Degenerative Cervical Myelopathy: Clinical Presentation, Assessment, and Natural History
Abstract
:1. Introduction
2. Topics
2.1. Pathophysiology
2.2. Presentation
2.3. Differential Diagnoses
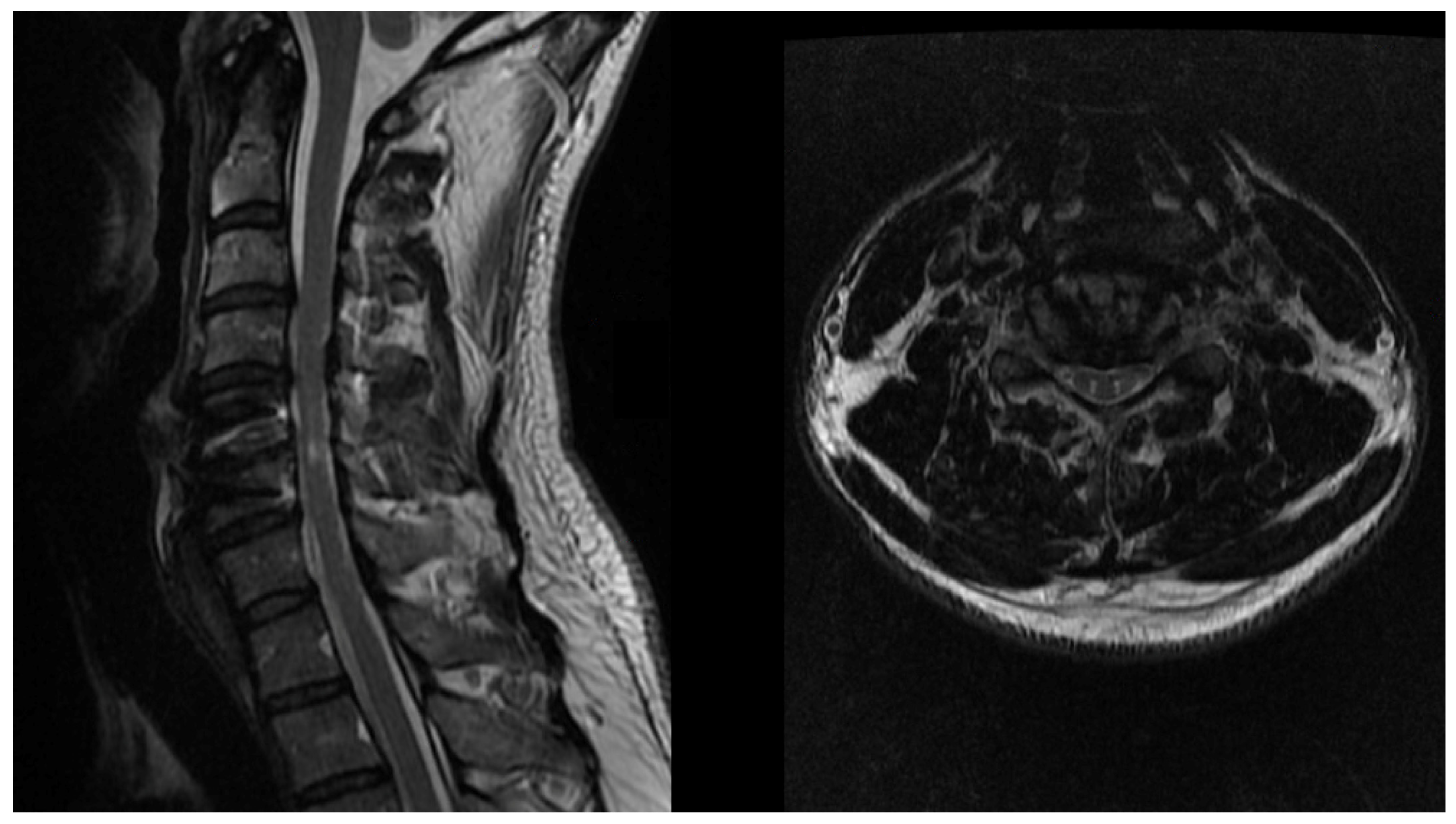
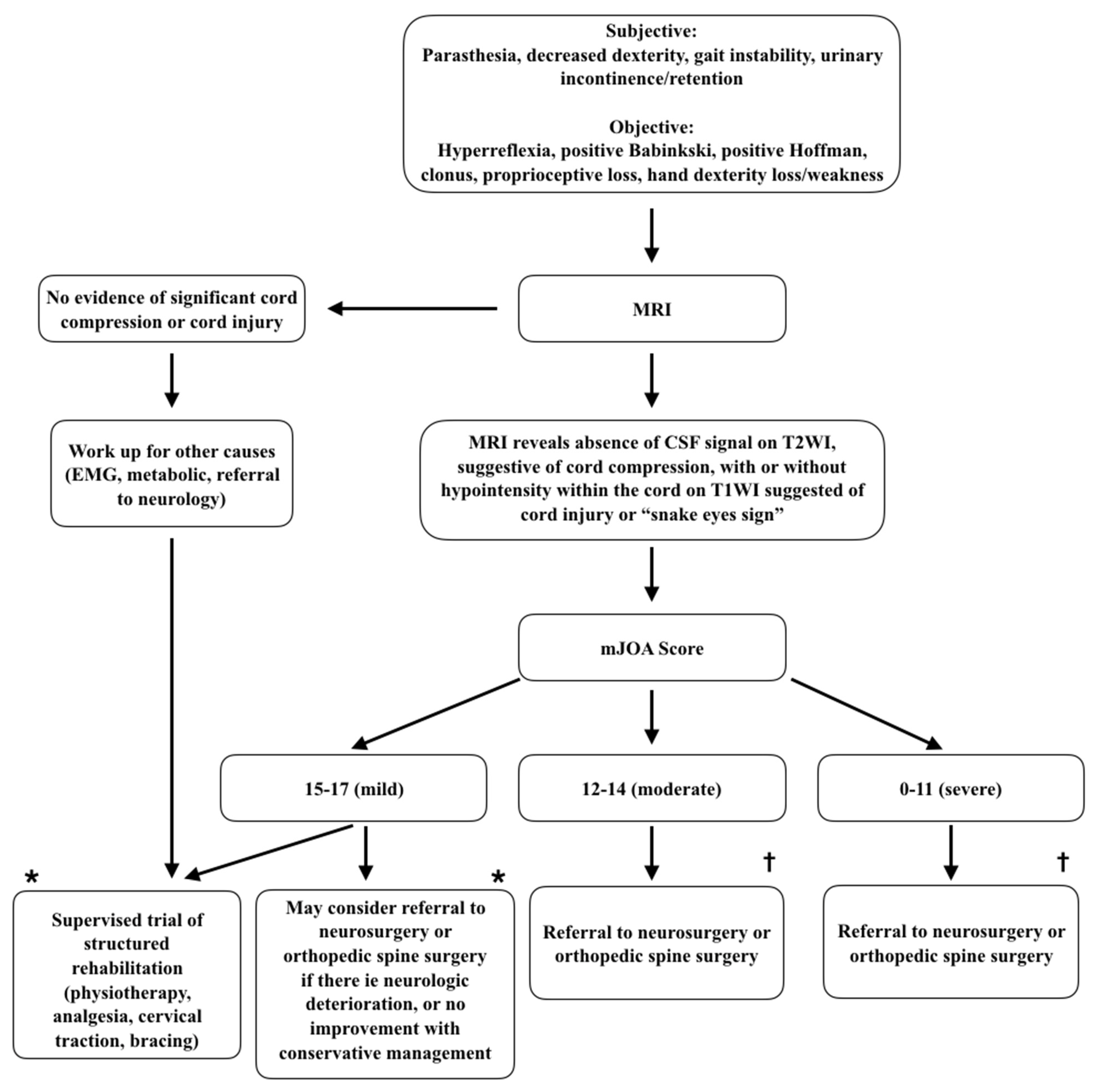
2.4. Diagnosis
2.5. Natural History and Conservative Management
2.6. Surgical Management
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kalsi-Ryan, S.; Karadimas, S.K.; Fehlings, M.G. Cervical Spondylotic Myelopathy: The Clinical Phenomenon and the Current Pathobiology of an Increasingly Prevalent and Devastating Disorder. Neuroscientist 2013, 19, 409–421. [Google Scholar] [CrossRef]
- Witiw, C.D.; Fehlings, M.G. Degenerative cervical myelopathy. Can. Med. Assoc. J. 2017, 189, E116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakhsheshian, J.; Mehta, V.A.; Liu, J.C. Current Diagnosis and Management of Cervical Spondylotic Myelopathy. Glob. Spine J. 2017, 7, 572–586. [Google Scholar] [CrossRef] [PubMed]
- Behrbalk, E.; Salame, K.; Regev, G.J.; Keynan, O.; Boszczyk, B.; Lidar, Z. Delayed diagnosis of cervical spondylotic myelopathy by primary care physicians. Neurosurg. Focus 2013, 35, E1. [Google Scholar] [CrossRef] [PubMed]
- Baptiste, D.C.; Fehlings, M.G. Pathophysiology of cervical myelopathy. Spine J. 2006, 6, S190–S197. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Skaf, G. A Review of the Pathophysiology of Cervical Spondylotic Myelopathy With Insights for Potential Novel Mechanisms Drawn From Traumatic Spinal Cord Injury. Spine 1998, 23, 2730–2736. [Google Scholar] [CrossRef]
- Vilaça, C.D.O.; Orsini, M.; Araujo Leite, M.A.; De Freitas, M.R.G.; Davidovich, E.; Fiorelli, R.; Fiorelli, S.; Fiorelli, C.; Oliveira, A.B.; Pessoa, B.L. Cervical spondylotic myelopathy: What the neurologist should know. Neurol. Int. 2016, 8, 69–73. [Google Scholar] [CrossRef] [Green Version]
- Milligan, J.; Ryan, K.; Fehlings, M.; Bauman, C. Degenerative cervical myelopathy: Diagnosis and management in primary care. Can. Fam. Physician Med. Fam. Can. 2019, 65, 619–624. [Google Scholar]
- Wang, C.; Laiwalla, A.; Salamon, N.; Ellingson, B.M.; Holly, L.T. Compensatory brainstem functional and structural connectivity in patients with degenerative cervical myelopathy by probabilistic tractography and functional MRI. Brain Res. 2020, 1749, 147129. [Google Scholar] [CrossRef]
- Peng, X.; Tan, Y.; He, L.; Ou, Y. Alterations of functional connectivity between thalamus and cortex before and after decompression in cervical spondylotic myelopathy patients: A resting-state functional MRI study. NeuroReport 2020, 31, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Huang, M.; Wu, L.; Tan, Y.; Guo, J.; Zhang, Y.; He, L.; Gong, H. Altered perfusion of the sensorimotor cortex in patients with cervical spondylotic myelopathy: An arterial spin labeling study. J. Pain Res. 2018, 11, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Bhagavatula, I.D.; Shukla, D.; Sadashiva, N.; Saligoudar, P.; Prasad, C.; Bhat, D.I. Functional cortical reorganization in cases of cervical spondylotic myelopathy and changes associated with surgery. Neurosurg. Focus 2016, 40, E2. [Google Scholar] [CrossRef]
- Zdunczyk, A.; Schwarzer, V.; Mikhailov, M.; Bagley, B.; Rosenstock, T.; Picht, T.; Vajkoczy, P. The Corticospinal Reserve Capacity: Reorganization of Motor Area and Excitability As a Novel Pathophysiological Concept in Cervical Myelopathy. Neurosurgery 2018, 83, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, N.S.; Toy, J.O.; Young, E.Y.; Ahn, N.U. Establishment of parameters for congenital stenosis of the cervical spine: An anatomic descriptive analysis of 1066 cadaveric specimens. Eur. Spine J. 2012, 21, 2467–2474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlov, H.; Torg, J.S.; Robie, B.; Jahre, C. Cervical spinal stenosis: Determination with vertebral body ratio method. Radiology 1987, 164, 771–775. [Google Scholar] [CrossRef]
- Countee, R.W.; Vijayanathan, T. Congenital stenosis of the cervical spine: Diagnosis and management. J. Natl. Med. Assoc. 1979, 71, 257–264. [Google Scholar] [PubMed]
- Bernhardt, M.; Hynes, R.A.; Blume, H.W.; White, A.A. Cervical spondylotic myelopathy. J. Bone Jt. Surg. Am. 1993, 75, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Tetreault, L.; Fehlings, M.; Fischer, D.; Skelly, A. Risk factors for development of cervical spondylotic myelopathy: Results of a systematic review. Evid. Based Spine Care J. 2013, 3, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nouri, A.; Tetreault, L.; Singh, A.; Karadimas, S.K.; Fehlings, M.G. Degenerative Cervical Myelopathy: Epidemiology, Genetics, and Pathogenesis. Spine 2015, 40, E675–E693. [Google Scholar] [CrossRef]
- Kato, S.; Oshima, Y.; Oka, H.; Chikuda, H.; Takeshita, Y.; Miyoshi, K.; Kawamura, N.; Masuda, K.; Kunogi, J.; Okazaki, R.; et al. Comparison of the Japanese Orthopaedic Association (JOA) Score and Modified JOA (mJOA) Score for the Assessment of Cervical Myelopathy: A Multicenter Observational Study. PLoS ONE 2015, 10, e0123022. [Google Scholar] [CrossRef]
- Tetreault, L.; Kopjar, B.; Nouri, A.; Arnold, P.; Barbagallo, G.; Bartels, R.; Qiang, Z.; Singh, A.; Zileli, M.; Vaccaro, A.; et al. The modified Japanese Orthopaedic Association scale: Establishing criteria for mild, moderate and severe impairment in patients with degenerative cervical myelopathy. Eur. Spine J. 2017, 26, 78–84. [Google Scholar] [CrossRef]
- Chiles, B.W.; Leonard, M.A.; Choudhri, H.F.; Cooper, P.R. Cervical spondylotic myelopathy: Patterns of neurological deficit and recovery after anterior cervical decompression. Neurosurgery 1999, 44, 762–769. [Google Scholar] [CrossRef] [Green Version]
- Hukuda, S.; Mochizuki, T.; Ogata, M.; Shichikawa, K.; Shimomura, Y. Operations for cervical spondylotic myelopathy. A comparison of the results of anterior and posterior procedures. J. Bone Jt. Surg. Br. Vol. 1985, 67, 609–615. [Google Scholar] [CrossRef]
- Herdmann, J.; Linzbach, M.; Krzan, M.; Dvorak, J.; Bock, W.J.; Bauer, B.L.; Brock, M.; Klinger, M. The European Myelopathy Score; Springer: Berlin/Heidelberg, Germany, 1994; p. 268. [Google Scholar]
- Keller, A.; Von Ammon, K.; Klaiber, R.; Waespe, W. [Spondylogenic cervical myelopathy: Conservative and surgical therapy]. Schweiz. Med. Wochenschr. 1993, 123, 1682–1691. [Google Scholar]
- Nurjck, S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain 1972, 95, 87–100. [Google Scholar] [CrossRef]
- Prolo, D.J.; Oklund, S.A.; Butcher, M. Toward uniformity in evaluating results of lumbar spine operations. A paradigm applied to posterior lumbar interbody fusions. Spine 1986, 11, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Tetreault, L.A.; Karpova, A.; Fehlings, M.G. Predictors of outcome in patients with degenerative cervical spondylotic myelopathy undergoing surgical treatment: Results of a systematic review. Eur. Spine J. 2015, 24, 236–251. [Google Scholar] [CrossRef] [Green Version]
- Vernon, H.; Mior, S. The Neck Disability Index: A study of reliability and validity. J. Manip. Physiol. Ther. 1991, 14, 409–415. [Google Scholar]
- Goyal, D.K.C.; Murphy, H.A.; Hollern, D.A.; Divi, S.N.; Nicholson, K.; Stawicki, C.; Kaye, I.D.; Schroeder, G.D.; Woods, B.I.; Kurd, M.F.; et al. Is the Neck Disability Index an Appropriate Measure for Changes in Physical Function After Surgery for Cervical Spondylotic Myelopathy? Int. J. Spine Surg. 2020, 14, 53–58. [Google Scholar] [CrossRef] [PubMed]
- El-Zuway, S.; Farrokhyar, F.; Kachur, E. Myelopathic signs and functional outcome following cervical decompression surgery: A proposed myelopathy scale. J. Neurosurg. Spine 2016, 24, 871–877. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.R.; Kalsi-Ryan, S.; Akbar, M.A.; Rienmueller, A.C.; Badhiwala, J.H.; Wilson, J.R.; Tetreault, L.A.; Nouri, A.; Massicotte, E.M.; Fehlings, M.G. Clinical outcomes of nonoperatively managed degenerative cervical myelopathy: An ambispective longitudinal cohort study in 117 patients. J. Neurosurg. Spine 2021, 34, 821–829. [Google Scholar] [CrossRef]
- Kim, H.J.; Tetreault, L.A.; Massicotte, E.M.; Arnold, P.M.; Skelly, A.C.; Brodt, E.D.; Riew, K.D. Differential Diagnosis for Cervical Spondylotic Myelopathy: Literature Review. Spine 2013, 38, S78–S88. [Google Scholar] [CrossRef]
- Nakashima, H.; Yukawa, Y.; Suda, K.; Yamagata, M.; Ueta, T.; Kato, F. Cervical Disc Protrusion Correlates With the Severity of Cervical Disc Degeneration: A Cross-Sectional Study of 1211 Relatively Healthy Volunteers. Spine 2015, 40, E774–E779. [Google Scholar] [CrossRef] [PubMed]
- Nouri, A.; Martin, A.R.; Kato, S.; Reihani-Kermani, H.; Riehm, L.E.; Fehlings, M.G. The Relationship Between MRI Signal Intensity Changes, Clinical Presentation, and Surgical Outcome in Degenerative Cervical Myelopathy: Analysis of a Global Cohort. Spine 2017, 42, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Fontanella, M.M.; Zanin, L.; Bergomi, R.; Fazio, M.; Zattra, C.M.; Agosti, E.; Saraceno, G.; Schembari, S.; De Maria, L.; Quartini, L.; et al. Snake-Eye Myelopathy and Surgical Prognosis: Case Series and Systematic Literature Review. J. Clin. Med. 2020, 9, 2197. [Google Scholar] [CrossRef]
- Rindler, R.S.; Chokshi, F.H.; Malcolm, J.G.; Eshraghi, S.R.; Mossa-Basha, M.; Chu, J.K.; Kurpad, S.N.; Ahmad, F.U. Spinal Diffusion Tensor Imaging in Evaluation of Preoperative and Postoperative Severity of Cervical Spondylotic Myelopathy: Systematic Review of Literature. World Neurosurg. 2017, 99, 150–158. [Google Scholar] [CrossRef] [PubMed]
- D’Avanzo, S.; Ciavarro, M.; Pavone, L.; Pasqua, G.; Ricciardi, F.; Bartolo, M.; Solari, D.; Somma, T.; de Divitiis, O.; Cappabianca, P.; et al. The Functional Relevance of Diffusion Tensor Imaging in Patients with Degenerative Cervical Myelopathy. J. Clin. Med. 2020, 9, 1828. [Google Scholar] [CrossRef] [PubMed]
- Hohenhaus, M.; Egger, K.; Klingler, J.-H.; Hubbe, U.; Reisert, M.; Wolf, K. Is microdiffusion imaging able to improve the detection of cervical myelopathy? Study protocol of a prospective observational trial (MIDICAM-Trial). BMJ Open 2019, 9, e029153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nardone, R.; Höller, Y.; Brigo, F.; Frey, V.N.; Lochner, P.; Leis, S.; Golaszewski, S.; Trinka, E. The contribution of neurophysiology in the diagnosis and management of cervical spondylotic myelopathy: A review. Spinal Cord 2016, 54, 756–766. [Google Scholar] [CrossRef]
- Malone, A.; Meldrum, D.; Gleeson, J.; Bolger, C. Electromyographic characteristics of gait impairment in cervical spondylotic myelopathy. Eur. Spine J. 2013, 22, 2538–2544. [Google Scholar] [CrossRef] [Green Version]
- El Negamy, E.; Sedgwick, E.M. Delayed cervical somatosensory potentials in cervical spondylosis. J. Neurol. Neurosurg. Psychiatry 1979, 42, 238–241. [Google Scholar] [CrossRef] [Green Version]
- Ganes, T. Somatosensory conduction times and peripheral, cervical and cortical evoked potentials in patients with cervical spondylosis. J. Neurol. Neurosurg. Psychiatry 1980, 43, 683–689. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.L.; Jones, S.J. Somatosensory evoked potentials in cervical spondylosis: Correlation of median, ulnar and posterior tibial nerve responses with clinical and radiological findings. Brain 1985, 108, 273–300. [Google Scholar] [CrossRef] [PubMed]
- Veilleux, M.; Daube, J.R. The value of ulnar somatosensory evoked potentials (SEPs) in cervical myelopathy. Electroencephalogr. Clin. Neurophysiol. Potentials Sect. 1987, 68, 415–423. [Google Scholar] [CrossRef]
- Abbruzzese, G.; Dall’Agata, D.; Morena, M.; Simonetti, S.; Spadavecchia, L.; Severi, P.; Andrioli, G.C.; Favale, E. Electrical stimulation of the motor tracts in cervical spondylosis. J. Neurol. Neurosurg. Psychiatry 1988, 51, 796–802. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.-C.; Yeh, I.-B.; Kannan, T.A.; Wilder-Smith, E. Trapezius motor evoked potential in evaluations of corticospinal tract lesions. Eur. J. Neurol. 2009, 16, 540–543. [Google Scholar] [CrossRef]
- Bednařík, J.; Kadaňka, Z.; Voháňka, S.; Novotný, O.; Šurelová, D.; Filipovičová, D.; Prokeš, B. The value of somatosensory and motor evoked potentials in pre-clinical spondylotic cervical cord compression. Eur. Spine J. 1998, 7, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Kalupahana, N.S.; Weerasinghe, V.S.; Dangahadeniya, U.; Senanayake, N. Abnormal parameters of magnetically evoked motor-evoked potentials in patients with cervical spondylotic myelopathy. Spine J. 2008, 8, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Tetreault, L.A.; Riew, K.D.; Middleton, J.W.; Aarabi, B.; Arnold, P.M.; Brodke, D.S.; Burns, A.S.; Carette, S.; Chen, R.; et al. A Clinical Practice Guideline for the Management of Patients With Degenerative Cervical Myelopathy: Recommendations for Patients With Mild, Moderate, and Severe Disease and Nonmyelopathic Patients With Evidence of Cord Compression. Glob. Spine J. 2017, 7, 70S–83S. [Google Scholar] [CrossRef] [Green Version]
- Lees, F.; Turner, J.W.A. Natural History and Prognosis of Cervical Spondylosis. BMJ 1963, 2, 1607–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karadimas, S.K.; Erwin, W.M.; Ely, C.G.; Dettori, J.R.; Fehlings, M.G. Pathophysiology and Natural History of Cervical Spondylotic Myelopathy. Spine 2013, 38, S21–S36. [Google Scholar] [CrossRef]
- Kadaňka, Z.; Bednařík, J.; Novotný, O.; Urbánek, I.; Dušek, L. Cervical spondylotic myelopathy: Conservative versus surgical treatment after 10 years. Eur. Spine J. 2011, 20, 1533–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadanka, Z.; Mares, M.; Bednarik, J.; Smrcka, V.; Krbec, M.; Stejskal, L.; Chaloupka, R.; Surelova, D.; Novotny, O.; Urbanek, I.; et al. Approaches to spondylotic cervical myelopathy: Conservative versus surgical results in a 3-year follow-up study. Spine 2002, 27, 2205–2210. [Google Scholar] [CrossRef] [PubMed]
- Sampath, P.; Bendebba, M.; Davis, J.D.; Ducker, T.B. Outcome of Patients Treated for Cervical Myelopathy: A Prospective, Multicenter Study With Independent Clinical Review. Spine 2000, 25, 670–676. [Google Scholar] [CrossRef]
- Khan, I.; Archer, K.R.; Wanner, J.P.; Bydon, M.; Pennings, J.S.; Sivaganesan, A.; Knightly, J.J.; Foley, K.T.; Bisson, E.F.; Shaffrey, C.; et al. Trajectory of Improvement in Myelopathic Symptoms From 3 to 12 Months Following Surgery for Degenerative Cervical Myelopathy. Neurosurgery 2020, 86, 763–768. [Google Scholar] [CrossRef]
- SaterenZoller, E.; Cannella, D.; Chyatte, D.; Fogelson, J.; Sharma, M. Diagnosis and medical and surgical management of cervical spondylotic myelopathy. JAAPA J. Am. Acad. Physician Assist. Lippincott Williams Wilkins 2015, 28, 29–36. [Google Scholar] [CrossRef]
- Samuel, A.M.; Moore, H.G.; Vaishnav, A.S.; McAnany, S.; Albert, T.; Iyer, S.; Katsuura, Y.; Gang, C.H.; Qureshi, S.A. Effect of Myelopathy on Early Clinical Improvement After Cervical Disc Replacement: A Study of a Local Patient Cohort and a Large National Cohort. Neurospine 2019, 16, 563–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heller, J.G.; Edwards, C.C.; Murakami, H.; Rodts, G.E. Laminoplasty Versus Laminectomy and Fusion for Multilevel Cervical Myelopathy: An Independent Matched Cohort Analysis. Spine 2001, 26, 1330–1336. [Google Scholar] [CrossRef]
- Di Martino, A.; Papalia, R.; Caldaria, A.; Torre, G.; Denaro, L.; Denaro, V. Should evoked potential monitoring be used in degenerative cervical spine surgery? A systematic review. J. Orthop. Traumatol. 2019, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Resnick, D.K.; Anderson, P.A.; Kaiser, M.G.; Groff, M.W.; Heary, R.F.; Holly, L.T.; Mummaneni, P.V.; Ryken, T.C.; Choudhri, T.F.; Vresilovic, E.J.; et al. Electrophysiological monitoring during surgery for cervical degenerative myelopathy and radiculopathy. J. Neurosurg. Spine 2009, 11, 245–252. [Google Scholar] [CrossRef]
| Category | Score | Description |
|---|---|---|
| Upper Extremity Motor | 0 | Unable to move hands |
| 1 | Unable to eat with spoon but able to move hands | |
| 2 | Unable to button shirt but able to eat with spoon | |
| 3 | Able to button shirt with great difficulty | |
| 4 | Able to button shirt with mild difficulty OR other mild fine motor dysfunction (marked change in handwriting, frequent dropping of objects, difficulty clasping jewelry, etc.) | |
| 5 | Normal hand coordination | |
| Lower Extremity Motor/Sensation | 0 | Complete loss of movement and sensation |
| 1 | Complete loss of movement, some sensation present | |
| 2 | Unable to walk but some movement | |
| 3 | Able to walk on flat ground with walking aid | |
| 4 | Able to walk without walking aid, must hold handrail on stairs | |
| 5 | Moderate to severe gait imbalance but able to take stairs without handrail | |
| 6 | Mild imbalance standing OR walking | |
| 7 | Normal walking | |
| Upper Extremity Sensory | 0 | Complete loss of hand sensation |
| 1 | Severe loss of hand sensation OR pain | |
| 2 | Mild loss of hand sensation | |
| 3 | Normal hand sensation | |
| Urinary function | 0 | Inability to voluntarily urinate (requiring catheterization) |
| 1 | Frequent urinary incontinence (more than once monthly) | |
| 2 | Urinary urgency OR occasional stress incontinence (less than once monthly) | |
| 3 | Normal urinary function |
| Differential Diagnosis | Differentiating Findings |
|---|---|
| Amyotrophic lateral sclerosis [7,33] | Presence of cranial nerve findings (e.g., dysphagia, dysarthria) Absence of sensory findings |
| Brain neoplasm | Presence of cranial nerve findings Lateralizing findings (e.g., unilateral weakness/sensory changes) Headache Vomiting Altered level of consciousness |
| Multiple sclerosis [7,33] | Visual changes Cranial nerve findings Fatigue |
| Peripheral nerve entrapment (e.g., carpal tunnel syndrome, ulnar neuropathy) [33] | Absence of upper motor neuron findings |
| Normal pressure hydrocephalus | Cognitive disturbances Speech or swallowing difficulty |
| Vitamin B deficiency [7,33] | Fatigue Cognitive disturbances Glossitis Visual changes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lannon, M.; Kachur, E. Degenerative Cervical Myelopathy: Clinical Presentation, Assessment, and Natural History. J. Clin. Med. 2021, 10, 3626. https://doi.org/10.3390/jcm10163626
Lannon M, Kachur E. Degenerative Cervical Myelopathy: Clinical Presentation, Assessment, and Natural History. Journal of Clinical Medicine. 2021; 10(16):3626. https://doi.org/10.3390/jcm10163626
Chicago/Turabian StyleLannon, Melissa, and Edward Kachur. 2021. "Degenerative Cervical Myelopathy: Clinical Presentation, Assessment, and Natural History" Journal of Clinical Medicine 10, no. 16: 3626. https://doi.org/10.3390/jcm10163626
APA StyleLannon, M., & Kachur, E. (2021). Degenerative Cervical Myelopathy: Clinical Presentation, Assessment, and Natural History. Journal of Clinical Medicine, 10(16), 3626. https://doi.org/10.3390/jcm10163626






