Unicompartmental Knee Replacement in Obese Patients: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Information Sources and Search
2.2. Eligibility Criteria and Study Selection
2.3. Data Collection Process
2.4. Methodological Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Literature Search
3.2. Patient Demographics
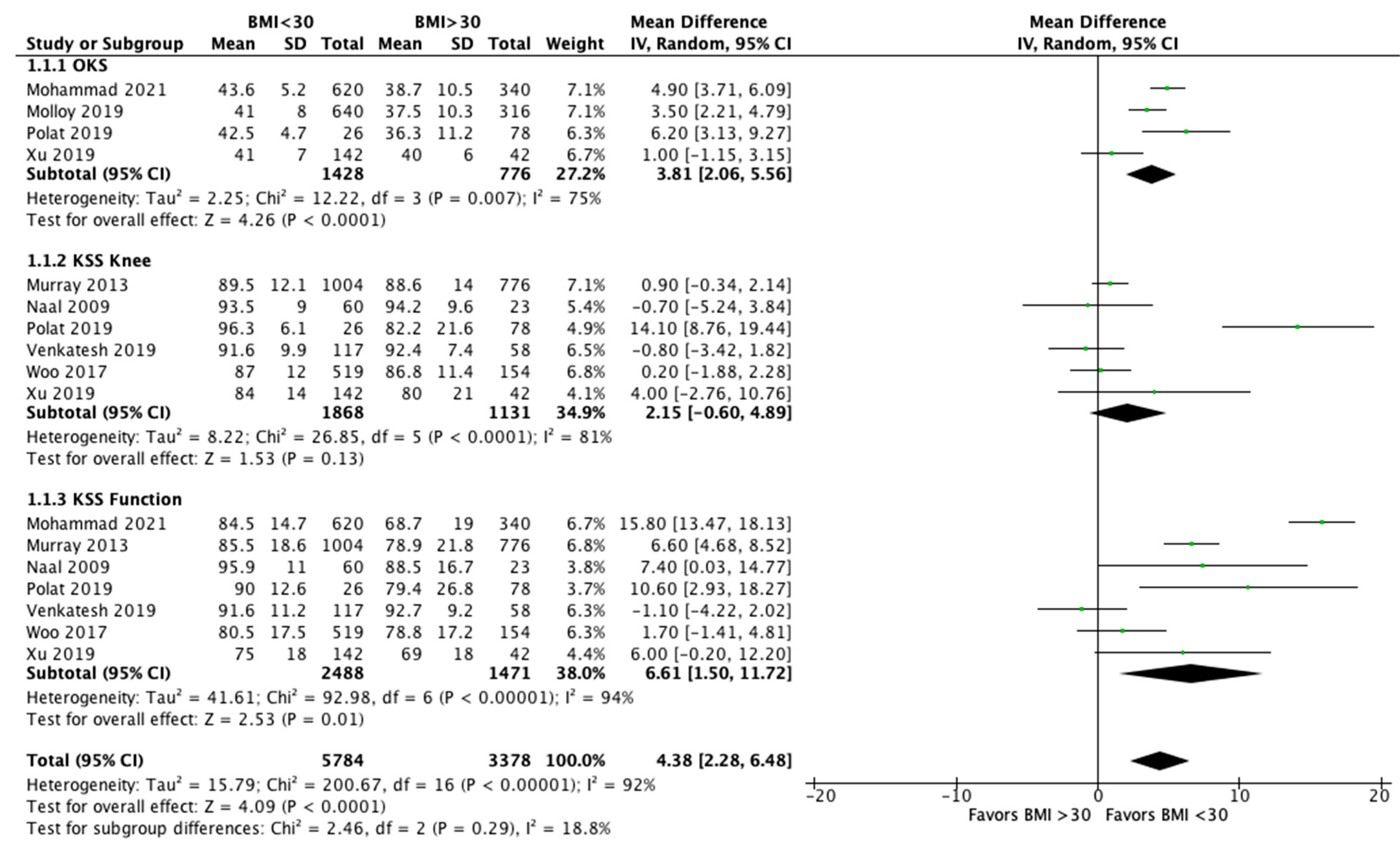
3.3. Clinical Outcome
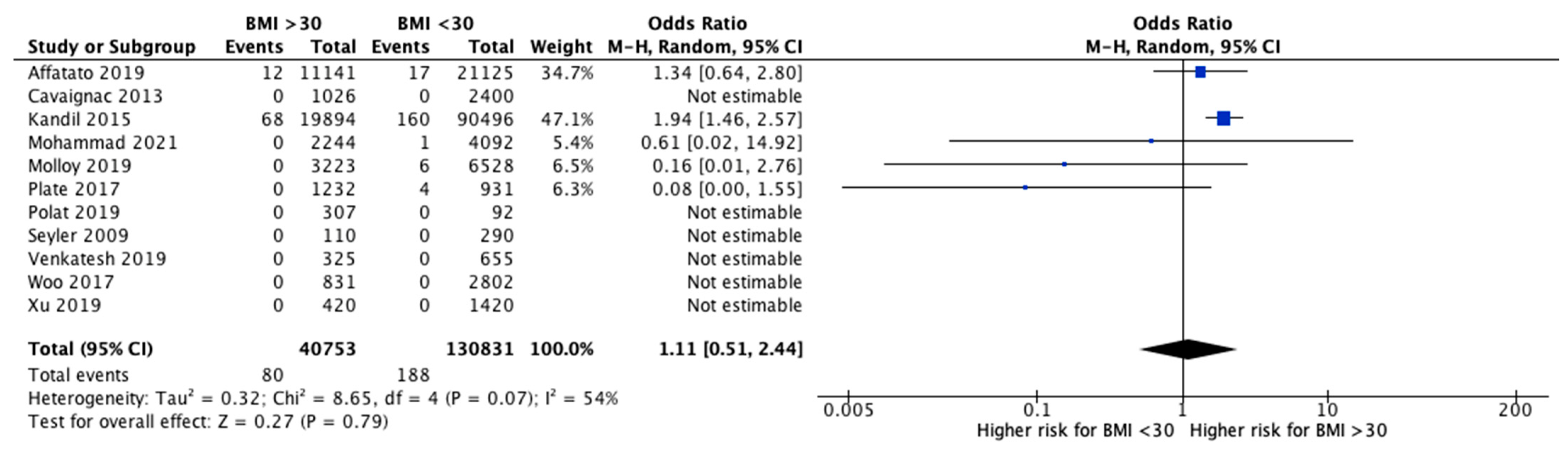
3.4. Failures and Revisions
3.5. Methodological Evaluation
3.6. Effect of Intervention
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
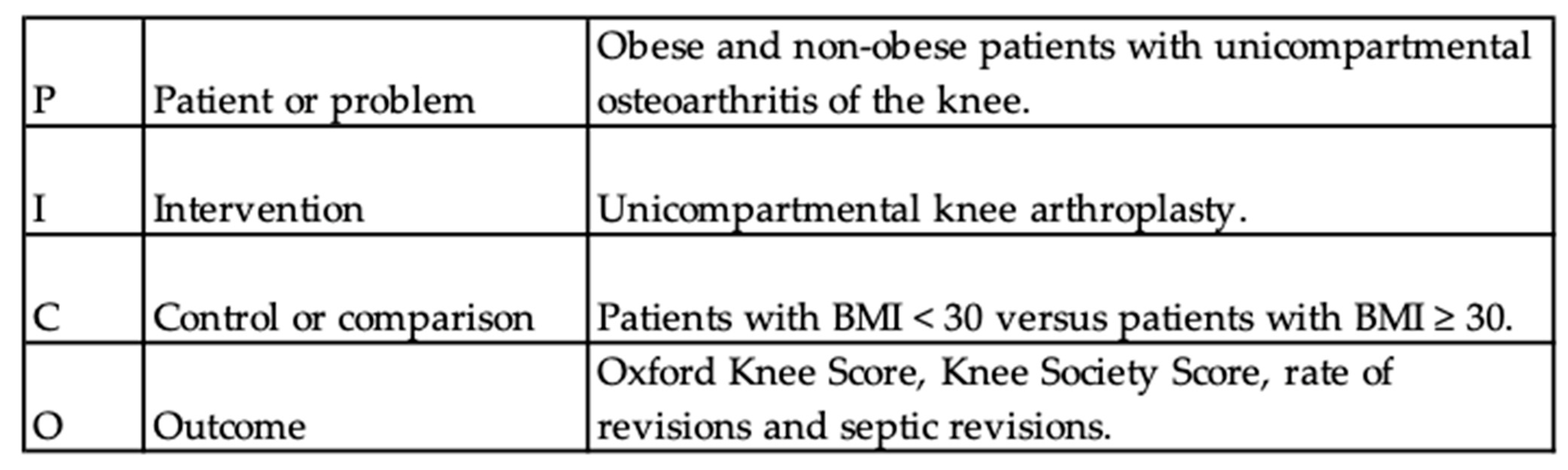
Appendix A. PICO Strategy
References
- Campi, S.; Tibrewal, S.; Cuthbert, R.; Tibrewal, S.B. Unicompartmental Knee Replacement—Current Perspectives. J. Clin. Orthop. Trauma 2018, 9, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Schwab, P.-E.; Lavand’homme, P.; Yombi, J.C.; Thienpont, E. Lower Blood Loss after Unicompartmental than Total Knee Arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 3494–3500. [Google Scholar] [CrossRef]
- Siman, H.; Kamath, A.F.; Carrillo, N.; Harmsen, W.S.; Pagnano, M.W.; Sierra, R.J. Unicompartmental Knee Arthroplasty vs Total Knee Arthroplasty for Medial Compartment Arthritis in Patients Older Than 75 Years: Comparable Reoperation, Revision, and Complication Rates. J. Arthroplast. 2017, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Engh, G.A. Orthopaedic Crossfire®—Can We Justify Unicondylar Arthroplasty as a Temporizing Procedure? In the Affirmative. J. Arthroplast. 2002, 17, 54–55. [Google Scholar] [CrossRef] [PubMed]
- Drager, J.; Hart, A.; Khalil, J.A.; Zukor, D.J.; Bergeron, S.G.; Antoniou, J. Shorter Hospital Stay and Lower 30-Day Readmission After Unicondylar Knee Arthroplasty Compared to Total Knee Arthroplasty. J. Arthroplast. 2016, 31, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Liddle, A.D.; Judge, A.; Pandit, H.; Murray, D.W. Adverse Outcomes after Total and Unicompartmental Knee Replacement in 101 330 Matched Patients: A Study of Data from the National Joint Registry for England and Wales. Lancet 2014, 384, 1437–1445. [Google Scholar] [CrossRef] [Green Version]
- Wiik, A.V.; Aqil, A.; Tankard, S.; Amis, A.A.; Cobb, J.P. Downhill Walking Gait Pattern Discriminates between Types of Knee Arthroplasty: Improved Physiological Knee Functionality in UKA versus TKA. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 1748–1755. [Google Scholar] [CrossRef] [PubMed]
- Fabre-Aubrespy, M.; Ollivier, M.; Pesenti, S.; Parratte, S.; Argenson, J.-N. Unicompartmental Knee Arthroplasty in Patients Older Than 75 Results in Better Clinical Outcomes and Similar Survivorship Compared to Total Knee Arthroplasty. A Matched Controlled Study. J. Arthroplast. 2016, 31, 2668–2671. [Google Scholar] [CrossRef]
- Burn, E.; Sanchez-Santos, M.T.; Pandit, H.G.; Hamilton, T.W.; Liddle, A.D.; Murray, D.W.; Pinedo-Villanueva, R. Ten-Year Patient-Reported Outcomes Following Total and Minimally Invasive Unicompartmental Knee Arthroplasty: A Propensity Score-Matched Cohort Analysis. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 1455–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lum, Z.C.; Lombardi, A.V.; Hurst, J.M.; Morris, M.J.; Adams, J.B.; Berend, K.R. Early Outcomes of Twin-Peg Mobile-Bearing Unicompartmental Knee Arthroplasty Compared with Primary Total Knee Arthroplasty. Bone Jt. J. 2016, 98-B, 28–33. [Google Scholar] [CrossRef] [Green Version]
- Zuiderbaan, H.A.; van der List, J.P.; Khamaisy, S.; Nawabi, D.H.; Thein, R.; Ishmael, C.; Paul, S.; Pearle, A.D. Unicompartmental Knee Arthroplasty versus Total Knee Arthroplasty: Which Type of Artificial Joint Do Patients Forget? Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 681–686. [Google Scholar] [CrossRef]
- Goodfellow, J.W.; O’Connor, J.J.; Murray, D.W. A Critique of Revision Rate as an Outcome Measure: Re-Interpretation of Knee Joint Registry Data. J. Bone Jt. Surg. Br. 2010, 92-B, 1628–1631. [Google Scholar] [CrossRef] [Green Version]
- Kozinn, S.C.; Scott, R. Unicondylar knee arthroplasty. J Bone Jt. Surg Am. 1989, 71, 145–150. [Google Scholar] [CrossRef]
- Goodfellow, J.; O’Connor, J.; Pandit, H.; Dodd, C.; Murray, D. Unicompartmental Arthroplasty with the Oxford Knee; Goodfellow Publishers: Oxford, UK, 2015; ISBN 978-1-910158-45-6. [Google Scholar] [CrossRef]
- Pandit, H.; Jenkins, C.; Gill, H.S.; Smith, G.; Price, A.J.; Dodd, C.A.F.; Murray, D.W. Unnecessary Contraindications for Mobile-Bearing Unicompartmental Knee Replacement. J. Bone Jt. Surg. Br. 2011, 93-B, 622–628. [Google Scholar] [CrossRef] [Green Version]
- Berend, K.R.; Lombardi, A.V.; Adams, J.B. Obesity, young age, patellofemoral disease, and anterior knee pain: Identifying the unicondylar arthroplasty patient in the United States. Orthopedics 2007, 30 (Suppl. 5), 19–23. [Google Scholar]
- Gelber, A.C.; Hochberg, M.C.; Mead, L.A.; Wang, N.-Y.; Wigley, F.M.; Klag, M.J. Body Mass Index in Young Men and the Risk of Subsequent Knee and Hip Osteoarthritis. Am. J. Med. 1999, 107, 542–548. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, Y.Q.; Torner, J.; Nevitt, M.; Lewis, C.E.; Aliabadi, P.; Sack, B.; Clancy, M.; Sharma, L.; Felson, D.T. Is Obesity a Risk Factor for Progressive Radiographic Knee Osteoarthritis? Arthritis Rheum. 2009, 61, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Messier, S.P.; Gutekunst, D.J.; Davis, C.; DeVita, P. Weight Loss Reduces Knee-Joint Loads in Overweight and Obese Older Adults with Knee Osteoarthritis. Arthritis Rheum. 2005, 52, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Dowsey, M.M.; Liew, D.; Stoney, J.D.; Choong, P.F. The Impact of Pre-Operative Obesity on Weight Change and Outcome in Total Knee Replacement. J. Bone Jt. Surg. 2010, 92, 8. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Morshed, S.; Joseph, T.; Bozic, K.; Ries, M.D. Clinical Impact of Obesity on Stability Following Revision Total Hip Arthroplasty. Clin. Orthop. 2006, 453, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Dewan, A.; Bertolusso, R.; Karastinos, A.; Conditt, M.; Noble, P.C.; Parsley, B.S. Implant Durability and Knee Function After Total Knee Arthroplasty in the Morbidly Obese Patient. J. Arthroplast. 2009, 24, 89–94.e3. [Google Scholar] [CrossRef]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological Index For Non-Randomized Studies (Minors): Development And Validation Of A New Instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Pandit, H.; Jenkins, C.; Gill, H.S.; Barker, K.; Dodd, C.A.F.; Murray, D.W. Minimally Invasive Oxford Phase 3 Unicompartmental Knee Replacement: RESULTS OF 1000 CASES. J. Bone Jt. Surg. Br. 2011, 93-B, 198–204. [Google Scholar] [CrossRef]
- Murray, D.W.; Pandit, H.; Weston-Simons, J.S.; Jenkins, C.; Gill, H.S.; Lombardi, A.V.; Dodd, C.A.F.; Berend, K.R. Does Body Mass Index Affect the Outcome of Unicompartmental Knee Replacement? Knee 2013, 20, 461–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nettrour, J.F.; Ellis, R.T.; Hansen, B.J.; Keeney, J.A. High Failure Rates for Unicompartmental Knee Arthroplasty in Morbidly Obese Patients: A Two-Year Minimum Follow-Up Study. J. Arthroplast. 2020, 35, 989–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polat, A.E.; Polat, B.; Gürpınar, T.; Çarkçı, E.; Güler, O. The Effect of Morbid Obesity (BMI ≥ 35 Kg/M2) on Functional Outcome and Complication Rate Following Unicompartmental Knee Arthroplasty: A Case-Control Study. J. Orthop. Surg. 2019, 14, 266. [Google Scholar] [CrossRef] [Green Version]
- Affatato, S.; Caputo, D.; Bordini, B. Does the Body Mass Index Influence the Long-Term Survival of Unicompartmental Knee Prostheses? A Retrospective Multi-Centre Study. Int. Orthop. 2019, 43, 1365–1370. [Google Scholar] [CrossRef]
- Woo, Y.L.; Chen, Y.Q.J.; Lai, M.C.; Tay, K.J.D.; Chia, S.-L.; Lo, N.N.; Yeo, S.J. Does Obesity Influence Early Outcome of Fixed-Bearing Unicompartmental Knee Arthroplasty? J. Orthop. Surg. 2017, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zengerink, I.; Duivenvoorden, T.; Niesten, D.; Verburg, H.; Bloem, R.; Mathijssen, N. Obesity Does Not Influence the Outcome after Unicompartmental Knee Arthroplasty. Acta Orthop. Belg. 2015, 81, 776–783. [Google Scholar] [PubMed]
- Kandil, A.; Werner, B.C.; Gwathmey, W.F.; Browne, J.A. Obesity, Morbid Obesity and Their Related Medical Comorbidities Are Associated with Increased Complications and Revision Rates after Unicompartmental Knee Arthroplasty. J. Arthroplast. 2015, 30, 456–460. [Google Scholar] [CrossRef]
- Thompson, S.A.J.; Liabaud, B.; Nellans, K.W.; Geller, J.A. Factors Associated With Poor Outcomes Following Unicompartmental Knee Arthroplasty. J. Arthroplast. 2013, 28, 1561–1564. [Google Scholar] [CrossRef]
- Cavaignac, E.; Lafontan, V.; Reina, N.; Pailhé, R.; Warmy, M.; Laffosse, J.M.; Chiron, P. Obesity Has No Adverse Effect on the Outcome of Unicompartmental Knee Replacement at a Minimum Follow-up of Seven Years. Bone Jt. J. 2013, 95-B, 1064–1068. [Google Scholar] [CrossRef]
- Xing, Z.; Katz, J.; Jiranek, W. Unicompartmental Knee Arthroplasty: Factors Influencing the Outcome. J. Knee Surg. 2012, 25, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Bonutti, P.M.; Goddard, M.S.; Zywiel, M.G.; Khanuja, H.S.; Johnson, A.J.; Mont, M.A. Outcomes of Unicompartmental Knee Arthroplasty Stratified by Body Mass Index. J. Arthroplast. 2011, 26, 1149–1153. [Google Scholar] [CrossRef]
- Kuipers, B.M.; Kollen, B.J.; Kaijser Bots, P.C.; Burger, B.J.; van Raay, J.J.A.M.; Tulp, N.J.A.; Verheyen, C.C.P.M. Factors Associated with Reduced Early Survival in the Oxford Phase III Medial Unicompartment Knee Replacement. Knee 2010, 17, 48–52. [Google Scholar] [CrossRef]
- Naal, F.D.; Neuerburg, C.; Salzmann, G.M.; Kriner, M.; von Knoch, F.; Preiss, S.; Drobny, T.; Munzinger, U. Association of Body Mass Index and Clinical Outcome 2 Years after Unicompartmental Knee Arthroplasty. Arch. Orthop. Trauma Surg. 2009, 129, 463–468. [Google Scholar] [CrossRef]
- Molloy, J.; Kennedy, J.; Jenkins, C.; Mellon, S.; Dodd, C.; Murray, D. Obesity Should Not Be Considered a Contraindication to Medial Oxford UKA: Long-Term Patient-Reported Outcomes and Implant Survival in 1000 Knees. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 2259–2265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Lim, W.-A.J.; Chen, J.Y.; Lo, N.N.; Chia, S.-L.; Tay, D.K.J.; Hao, Y.; Yeo, S.J. The Influence of Obesity on Clinical Outcomes of Fixed-Bearing Unicompartmental Knee Arthroplasty: A Ten-Year Follow-up Study. Bone Jt. J. 2019, 101-B, 213–220. [Google Scholar] [CrossRef]
- Venkatesh, H.K.; Maheswaran, S.S. Age and Body Mass Index Has No Adverse Effect on Clinical Outcome of Unicompartmental Knee Replacement—Midterm Followup Study. Indian J. Orthop. 2019, 53, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Seyler, T.M.; Mont, M.A.; Lai, L.P.; Xie, J.; Marker, D.R.; Zywiel, M.G.; Bonutti, P.M. Mid-Term Results and Factors Affecting Outcome of a Metal-Backed Unicompartmental Knee Design: A Case Series. J. Orthop. Surg. 2009, 4, 39. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, H.R.; Mellon, S.; Judge, A.; Dodd, C.; Murray, D. The Effect of Body Mass Index on the Outcomes of Cementless Medial Mobile-Bearing Unicompartmental Knee Replacements. Knee Surg. Sports Traumatol. Arthrosc. 2021. [Google Scholar] [CrossRef]
- Seth, A.; Dobransky, J.; Albishi, W.; Dervin, G.F. Mid-Term Evaluation of the Unicompartmental Knee Arthroplasty in Patients with BMI of 40 or Greater. J. Knee Surg. 2021, 34, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Plate, J.F.; Augart, M.A.; Seyler, T.M.; Bracey, D.N.; Hoggard, A.; Akbar, M.; Jinnah, R.H.; Poehling, G.G. Obesity Has No Effect on Outcomes Following Unicompartmental Knee Arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Cepni, S.K. Mid-Term Results of Oxford Phase 3 Unicompartmental Knee Arthroplasty in Obese Patients. Acta Orthop. Traumatol. Turc. 2014, 48, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Berend, K.R.; Lombardi, A.V.; Mallory, T.H.; Adams, J.B.; Groseth, K.L. Early Failure of Minimally Invasive Unicompartmental Knee Arthroplasty Is Associated with Obesity. Clin. Orthop. 2005, 440, 60–66. [Google Scholar] [CrossRef]
- van der List, J.P.; Chawla, H.; Zuiderbaan, H.A.; Pearle, A.D. The Role of Preoperative Patient Characteristics on Outcomes of Unicompartmental Knee Arthroplasty: A Meta-Analysis Critique. J. Arthroplast. 2016, 31, 2617–2627. [Google Scholar] [CrossRef]
- Agarwal, N.; To, K.; Zhang, B.; Khan, W. Obesity Does Not Adversely Impact the Outcome of Unicompartmental Knee Arthroplasty for Osteoarthritis: A Meta-Analysis of 80,798 Subjects. Int. J. Obes. 2021, 45, 715–724. [Google Scholar] [CrossRef]
- Musbahi, O.; Hamilton, T.W.; Crellin, A.J.; Mellon, S.J.; Kendrick, B.; Murray, D.W. The Effect of Obesity on Revision Rate in Unicompartmental Knee Arthroplasty: A Systematic Review and Meta-Analysis. Knee Surg. Sports Traumatol. Arthrosc. 2020, 1–11. [Google Scholar] [CrossRef]
- Chaudhry, H.; Ponnusamy, K.; Somerville, L.; McCalden, R.W.; Marsh, J.; Vasarhelyi, E.M. Revision Rates and Functional Outcomes Among Severely, Morbidly, and Super-Obese Patients Following Primary Total Knee Arthroplasty: A Systematic Review and Meta-Analysis. JBJS Rev. 2019, 7, e9. [Google Scholar] [CrossRef]


| Study | Year | Study Design | LOE | Cohort | Patients | Number of UKA | Mean Age, Years (Range) | Mean BMI, kg/m2 (Range) | Mean Follow-Up (Range) |
|---|---|---|---|---|---|---|---|---|---|
| Nettrour et al. | 2019 | RS | II | Not Morbidly Obese (BMI < 40) | 81 | 101 | 57.6 ± 8.3 (40–83) | 33.1 ± 5 (20–39) | 3.5 ± 1.3 years (2–6.8) |
| Morbidly Obese (BMI ≥ 40) | 71 | 89 | 55.3 ± 9.1 (40–79) | 45.8 ± 5.6 (>40) | 3.2 ± 1.1 years (2–6.8) | ||||
| Polat et al. | 2019 | RS | II | Normal and Overweight (BMI < 30) | 86 | 26 | 61.5 ± 7.3 | 27.3 ± 2.3 | 42.7 ± 14.1 months |
| Obese (BMI = 30–34.9) | 40 | 60.5 ± 7.7 | 32.7 ± 1.5 | 40.6 ± 13.5 months | |||||
| Morbidly Obese (BMI ≥ 35) | 38 | 59.0 ± 7.1 | 40.9 ± 5.6 | 53.9 ± 12.7 months | |||||
| Seth et al. | 2019 | CS | IV | Morbidly Obese (BMI ≥ 40) | 103 | 121 | 58 (43–75) | 43 (40–51) | 7 years (2 months–15 y) |
| Molloy et al. | 2019 | PS | III | Normal (BMI < 25) | 202 | 207 | 70.3 ± 10 | 22.6 ± 3 | 10.2 years (5–16) |
| Overweight (BMI = 25–29.9) | 427 | 433 | 66.4 ± 10 | 27.3 ± 1 | |||||
| Obese (BMI = 30–34.9) | 218 | 220 | 64.9 ± 9 | 32.1 ± 1 | |||||
| Morbidly Obese (BMI ≥ 35) | 94 | 96 | 61.7 ± 8 | 39.0 ± 4 | |||||
| Affatato et al. | 2019 | RS | III | Normal (BMI < 30) | 3976 | 3250 | 67.8 (24–90) | NR | 6.5 years (0–16.3) |
| Obese (BMI = 30–39.9) | 1636 | 65.7 (28–89) | NR | ||||||
| Morbidly Obese (BMI ≥ 40) | 78 | 61.2 (47–79) | NR | ||||||
| Xu et al. | 2019 | PS | I | Control (BMI < 30) | 142 | 142 | 62.4 ± 7.8 | 25.6 ± 2.9 | minimum 10 years |
| Obese (BMI≥ 30) | 42 | 42 | 56.5 ± 6.4 | 33.4 ± 3 | |||||
| Venkatesh et al. | 2019 | PS | I | BMI < 30 | 148 | 117 | 61.7 (44–80) | 29.2 kg/m2 (21–38) | 5.6 years (2–10) |
| BMI ≥ 30 | 58 | ||||||||
| Plate et al. | 2017 | CS | IV | Underweight (BMI < 18.5) | 672 | 1 | 64 ± 11 | 32.1 ± 6.5 | 34.6 ± 7.8 months |
| Normal (BMI = 18.5–24.9) | 91 | ||||||||
| Overweight (BMI = 25–29.9) | 229 | ||||||||
| Obese (BMI = 30–34.9) | 227 | ||||||||
| Severely Obese (BMI = 35–39.9) | 115 | ||||||||
| Morbidly Obese (BMI = 40–44.9) | 42 | ||||||||
| Super Obese (BMI ≥ 45) | 41 | ||||||||
| Woo et al. | 2017 | RS | II | Normal (BMI <25) | 230 | 230 | 65 ± 8 | 22.6 ± 1.8 | 5.4 years (2.5–8.5) |
| Overweight (BMI = 25–29.9) | 289 | 289 | 62 ± 8 | 27.4 ± 1.3 | |||||
| Obese (BMI = 30–34.9) | 124 | 124 | 61 ± 8 | 31.9 ± 1.4 | |||||
| Severely Obese (BMI = 35–39.9) | 30 | 30 | 58 ± 9 | 38.5 ± 3.6 | |||||
| Zengerink et al. | 2015 | RS | II | Not Obese (BMI < 30) | 122 | 63 | 60.0 (± 8.1) | 26.9 (± 2.3) | 3.9 years (2.0–12.2) |
| Obese (BMI ≥ 30) | 64 | 60.9 (± 6.6) | 33.6 (± 3.2) | 5.1 years (2.0–10.8) | |||||
| Kandil et al. | 2015 | RS | II | Non-Obese (BMI < 30) | 12,928 | NR | NR | NR | 7 years |
| Obese (BMI = 30–39.9) | 1823 | NR | NR | NR | |||||
| Morbidly Obese (BMI ≥ 40) | 1019 | NR | NR | NR | |||||
| Cepni et al. | 2014 | CS | IV | BMI > 30 | 67 | 67 | 61 ± 7.3 | 35.7 ± 2.6 | 67.5 months ± 15.4 |
| Murray et al. | 2013 | CS | IV | Normal (BMI < 25) | 2438 | 378 | 69 (38–91) | 23 (15–24.9) | 4.6 years (1–12) |
| Overweight (BMI = 25–29.9) | 856 | 65 (33–89) | 27 | ||||||
| Obese (BMI = 30–34.9) | 712 | 61 (34–88) | 32 | ||||||
| Severely Obese (BMI = 35–39.9) | 286 | 61 (34–87) | 37 | ||||||
| Morbidly Obese (BMI = 40–44.9) | 126 | 58 (41–87) | 42 | ||||||
| Super Obese (BMI ≥ 45) | 80 | 59 (41–78) | 50 (45–69) | ||||||
| Thompson et al. | 2013 | RS | II | BMI < 35 | 173 | 229 | 66 (33–89) | 29.3 (18.4–48.7) | 2 years |
| BMI ≥ 35 | 32 | ||||||||
| Cavaignac et al. | 2013 | RS | II | Not Obese (BMI < 30) | 254 | 200 | 66.5 (39–92) | 27 (19–29) | 12 years (7–22) |
| Obese (BMI ≥ 30) | 90 | 65.8 (55–84) | 34 (30–43.2) | 11.4 years (7–17) | |||||
| Xing et al. | 2012 | RS | II | BMI < 30 | 140 | 178 | 67 (36–90) | 28.8 (19.7–48.5) | 54 months (24–77) |
| BMI = 30–34.9 | |||||||||
| BMI = 35–39.9 | |||||||||
| BMI ≥ 40 | |||||||||
| Bonutti et al. | 2011 | RS | II | Not Obese (BMI < 35) | 33 | 40 | 68 (48–79) | 28 (23–34) | 3 years (2–7) |
| Obese (BMI ≥ 35) | 34 | 40 | 65 (45–81) | 38 (35–47) | 3 years (2–6) | ||||
| Kuipers et al. | 2010 | RS | II | BMI < 30 | 437 | 437 | 62.8 (39.3–84.6) | 30.1 (17.7–47.3) | 2.6 years (0.1–7.9) |
| BMI ≥ 30 | |||||||||
| Seyler et al. | 2009 | PS | IV | Not Obese (BMI < 30) | 68 | 58 | 72 (44–91) | 27 (17–39) | 60 months (24–68) |
| Obese (BMI ≥ 30) | 22 | ||||||||
| Naal et al. | 2009 | RS | II | Normal (BMI = 18.5–24.9) | 77 | 13 | 66 (46–84) | 27.8 (20.2–39.2) | 2 years |
| Overweight (BMI = 25–29.9) | 47 | ||||||||
| Obese (BMI = 30–34.9) | 23 | ||||||||
| Berend et al. | 2005 | CS | IV | Not Obese (BMI < 32) | 61 | 73 | 66.3 (43–83) | 31.65 (19–50) | 40 months (24–69) |
| Obese (BMI ≥ 32) | |||||||||
| Mohammad et al. | 2021 | PS | I | Normal (BMI = 18.5–24.9) | 756 | 186 | 69.1 ± 10.4 | 23.2 ± 1.4 | 6.6 years (5–10) ± 2.7 |
| Overweight (BMI = 25–29.9) | 434 | 66.5 ± 10.1 | 27.5 ± 1.4 | ||||||
| Obese Class 1 (BMI = 30–34.9) | 213 | 64.6 ± 9.4 | 32.2 ± 1.4 | ||||||
| Obese Class 2 (BMI ≥ 35) | 127 | 63.6 ± 8.6 | 38.3 ± 3.5 |
| Study | Cohort | KSS | KSS Knee | KSS Function | KSS Objective | OKS | ROM (°) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | ||
| Polat | Normal and Overweight (BMI < 30) | 44.0 ± 4.3 | 96.3 ± 6.1 | 35.8± 22.2 | 90 ± 12.6 | 11.4 ± 7.8 | 42.5 ± 4.7 | 116.3 ± 12.0 | 128.3 ± 19.3 | ||||
| Obese (BMI = 30–34.9) | 42.1 ± 11.8 | 88.8 ± 10.8 | 32.3 ± 21.2 | 87.8± 12.4 | 11.4 ± 8.0 | 39.3 ± 7.2 | 106.9 ± 11.2 | 124.5 ± 11.8 | |||||
| Morbidly Obese (BMI ≥ 35) | 43.9 ± 9.8 | 75.2 ± 27.2 | 36.6 ± 13.5 | 70.5 ± 34 | 15.1 ± 7.0 | 33.1 ± 13.6 | 113.2 ± 12.5 | 117.4 ± 18.3 | |||||
| Molloy | Normal (BMI < 25) | 26.1 ± 10 | 40.6 ± 8 | ||||||||||
| Overweight (BMI = 25–29.9) | 25.5 ± 9 | 41.2 ± 8 | |||||||||||
| Obese (BMI = 30–34.9) | 23.3 ± 8 | 36.6 ± 11 | |||||||||||
| Morbidly Obese (BMI ≥ 35) | 22.2 ± 9 | 39.5 ± 8 | |||||||||||
| Xu | Control (BMI < 30) | 47 ± 18 | 84 ± 14 | 60 ± 17 | 75 ± 18 | 28 ± 7 | 41 ± 7 | 123 ± 17 | 127 ± 13 | ||||
| Obese (BMI ≥ 30) | 44 ± 20 | 80 ± 21 | 60 ± 14 | 69 ± 18 | 27 ± 8 | 40 ± 6 | 116 ± 15 | 116 ± 13 | |||||
| Woo | Normal (BMI < 25) | 44 ± 18 | 87 ± 12 | 62 ± 17 | 81 ± 18 | 32 ± 8 | 17 ± 5 | ||||||
| Overweight (BMI = 25–29.9) | 43 ± 17 | 87 ± 12 | 61 ± 16 | 80 ± 17 | 33 ± 8 | 18 ± 5 | |||||||
| Obese (BMI = 30–34.9) | 46 ± 18 | 88 ± 10 | 60 ± 16 | 80 ± 17 | 33 ± 7 | 18 ± 5 | |||||||
| Severely Obese (BMI = 35–39.9) | 33 ± 17 | 82 ± 15 | 54 ± 16 | 74 ± 17 | 37 ± 6 | 20 ± 6 | |||||||
| Zengerink | Not Obese (BMI < 30) | 29.2 ± 11.4 | |||||||||||
| Obese (BMI ≥ 30) | 27.9 ± 10.8 | ||||||||||||
| Cepni | BMI > 30 | 18.5 ± 4.7 | 40 ± 5 | 117.6 ± 5 | 127 ± 5.2 | ||||||||
| Murray | Normal (BMI < 25) | 84 ± 18.5 */85± 17.8 ** | 86 ± 9.9 */94 ± 8.8 ** | 27 ± 9.2 | 42 ± 6.8 | ||||||||
| Overweight (BMI = 25–29.9) | 86 ± 19.1 */87 ± 18.8 ** | 85 ± 10 */92 ± 13.8 ** | 25 ± 8.5 | 41 ± 7.5 | |||||||||
| Obese (BMI = 30–34.9) | 80 ± 21.2 */81 ± 20.9 ** | 84 ± 14.9 */91 ± 13.3 ** | 23 ± 7.9 | 39 ± 8.9 | |||||||||
| Severely Obese (BMI = 35–39.9) | 69 ± 27.4 */79 ± 23.1 ** | 82 ± 13.6 */91 ± 12.3 ** | 19 ± 5.9 | 39 ± 9.3 | |||||||||
| Morbidly Obese (BMI = 40–44.9) | 79 ± 21.1 */76 ± 20.8 ** | 93 ± 6.1 */91 ± 14.7 ** | 19 ± 8.4 | 39 ± 7.7 | |||||||||
| Super Obese (BMI ≥ 45) | 76 ± 13.6 */73 ± 24 ** | 84 ± 19.8 */89 ± 13.7 ** | 23 ± 6.2 | 41 ± 3.7 | |||||||||
| Scott | BMI < 35 | 53 ± 20 | 81 ± 22 | 117 | 124 | ||||||||
| BMI ≥ 35 | |||||||||||||
| Cavaignac | Not Obese (BMI < 30) | 80 (70–96) | 85 (50 -100) | ||||||||||
| Obese (BMI ≥ 30) | 78 (64–90) | 77 (50–100) | |||||||||||
| Bonutti | Not Obese (BMI < 35) | 49 (20–70) | 97 (80–100) | ||||||||||
| Obese (BMI ≥ 35) | 50 (30–70) | 95 (70–100) | |||||||||||
| Seyler | Not Obese (BMI < 30) | 48 ± 9 | 92 ± 7 | 49 ± 9 | 95 ± 4 | ||||||||
| Obese (BMI ≥ 30) | |||||||||||||
| Naal | Normal (BMI = 18.5–24.9) | 132 ± 24.5 | 190.5 ± 13.7 | 57.5 ± 14.8 | 95.1 ± 4.7 | 74.5 ± 16.8 | 95.4 ± 9.7 | 123.7 ± 12.2 | 128.1 ± 5.2 | ||||
| Overweight (BMI = 25–29.9) | 189.1 ± 14.8 | 93.1 ± 9.8 | 96 ± 11.4 | 129.9 ± 7.1 | |||||||||
| Obese (BMI = 30–34.9) | 182.7 ± 23.4 | 94.2 ± 9.6 | 88.5 ± 16.7 | 125.7 ± 6.6 | |||||||||
| Berend | Not Obese (BMI < 32) | 54 (34–92) | 87 | 48 (20–90) | 63 | ||||||||
| Obese (BMI ≥ 32) | |||||||||||||
| Venkatesh | BMI < 30 | 47.4 ± 5.5 | 91.6 ± 9.9 | 55.3 ± 4.6 | 91.6 ± 11.2 | 111.3 ± 11.7 | 118.4 ± 11.8 | ||||||
| BMI ≥ 30 | 46.2 ± 5.6 | 92.4 ± 7.43 | 54.9 ± 4.6 | 92.7 ± 9.2 | 108.7 ± 10.1 | 118.3 ± 12.1 | |||||||
| Mohammad | Normal (BMI < 25) | 71.9 ± 14.8 | 80.9 ± 16 | 61.9 ± 16.8 | 90.5 ± 12.1 | 26.9 ± 8.4 | 42 ± 5.6 | 133.9 ± 10.8 | |||||
| Overweight (BMI = 25–29.9) | 73.8 ± 16.8 | 86.0 ± 13.9 | 62.3 ± 14.5 | 95.6 ± 4.8 | 26.7 ± 7.5 | 44.3 ± 4.9 | 128.5 ± 9.7 | ||||||
| Obese (BMI = 30–34.9) | 68.9 ± 16.9 | 67.9 ± 11.8 | 59.2 ± 15.8 | 87.5 ± 17.5 | 24.2 ± 8.5 | 40.1 ± 9.7 | 126.4 ± 5 | ||||||
| Severely Obese (BMI = 35–39.9) | 63.7 ± 17.8 | 70 ± 27.1 | 53.9 ± 13.8 | 83.1 ± 16.4 | 20.8 ± 8.8 | 36.4 ± 11.4 | 125.6 ± 9.1 | ||||||
| Study | Cohort | Survival Rate | Number of Revision (%) | Causes of Failure, Reoperation |
|---|---|---|---|---|
| Nettrour | Not Morbidly Obese (BMI < 40) | NR | 6 (6%) | Minor procedures-aseptic: 2 (2%) |
| Lateral/anterior compartment progression: 1 (1%) | ||||
| Loose tibial component: 2 (2%) | ||||
| Infection: 1 (1%) | ||||
| Morbidly Obese (BMI ≥ 40) | NR | 19 (21.3%) | Minor procedures-aseptic: 3 (3.4%) | |
| Lateral/anterior compartment progression: 7 (7.8%) | ||||
| Bearing instability: 5 (5.6%) | ||||
| Loose tibial component: 2 (2.2%) | ||||
| Infection: 2 (2.2%) | ||||
| Polat | Normal and Overweight (BMI < 30) | NR | 0 | - |
| Obese (BMI = 30–34.9) | NR | 3 (27%) | Tibial + femoral loosening: 3 | |
| Morbidly Obese (BMI ≥ 35) | NR | 8 (72.7%) | Tibial loosening: 3 | |
| Tibial + femoral loosening: 3 | ||||
| Tibial component collapse: 2 | ||||
| Seth | Morbidly Obese (BMI ≥ 40) | 91.7% at 2 years, 86.3% at 5 years | 19 | Improper patient selection: 1 |
| OA progression: 4 | ||||
| Issue in technique: 9 | ||||
| Unexplained pain: 2 | ||||
| Aseptic loosening of tibial component: 2 | ||||
| Traumatic liner dislocation: 1 | ||||
| Molloy | Normal (BMI < 25) | 92% at 10 years | 13 (6.3%) | OA progression: 26 |
| Unexplained pain: 7 | ||||
| Overweight (BMI = 25–29.9) | 95% at 10 years | 18 (4.2%) | Bearing dislocation: 7 | |
| Infection: 6 | ||||
| Obese (BMI = 30–34.9) | 94% at 10 years | 10 (4.5%) | Aseptic loosening: 2 | |
| Instability: 1 | ||||
| Morbidly Obese (BMI ≥ 35) | 93% at 10 years | 6 (6.3%) | Malposition: 1 | |
| ACL injury: 1 | ||||
| Unknown: 1 | ||||
| Affatato | Normal (BMI < 30) | 92.6% at 5 years, 87.4% at 10 years | 265 (8.1%) | Total aseptic loosening: 121 |
| Pain without loosening: 53 | ||||
| Tibial aseptic loosening: 35 | ||||
| Septic loosening: 17 | ||||
| Femoral aseptic loosening: 16 | ||||
| Insert wear: 12 | ||||
| Breakage of prosthesis: 7 | ||||
| Dislocation: 4 | ||||
| Obese (BMI = 30–39.9) | 91.4% at 5 years, 86.7% at 10 years | 145 (8.8%) | Total aseptic loosening: 55 | |
| Pain without loosening: 41 | ||||
| Tibial aseptic loosening: 27 | ||||
| Septic loosening: 12 | ||||
| Femoral aseptic loosening: 1 | ||||
| Insert wear: 1 | ||||
| Breakage of prosthesis: 3 | ||||
| Dislocation: 5 | ||||
| Morbidly Obese (BMI ≥ 40) | 95.5% at 5 years, 87.5% at 10 years | 5 (6.4%) | Total aseptic loosening:2 | |
| Pain without loosening:1 | ||||
| Tibial aseptic loosening:1 | ||||
| Dislocation:1 | ||||
| Xu | Control (BMI < 30) | 98.6% at 10 years | 2 | OA progression: 2 |
| Obese (BMI ≥ 30) | 88.1% at 10 years | 5 | OA progression: 2 | |
| Subsidence of tibial component: 2 | ||||
| Polyetilene wear:1 | ||||
| Plate | Underweight (BMI < 18.5) | NR | 0–0 | Revision to TKA: Persistent knee pain (46%), Unknown (21%), Tibial component loosening (12%), Progression of DJD to adjacent compartment (9%), Tibial component subsidence (7%), Infection (5%) |
| Normal (BMI = 18.5–24.9) | 2 (2.2%)–1 (1.1%) | |||
| Overweight (BMI = 25–29.9) | 14 (6.1%)–3 (1.3%) | |||
| Obese (BMI = 30–34.9) | 13 (5.7%)–4 (1.8%) | Conversion from InLay to OnLay: Tibial component subsidence (46%), Tibial component loosening (27%), Persistent knee pain (9%), Undersized tibial component (9%), Infection (9%) | ||
| Severely Obese (BMI = 35–39.9) | 10 (8.7%)–2 (1.7%) | |||
| Morbidly Obese (BMI = 40–44.9) | 4 (9.5%)–0 | |||
| Super Obese (BMI ≥ 45) | 0–1 (2.4%) | |||
| Woo | Normal (BMI < 25) | NR | 1 | Subsidence: 1 |
| Overweight (BMI = 25–29.9) | 4 | OA progression: 3 | ||
| Persisiting pain: 1 | ||||
| Obese (BMI = 30–34.9) | 2 | OA progression: 2 | ||
| Severely Obese (BMI = 35–39.9) | 2 | OA progression: 1 | ||
| Fracture: 1 | ||||
| Zengerink | Not Obese (BMI < 30) | 87% | 18 | Unexplained pain: 8 |
| OA progression: 2 | ||||
| Instability: 3 | ||||
| Aseptic loosening: 2 | ||||
| Obese (BMI ≥ 30) | Traumatic loosening of tibial component: 1 | |||
| Atraumatic migration of tibial component: 1 | ||||
| Unknown reason: 1 | ||||
| Kandil | Non-Obese (BMI < 30) | NR | 345 (2.7%) | Major complications: 303 (2.3%) |
| Minor complications: 532 (4.1%) | ||||
| Local complications: 439 (3.4%) | ||||
| Medical complications: 256 (2.0%) | ||||
| Obese (BMI = 30–39.9) | 84 (4.6%) | Major complications: 97 (5.3%) | ||
| Minor complications: 179 (9.8%) | ||||
| Local complications: 68 (3.7%) | ||||
| Medical complications: 142 (7.8%) | ||||
| Morbidly Obese (BMI ≥ 40) | 57 (5.6%) | Major complications: 73 (7.2%) | ||
| Minor complications: 132 (13%) | ||||
| Local complications: 68 (6.7%) | ||||
| Medical complications: 106 (10.4%) | ||||
| Cepni | BMI > 30 | 95.6% at 5 years | 3 | Insert dislocation: 3 |
| Murray | Normal (BMI < 25) | 97.6% at 5 years, 94.9% at 10 years | 9 | Unexplained pain: 3 |
| Infection: 2 | ||||
| OA progression: 2 | ||||
| Aseptic loosening: 1 | ||||
| Bearing dislocation: 1 | ||||
| Overweight (BMI = 25–29.9) | 96.8% at 5 years, 93% at 10 years | 25 | Unexplained pain: 7 | |
| Aseptic loosening: 5 | ||||
| Infection: 4 | ||||
| OA progression: 3 | ||||
| Bearing dislocation: 3 | ||||
| Traumatic ACL rupture: 1 | ||||
| AVN of lateral femoral condyle: 1 | ||||
| Fracture: 1 | ||||
| Obese (BMI = 30–34.9) | 95.3% at 5 years, 95.3% at 10 years | 18 | Unexplained pain: 6 | |
| Aseptic loosening: 5 | ||||
| OA progression: 3 | ||||
| Bearing dislocation: 3 | ||||
| Periprothetic fracture: 1 | ||||
| Severely Obese (BMI = 35–39.9) | 93.8% at 5 years, 93.8% at 10 years | 7 | Aseptic loosening: 4 | |
| Unexplained pain: 1 | ||||
| Infection: 1 | ||||
| Bearing dislocation: 1 | ||||
| Morbidly Obese (BMI = 40–44.9) | 95.2% at 5 years | 4 | Aseptic loosening: 2 | |
| Unexplained pain: 1 | ||||
| Infection: 1 | ||||
| Super Obese (BMI ≥ 45) | 100% at 5 years | 0 | - | |
| Thompson | BMI < 35 BMI ≥ 35 | NR | 8 (3.5%) | OA progression: 2 |
| Tibial plateau fracture: 2 | ||||
| Persistent pain: 2 | ||||
| Subsidence of tibial component: 1 | ||||
| Malposition of components: 1 | ||||
| Cavaignac | Not Obese (BMI < 30) | 92% at 10 years | 11 | Aseptic tibial loosening: 3 |
| OA progression: 4 | ||||
| Polyethylene wear: 1 | ||||
| Unexplained pain: 1 | ||||
| Impingement with LCM: 1 | ||||
| Impingement with intercondylar eminence: 1 | ||||
| Obese (BMI ≥ 30) | 94% at 10 years | 4 | OA progression: 3 | |
| Unexplained pain: 1 | ||||
| BMI < 30 | 96.2% | 6 (3.8%) | Implant loosening: 3 | |
| (BMI = 30–34.9) | Persisiting pain: 1 | |||
| BMI = 35–39.9 | OA progression: 2 | |||
| BMI ≥ 40 | ||||
| Bonutti | Not Obese (BMI < 35) | 88% | 5 | Progression of OA: 2 |
| Tibial component loosening: 2 | ||||
| Intractabile pain: 1 | ||||
| Obese (BMI ≥ 35) | 100% | 0 | ||
| Kuipers | BMI > 30 BMI ≥ 30 | 84.7% at 5 years | 45 (10.3%) | Persisiting pain: 13 |
| Aseptic loosening: 12 | ||||
| OA progression: 9 | ||||
| Recurrent luxation of meniscal bearing: 4 | ||||
| Deep infection: 2 | ||||
| Periprosthetic fracture: 3 | ||||
| Traumatic instability of MCL: 1 | ||||
| Malpositioning of tibial component: 1 | ||||
| Seyler | Not Obese (BMI < 30) | 92% at 5 years, 84% at 10 years | 5 | Aseptic loosening: 2 |
| Patellofemoral/lateral pain: 3 | ||||
| Obese (BMI ≥ 30) | 4 | Polyethylene wear: 2 | ||
| Progression of OA: 1 | ||||
| Tibial plateau fracture: 1 | ||||
| Naal | Normal (BMI <25) | NR | 3 (3.6%) | Loosening of the tibial component: 1 |
| Overweight (BMI = 25–29.9) | Loosening of the femoral component: 1 | |||
| Obese (BMI ≥ 30) | Intractabile pain: 1 | |||
| Berend | Not Obese (BMI < 32) Obese (BMI ≥ 32) | 78% at 3 years | 16 | Deep infection: 2 (2.7%) |
| Tibial plateau fracture: 3 (4.1%) | ||||
| Intractabile pain: 4 (5.5%) | ||||
| Progression of OA: 1 (1.4%) | ||||
| Aseptic loosening: 6 (8.2%) | ||||
| Venkatesh | BMI < 30 | 96% at 10.9 years | 5 (4.27%) | Unexplained pain: 2 |
| Loosening of component: 2 | ||||
| Polyethylene wear: 1 | ||||
| BMI ≥ 30 | 2 | Unexplained pain: 2 | ||
| Mohammad | Normal (BMI < 25) | 97.3% at 10 years | 4 | Bearing dislocation: 1 |
| Tibial avascular necrosis: 1 | ||||
| Disease progression: 1 | ||||
| Lateral meniscal tear: 1 | ||||
| Overweight (BMI = 25–29.9) | 96.2% at 10 years | 13 | Bearing dislocation: 4 | |
| Disease progression: 3 | ||||
| Suspected infection: 1 | ||||
| Pain: 2 | ||||
| Loose body: 1 | ||||
| Sweling: 1 | ||||
| Wound dehiscence: 1 | ||||
| Obese (BMI = 30–34.9) | 94.8% at 10 years | 9 | Bearing dislocation: 3 | |
| Pain: 4 | ||||
| Femoral component loosening: 1 | ||||
| Disease progression: 1 | ||||
| Severely Obese (BMI = 35–39.9) | 98.3% at 10 years | 2 | Lateral tibial fracture: 1 | |
| Disease progression: 1 |
| Study | Year | A Clearly Stated Aim | Inclusion of Consecutive Patients | Prospective Collection of Data | Endpoints Appropriate to the Aim of the Study | Unbiased Assessment of the Study Endpoint | Follow-Up Period Appropriate to the Aim of the Study | Loss to Follow-Up Less Than 5% | Prospective Calculation of the Study Size | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Mohammad | 2021 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 1 | 13 |
| Nettrour | 2019 | 2 | 2 | 1 | 2 | 0 | 2 | 2 | 1 | 12 |
| Polat | 2019 | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 0 | 12 |
| Seth | 2019 | 2 | 2 | 0 | 2 | 1 | 1 | 2 | 0 | 10 |
| Molloy | 2019 | 2 | 2 | 1 | 2 | 0 | 2 | 2 | 1 | 12 |
| Affatato | 2019 | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | 10 |
| Xu | 2019 | 2 | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 14 |
| Venkatesh | 2019 | 2 | 2 | 1 | 2 | 0 | 2 | 2 | 1 | 12 |
| Plate | 2017 | 2 | 2 | 1 | 2 | 0 | 1 | 2 | 1 | 11 |
| Woo | 2017 | 2 | 2 | 1 | 2 | 0 | 1 | 2 | 1 | 11 |
| Zengerink | 2015 | 2 | 2 | 0 | 2 | 0 | 1 | 1 | 1 | 9 |
| Kandil | 2015 | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 2 | 12 |
| Cepni | 2014 | 2 | 1 | 0 | 1 | 0 | 1 | 2 | 0 | 7 |
| Thompson | 2013 | 2 | 2 | 0 | 2 | 0 | 1 | 2 | 0 | 9 |
| Murray | 2013 | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 2 | 12 |
| Cavaignac | 2013 | 2 | 2 | 1 | 2 | 0 | 2 | 2 | 2 | 13 |
| 2012 | 2 | 2 | 1 | 2 | 0 | 1 | 1 | 0 | 9 | |
| Bonutti | 2011 | 2 | 2 | 1 | 2 | 0 | 1 | 2 | 2 | 12 |
| Kuipers | 2010 | 2 | 2 | 0 | 2 | 1 | 1 | 2 | 2 | 12 |
| Seyler | 2009 | 2 | 2 | 2 | 2 | 0 | 1 | 2 | 1 | 12 |
| Naal | 2009 | 2 | 2 | 1 | 2 | 0 | 1 | 1 | 1 | 10 |
| Berend | 2005 | 2 | 2 | 0 | 2 | 0 | 1 | 1 | 1 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campi, S.; Papalia, G.F.; Esposito, C.; Albo, E.; Cannata, F.; Zampogna, B.; Papalia, R.; Denaro, V. Unicompartmental Knee Replacement in Obese Patients: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 3594. https://doi.org/10.3390/jcm10163594
Campi S, Papalia GF, Esposito C, Albo E, Cannata F, Zampogna B, Papalia R, Denaro V. Unicompartmental Knee Replacement in Obese Patients: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2021; 10(16):3594. https://doi.org/10.3390/jcm10163594
Chicago/Turabian StyleCampi, Stefano, Giuseppe Francesco Papalia, Carlo Esposito, Erika Albo, Francesca Cannata, Biagio Zampogna, Rocco Papalia, and Vincenzo Denaro. 2021. "Unicompartmental Knee Replacement in Obese Patients: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 10, no. 16: 3594. https://doi.org/10.3390/jcm10163594
APA StyleCampi, S., Papalia, G. F., Esposito, C., Albo, E., Cannata, F., Zampogna, B., Papalia, R., & Denaro, V. (2021). Unicompartmental Knee Replacement in Obese Patients: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 10(16), 3594. https://doi.org/10.3390/jcm10163594







