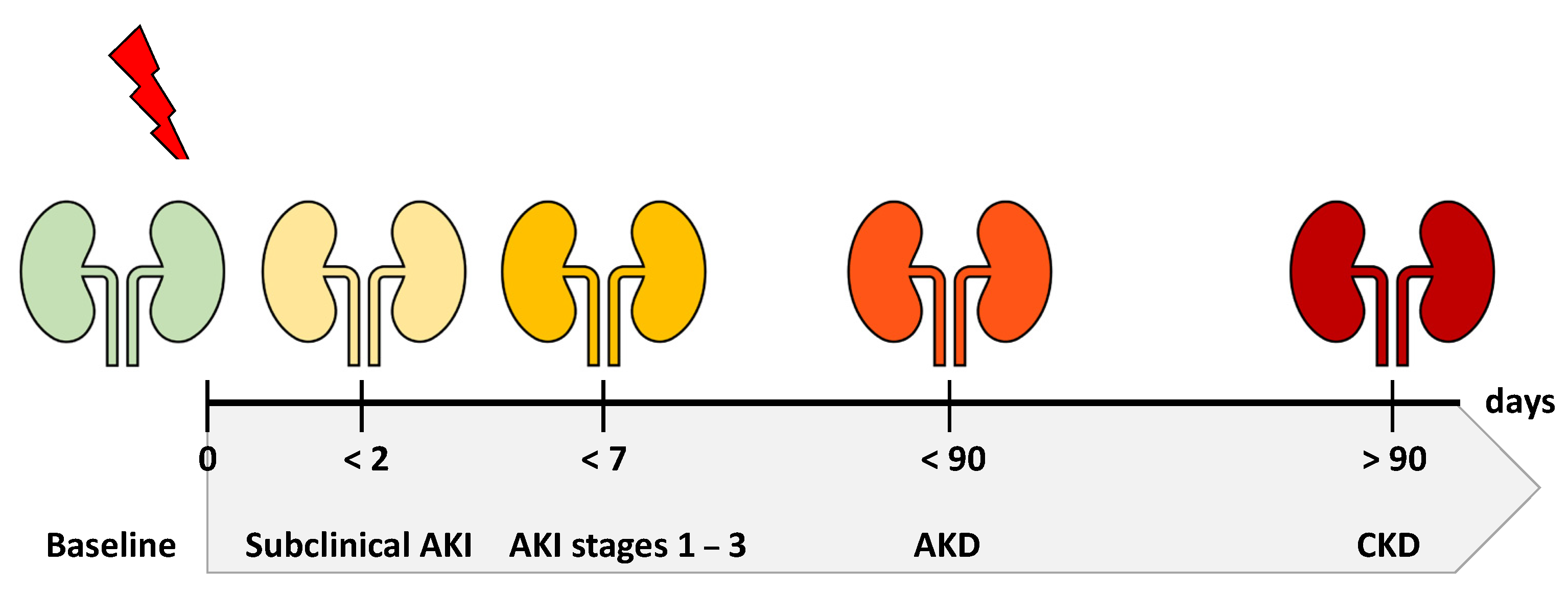
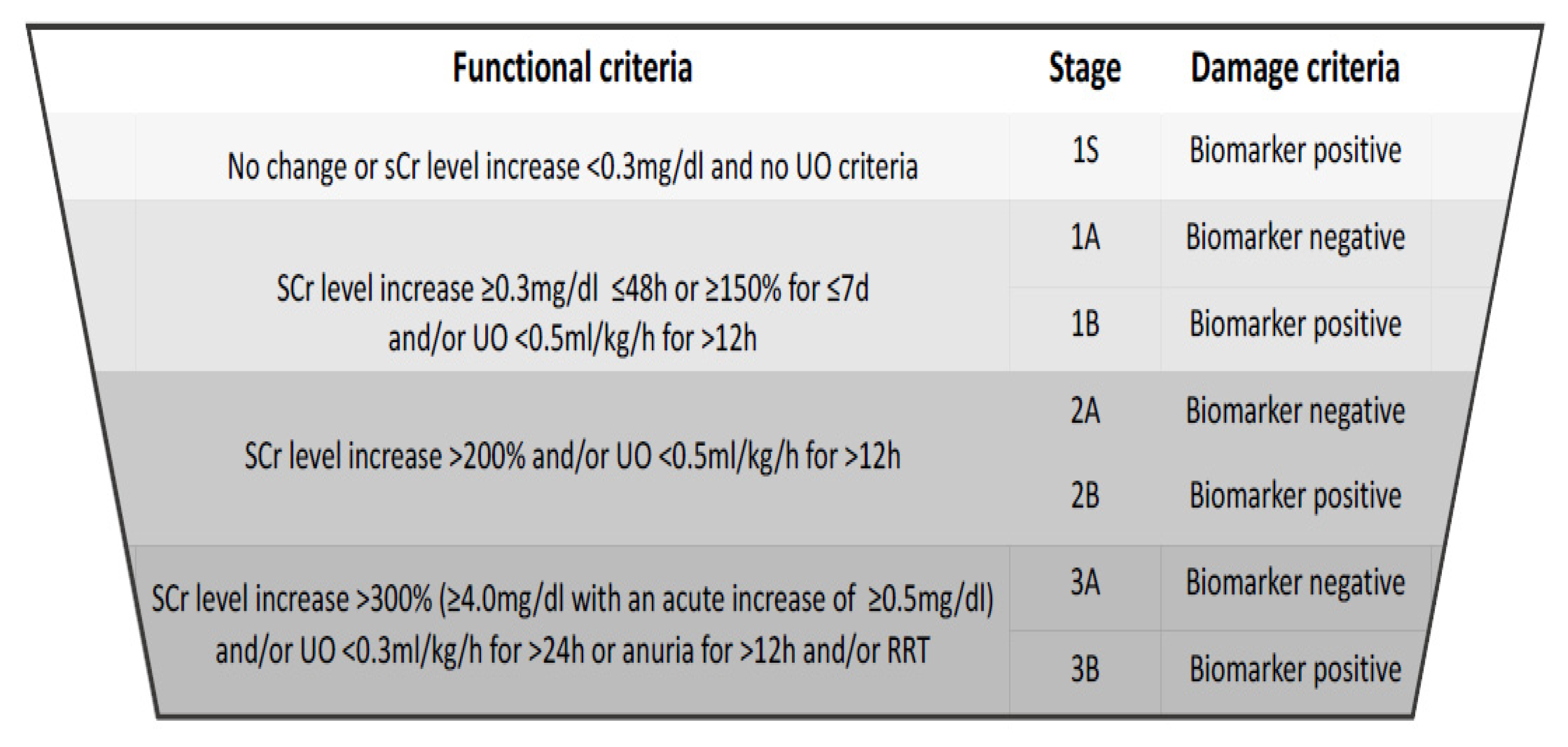
3. Diagnostic Criteria and the Spectrum of Disease
4. Serum Creatinine and Urine Output
5. Biomarkers of CSA-AKI
5.1. NGAL
5.2. KIM-1
5.3. [TIMP-2] × [IGFBP7]
6. Novel Candidates
6.1. Dickkopf-3
6.2. CCL-14
6.3. Renin
6.4. Free Hemoglobin
7. Biomarker-Enhanced Diagnostic Criteria?
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Jaghbeer, M.; Dealmeida, D.; Bilderback, A.; Ambrosino, R.; Kellum, J.A. Clinical Decision Support for In-Hospital AKI. J. Am. Soc. Nephrol. 2018, 29, 654–660. [Google Scholar] [CrossRef]
- Hoste, E.A.J.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, P.; Zarbock, A.; Izawa, J.; Gleason, T.G.; Renfurm, R.W.; Kellum, J.A. The impact of acute kidney injury by serum creatinine or urine output criteria on major adverse kidney events in cardiac surgery patients. J. Thorac. Cardiovasc. Surg. 2020, 162, 143–151.e7. [Google Scholar] [CrossRef]
- Grams, M.E.; Sang, Y.; Coresh, J.; Ballew, S.; Matsushita, K.; Molnar, M.Z.; Szabo, Z.; Kalantar-Zadeh, K.; Kovesdy, C.P. Acute Kidney Injury After Major Surgery: A Retrospective Analysis of Veterans Health Administration Data. Am. J. Kidney Dis. 2016, 67, 872–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakar, C.V.; Worley, S.; Arrigain, S.; Yared, J.-P.; Paganini, E.P. Improved survival in acute kidney injury after cardiac surgery. Am. J. Kidney Dis. 2007, 50, 703–711. [Google Scholar] [CrossRef]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute kidney injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef]
- Lopez-Delgado, J.C.; Esteve, F.; Torrado, H.; Rodríguez-Castro, D.; Carrio, M.L.; Farrero, E.; Javierre, C.; Ventura, J.L.; Manez, R. Influence of acute kidney injury on short- and long-term outcomes in patients undergoing cardiac surgery: Risk factors and prognostic value of a modified RIFLE classification. Crit. Care 2013, 17, R293. [Google Scholar] [CrossRef] [Green Version]
- Thakar, C.V.; Arrigain, S.; Worley, S.; Yared, J.-P.; Paganini, E.P. A Clinical Score to Predict Acute Renal Failure after Cardiac Surgery. J. Am. Soc. Nephrol. 2004, 16, 162–168. [Google Scholar] [CrossRef] [Green Version]
- Mehta, R.H.; Grab, J.D.; O’Brien, S.M.; Bridges, C.R.; Gammie, J.S.; Haan, C.K.; Ferguson, T.B.; Peterson, E.D.; Society of Thoracic Surgeons National Cardiac Surgery Database Investigators. Bedside Tool for Predicting the Risk of Postoperative Dialysis in Patients Undergoing Cardiac Surgery. Circulation 2006, 114, 2208–2216. [Google Scholar] [CrossRef]
- Birnie, K.; Verheyden, V.; Pagano, D.; Bhabra, M.; Tilling, K.; Sterne, J.A.; Murphy, G.J.; UK AKI in Cardiac Surgery Collaborators. Predictive models for kidney disease: Improving global outcomes (KDIGO) defined acute kidney injury in UK cardiac surgery. Crit. Care 2014, 18, 606. [Google Scholar] [CrossRef] [Green Version]
- O’Neal, J.B.; Shaw, A.D.; Billings, F.T., IV. Acute kidney injury following cardiac surgery: Current understanding and future directions. Crit. Care 2016, 20, 187. [Google Scholar] [CrossRef] [Green Version]
- Presta, P.; Bolignano, D.; Coppolino, G.; Serraino, F.; Mastroroberto, P.; Andreucci, M.; Fuiano, G. Antecedent ACE-inhibition, inflammatory response, and cardiac surgery associated acute kidney injury. Rev. Cardiovasc. Med. 2021, 22, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-Y.; Yiang, G.-T.; Liao, W.-T.; Tsai, A.P.-Y.; Cheng, Y.-L.; Cheng, P.-W.; Li, C.-Y.; Li, C.-J. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell. Physiol. Biochem. 2018, 46, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Serraino, G.F.; Provenzano, M.; Jiritano, F.; Michael, A.; Ielapi, N.; Mastroroberto, P.; Andreucci, M.; Serra, R. Risk factors for acute kidney injury and mortality in high risk patients undergoing cardiac surgery. PLoS ONE 2021, 16, e0252209. [Google Scholar] [CrossRef]
- Küllmar, M.; Saadat-Gilani, K.; Weiss, R.; Massoth, C.; Lagan, A.; Cortés, M.N.; Gerss, J.; Chawla, L.S.; Fliser, D.; Meersch, M.; et al. Kinetic Changes of Plasma Renin Concentrations Predict Acute Kidney Injury in Cardiac Surgery Patients. Am. J. Respir. Crit. Care Med. 2021, 203, 1119–1126. [Google Scholar] [CrossRef]
- Bellomo, R.; Ronco, C.; Kellum, J.A.; Mehta, R.L.; Palevsky, P.; Acute Dialysis Quality Initiative Workgroup. Acute renal failure—Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 2004, 8, R204–R212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C.; Warnock, D.G.; Levin, A.; Acute Kidney Injury Network, the A.K.I. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 2007, 11, R31. [Google Scholar] [CrossRef] [Green Version]
- KDIGO AKI Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury; Elsevier: Amsterdam, The Netherlands, 2012; Volume 2. [Google Scholar] [CrossRef] [Green Version]
- Kellum, J.A.; Ronco, C.; Bellomo, R. Conceptual advances and evolving terminology in acute kidney disease. Nat. Rev. Nephrol. 2021, 17, 493–502. [Google Scholar] [CrossRef]
- Levey, A.S.; Eckardt, K.-U.; Dorman, N.M.; Christiansen, S.L.; Hoorn, E.J.; Ingelfinger, J.R.; Inker, L.A.; Levin, A.; Mehrotra, R.; Palevsky, P.M.; et al. Nomenclature for kidney function and disease: Report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2020, 97, 1117–1129. [Google Scholar] [CrossRef]
- Biswas, A.; Parikh, C.R.; Feldman, H.I.; Garg, A.X.; Latham, S.; Lin, H.; Palevsky, P.M.; Ugwuowo, U.; Wilson, F.P. Identification of Patients Expected to Benefit from Electronic Alerts for Acute Kidney Injury. Clin. J. Am. Soc. Nephrol. 2018, 13, 842–849. [Google Scholar] [CrossRef]
- Mutter, M.; Martin, M.; Yamamoto, Y.; Biswas, A.; Etropolski, B.; Feldman, H.; Garg, A.; Gourlie, N.; Latham, S.; Lin, H.; et al. Electronic Alerts for Acute Kidney Injury Amelioration (ELAIA-1): A completely electronic, multicentre, randomised controlled trial: Design and rationale. BMJ Open 2019, 9, e025117. [Google Scholar] [CrossRef] [Green Version]
- Wijeysundera, D.N.; Rao, V.; Beattie, W.S.; Ivanov, J.; Karkouti, K. Evaluating surrogate measures of renal dysfunction after cardiac surgery. Anesth. Analg. 2003, 96, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Sileanu, F.E.; Murugan, R.; Lucko, N.; Shaw, A.D.; Clermont, G. Classifying AKI by Urine Output versus Serum Creatinine Level. J. Am. Soc. Nephrol. 2015, 26, 2231–2238. [Google Scholar] [CrossRef] [PubMed]
- Howitt, S.H.; Grant, S.W.; Caiado, C.; Carlson, E.; Kwon, D.; Dimarakis, I.; Malagon, I.; McCollum, C. The KDIGO acute kidney injury guidelines for cardiac surgery patients in critical care: A validation study. BMC Nephrol. 2018, 19, 149. [Google Scholar] [CrossRef] [PubMed]
- McIlroy, D.R.; Argenziano, M.; Farkas, D.; Umann, T.; Sladen, R.N. Incorporating oliguria into the diagnostic criteria for acute kidney injury after on-pump cardiac surgery: Impact on incidence and outcomes. J. Cardiothorac. Vasc. Anesth. 2013, 27, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Murugan, R.; Sileanu, F.E.; Foldes, E.; Priyanka, P.; Clermont, G.; Kellum, J.A. Intensive Monitoring of Urine Output Is Associated with Increased Detection of Acute Kidney Injury and Improved Outcomes. Chest 2017, 152, 972–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rewa, O.G.; Bagshaw, S.M.; Wang, X.; Wald, R.; Smith, O.; Shapiro, J.; McMahon, B.; Liu, K.D.; Trevino, S.A.; Chawla, L.S.; et al. The furosemide stress test for prediction of worsening acute kidney injury in critically ill patients: A multicenter, prospective, observational study. J. Crit. Care 2019, 52, 109–114. [Google Scholar] [CrossRef]
- Koyner, J.L.; Davison, D.L.; Brasha-Mitchell, E.; Chalikonda, D.M.; Arthur, J.M.; Shaw, A.D.; Tumlin, J.A.; Trevino, S.A.; Bennett, M.R.; Kimmel, P.L.; et al. Furosemide Stress Test and Biomarkers for the Prediction of AKI Severity. J. Am. Soc. Nephrol. 2015, 26, 2023–2031. [Google Scholar] [CrossRef] [PubMed]
- Lumlertgul, N.; Peerapornratana, S.; Trakarnvanich, T.; Pongsittisak, W.; Surasit, K.; Chuasuwan, A.; Tankee, P.; Tiranathanagul, K.; Praditpornsilpa, K.; Tungsanga, K.; et al. Early versus standard initiation of renal replacement therapy in furosemide stress test non-responsive acute kidney injury patients (the FST trial). Crit. Care 2018, 22, 101. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.-C.; Lei, S.-H.; Yang, X.; Zhang, Y.; Qiu, S.-D.; Liu, W.-F.; Li, C.; Liu, K.-X. Assessment of prognostic value of intraoperative oliguria for postoperative acute kidney injury: A retrospective cohort study. Br. J. Anaesth. 2021, 126, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Chaudery, H.; Macdonald, N.; Ahmad, T.; Chandra, S.; Tantri, A.; Sivasakthi, V.; Mansor, M.; Matos, R.; Pearse, R.M.; Prowle, J.R. Acute kidney injury and risk of death after elective surgery: Prospective analysis of data from an international cohort study. Anesth. Analg. 2019, 128, 1022–1029. [Google Scholar] [CrossRef]
- Lassnigg, A.; Schmid, E.R.; Hiesmayr, M.; Falk, C.; Druml, W.; Bauer, P.; Schmidlin, D. Impact of minimal increases in serum creatinine on outcome in patients after cardiothoracic surgery: Do we have to revise current definitions of acute renal failure? Crit. Care Med. 2008, 36, 1129–1137. [Google Scholar] [CrossRef]
- Davidson, J.A.; Urban, T.T.; Baird, C.; Tong, S.; Woodruff, A.; Twite, M.; Jaggers, J.; Simões, E.A.F.; Wischmeyer, P. Alkaline Phosphatase in Infant Cardiopulmonary Bypass: Kinetics and Relationship to Organ Injury and Major Cardiovascular Events. J. Pediatr. 2017, 190, 49–55.e2. [Google Scholar] [CrossRef] [PubMed]
- Lannemyr, L.; Lundin, E.; Reinsfelt, B.; Bragadottir, G.; Redfors, B.; Oras, J.; Ricksten, S.-E. Renal tubular injury during cardiopulmonary bypass as assessed by urinary release of N-acetyl-ß-D-glucosaminidase. Acta Anaesthesiol. Scand. 2017, 61, 1075–1083. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, W.; Zhang, J.; Xu, C.; Yu, S.; Mao, Z.; Wu, J.; Ye, C.; Mei, C.; Dai, B. Urinary interleukin 18 for detection of acute kidney injury: A meta-analysis. Am. J. Kidney Dis. 2013, 62, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Silverton, N.A.; Hall, I.E.; Melendez, N.P.; Harris, B.; Harley, J.S.; Parry, S.R.; Lofgren, L.R.; Stoddard, G.J.; Hoareau, G.L.; Kuck, K. Intraoperative Urinary Biomarkers and Acute Kidney Injury After Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2021, 35, 1691–1700. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, X.; Xie, B.; Huang, R.; Yan, Y.; Liu, S.; Zhu, M.; Lu, R.; Qian, J.; Ni, Z.; et al. Early serum cystatin C-enhanced risk prediction for acute kidney injury post cardiac surgery: A prospective, observational, cohort study. Biomarkers 2020, 25, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Załęska-Kocięcka, M.; Skrobisz, A.; Wojtkowska, I.; Grabowski, M.; Dąbrowski, M.; Kuśmierski, K.; Piotrowska, K.; Imiela, J.; Stępińska, J. Serum beta-2 microglobulin levels for predicting acute kidney injury complicating aortic valve replacement. Interact. Cardiovasc. Thorac. Surg. 2017, 25, 533–540. [Google Scholar] [CrossRef] [Green Version]
- Che, M.; Xie, B.; Xue, S.; Dai, H.; Qian, J.; Ni, Z.; Axelsson, J.; Yan, Y. Clinical usefulness of novel biomarkers for the detection of acute kidney injury following elective cardiac surgery. Nephron. Clin. Pract. 2010, 115, c66–c72. [Google Scholar] [CrossRef]
- Cruz, D.N.; Ronco, C.; Katz, N. Neutrophil gelatinase-associated lipocalin: A promising biomarker for detecting cardiac surgery–associated acute kidney injury. J. Thorac. Cardiovasc. Surg. 2010, 139, 1101–1106. [Google Scholar] [CrossRef] [Green Version]
- Koyner, J.L.; Bennett, M.R.; Worcester, E.M.; Ma, Q.; Raman, J.; Jeevanandam, V.; Kasza, K.E.; O’Connor, M.F.; Konczal, D.J.; Trevino, S.; et al. Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int. 2008, 74, 1059–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagener, G.; Gubitosa, G.; Wang, S.; Borregaard, N.; Kim, M.; Lee, H.T. Urinary neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery. Am. J. Kidney Dis. 2008, 52, 425–433. [Google Scholar] [CrossRef]
- Tuladhar, S.M.; Püntmann, V.O.; Soni, M.; Punjabi, P.P.; Bogle, R.G. Rapid detection of acute kidney injury by plasma and urinary neutrophil gelatinase-associated lipocalin after cardiopulmonary bypass. J. Cardiovasc. Pharmacol. 2009, 53, 261–266. [Google Scholar] [CrossRef]
- Mishra, J.; Ma, Q.; Prada, A.; Mitsnefes, M.; Zahedi, K.; Yang, J.; Barasch, J.; Devarajan, P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J. Am. Soc. Nephrol. 2003, 14, 2534–2543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, F.; Luo, Q.; Wang, L.; Han, L. Diagnostic value of neutrophil gelatinase-associated lipocalin for early diagnosis of cardiac surgery-associated acute kidney injury: A meta-analysis. Eur. J. Cardiothorac. Surg. 2016, 49, 746–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, J.; Qiu, Y.; Qin, Z.; Su, B. The value of kidney injury molecule 1 in predicting acute kidney injury in adult patients: A systematic review and Bayesian meta-analysis. J. Transl. Med. 2021, 19, 105. [Google Scholar] [CrossRef] [PubMed]
- Meersch, M.; Schmidt, C.; Van Aken, H.; Martens, S.; Rossaint, J.; Singbartl, K.; Görlich, D.; Kellum, J.A.; Zarbock, A. Urinary TIMP-2 and IGFBP7 as Early Biomarkers of Acute Kidney Injury and Renal Recovery following Cardiac Surgery. PLoS ONE 2014, 9, e93460. [Google Scholar] [CrossRef] [Green Version]
- Ilaria, G.; Kianoush, K.; Ruxandra, B.; Francesca, M.; Mariarosa, C.; Davide, G.; Claudio, R. Clinical adoption of Nephrocheck® in the early detection of acute kidney injury. Ann. Clin. Biochem. 2021, 58, 6–15. [Google Scholar] [CrossRef]
- Tai, Q.; Yi, H.; Wei, X.; Xie, W.; Zeng, O.; Zheng, D.; Sun, J.; Wang, G.; Wang, S.; Liu, G. The Accuracy of Urinary TIMP-2 and IGFBP7 for the Diagnosis of Cardiac Surgery-Associated Acute Kidney Injury: A Systematic Review and Meta-Analysis. J. Intensive Care Med. 2020, 35, 1013–1025. [Google Scholar] [CrossRef]
- Cummings, J.J.; Shaw, A.D.; Shi, J.; Lopez, M.G.; O’Neal, J.B.; Billings, F.T. Intraoperative prediction of cardiac surgery-associated acute kidney injury using urinary biomarkers of cell cycle arrest. J. Thorac. Cardiovasc. Surg. 2019, 157, 1545–1553.e5. [Google Scholar] [CrossRef] [PubMed]
- Grieshaber, P.; Möller, S.; Arneth, B.; Roth, P.; Niemann, B.; Renz, H.; Böning, A. Predicting Cardiac Surgery-Associated Acute Kidney Injury Using a Combination of Clinical Risk Scores and Urinary Biomarkers. Thorac. Cardiovasc. Surg. 2020, 68, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Schunk, S.J.; Zarbock, A.; Meersch, M.; Küllmar, M.; Kellum, J.A.; Schmit, D.; Wagner, M.; Triem, S.; Wagenpfeil, S.; Gröne, H.-J.; et al. Association between urinary dickkopf-3, acute kidney injury, and subsequent loss of kidney function in patients undergoing cardiac surgery: An observational cohort study. Lancet 2019, 394, 488–496. [Google Scholar] [CrossRef]
- Massoth, C.; Küllmar, M.; Enders, D.; Kellum, J.A.; Forni, L.G.; Meersch, M.; Zarbock, A.; Progressive AKI Group. Comparison of C-C motif chemokine ligand 14 with other biomarkers for adverse kidney events after cardiac surgery. J. Thorac. Cardiovasc. Surg. 2021, 10, S0022-5223(21)00436-0. [Google Scholar] [CrossRef]
- Hoste, E.; Bihorac, A.; Al-Khafaji, A.; Ortega, L.M.; Ostermann, M.; Haase, M.; Zacharowski, K.; Wunderink, R.; Heung, M.; Lissauer, M.; et al. Identification and validation of biomarkers of persistent acute kidney injury: The RUBY study. Intensive Care Med. 2020, 46, 943–953. [Google Scholar] [CrossRef] [Green Version]
- Paul, M.; Poyan Mehr, A.; Kreutz, R. Physiology of local renin-angiotensin systems. Physiol. Rev. 2006, 86, 747–803. [Google Scholar] [CrossRef]
- Hu, J.; Rezoagli, E.; Zadek, F.; Bittner, E.A.; Lei, C.; Berra, L. Free Hemoglobin Ratio as a Novel Biomarker of Acute Kidney Injury After On-Pump Cardiac Surgery: Secondary Analysis of a Randomized Controlled Trial. Anesth. Analg. 2021, 132, 1548–1558. [Google Scholar] [CrossRef]
- Meersch, M.; Schmidt, C.; Hoffmeier, A.; Van Aken, H.; Wempe, C.; Gerss, J.; Zarbock, A. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: The PrevAKI randomized controlled trial. Intensive Care Med. 2017, 43, 1551–1561. [Google Scholar] [CrossRef] [Green Version]
- Engelman, D.T.; Ben Ali, W.; Williams, J.B.; Perrault, L.P.; Reddy, V.S.; Arora, R.C.; Roselli, E.E.; Khoynezhad, A.; Gerdisch, M.; Levy, J.H.; et al. Guidelines for Perioperative Care in Cardiac Surgery: Enhanced Recovery after Surgery Society Recommendations. JAMA Surg. 2019, 154, 755–766. [Google Scholar] [CrossRef]
- Zarbock, A.; Küllmar, M.; Ostermann, M.; Lucchese, G.; Baig, K.; Cennamo, A.; Rajani, R.; McCorkell, S.; Arndt, C.; Wulf, H.; et al. Prevention of Cardiac Surgery–Associated Acute Kidney Injury by Implementing the KDIGO Guidelines in High-Risk Patients Identified by Biomarkers: The PrevAKI-Multicenter Randomized Controlled Trial. Anesth. Analg. 2021, 133, 292–302. [Google Scholar] [CrossRef]
- Engelman, D.T.; Crisafi, C.; Germain, M.; Greco, B.; Nathanson, B.H.; Engelman, R.M.; Schwann, T.A. Using urinary biomarkers to reduce acute kidney injury following cardiac surgery. J. Thorac. Cardiovasc. Surg. 2020, 160, 1235–1246.e2. [Google Scholar] [CrossRef] [PubMed]
- Göcze, I.; Jauch, D.; Götz, M.; Kennedy, P.; Jung, B.; Zeman, F.; Gnewuch, C.; Graf, B.M.; Gnann, W.; Banas, B.; et al. Biomarker-guided Intervention to Prevent Acute Kidney Injury after Major Surgery: The Prospective Randomized BigpAK Study. Ann. Surg. 2018, 267, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, M.; Zarbock, A.; Goldstein, S.; Kashani, K.; Macedo, E.; Murugan, R.; Bell, M.; Forni, L.; Guzzi, L.; Joannidis, M.; et al. Recommendations on Acute Kidney Injury Biomarkers from the Acute Disease Quality Initiative Consensus Conference. JAMA Netw. Open 2020, 3, e2019209. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massoth, C.; Zarbock, A. Diagnosis of Cardiac Surgery-Associated Acute Kidney Injury. J. Clin. Med. 2021, 10, 3664. https://doi.org/10.3390/jcm10163664
Massoth C, Zarbock A. Diagnosis of Cardiac Surgery-Associated Acute Kidney Injury. Journal of Clinical Medicine. 2021; 10(16):3664. https://doi.org/10.3390/jcm10163664
Chicago/Turabian StyleMassoth, Christina, and Alexander Zarbock. 2021. "Diagnosis of Cardiac Surgery-Associated Acute Kidney Injury" Journal of Clinical Medicine 10, no. 16: 3664. https://doi.org/10.3390/jcm10163664
APA StyleMassoth, C., & Zarbock, A. (2021). Diagnosis of Cardiac Surgery-Associated Acute Kidney Injury. Journal of Clinical Medicine, 10(16), 3664. https://doi.org/10.3390/jcm10163664





