Automatic Segmentation of Pancreatic Tumors Using Deep Learning on a Video Image of Contrast-Enhanced Endoscopic Ultrasound
Abstract
1. Backgrounds
2. Patients and Methods
2.1. Patients Selection
2.2. Contrast Enhanced-Endoscopic Ultrasound
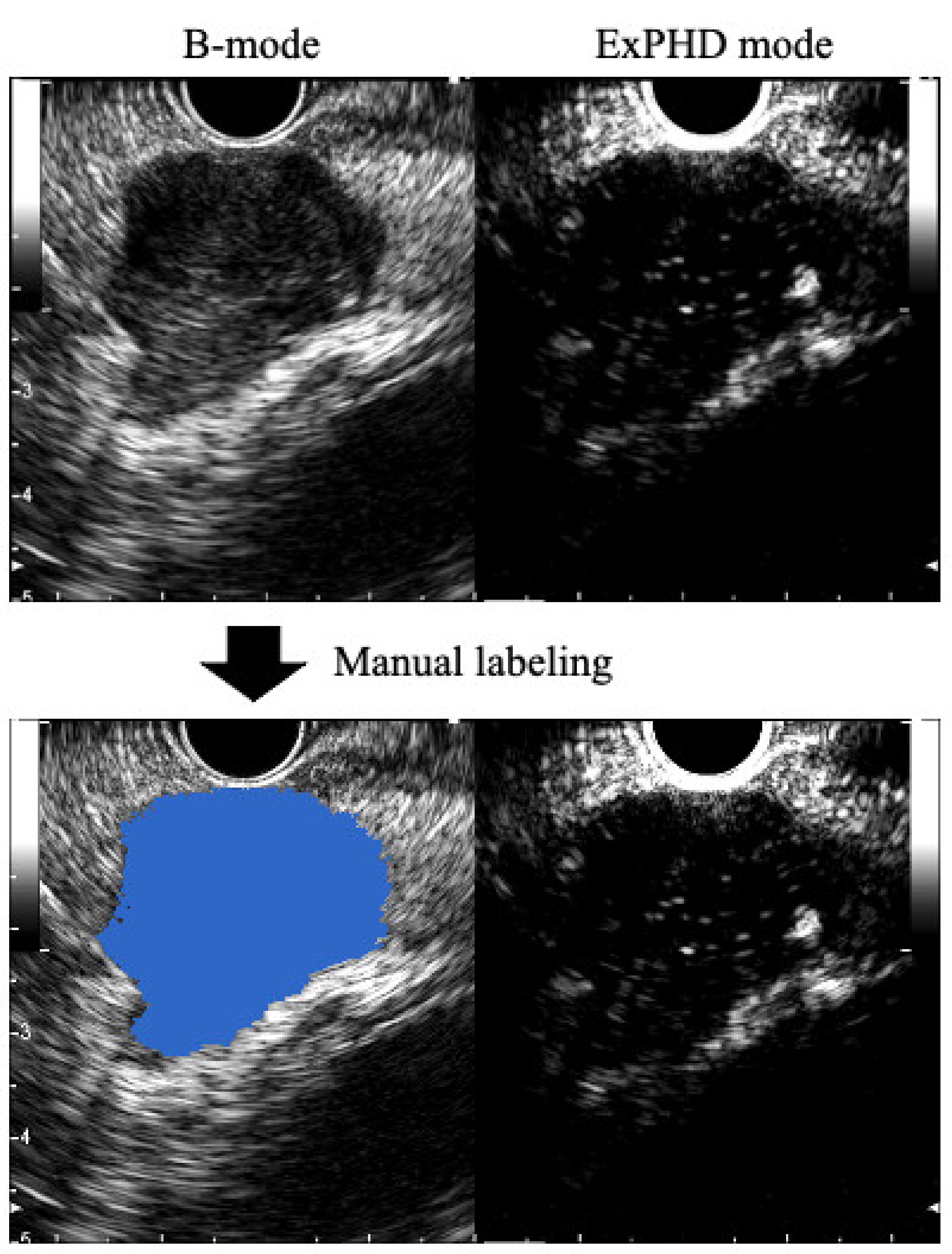
2.3. Preparation of the Training Data Sets
2.4. Deep Learning and Automatic Segmentation
2.5. Study Outcomes and Statistical Analysis
3. Results
3.1. Characteristics of Patients
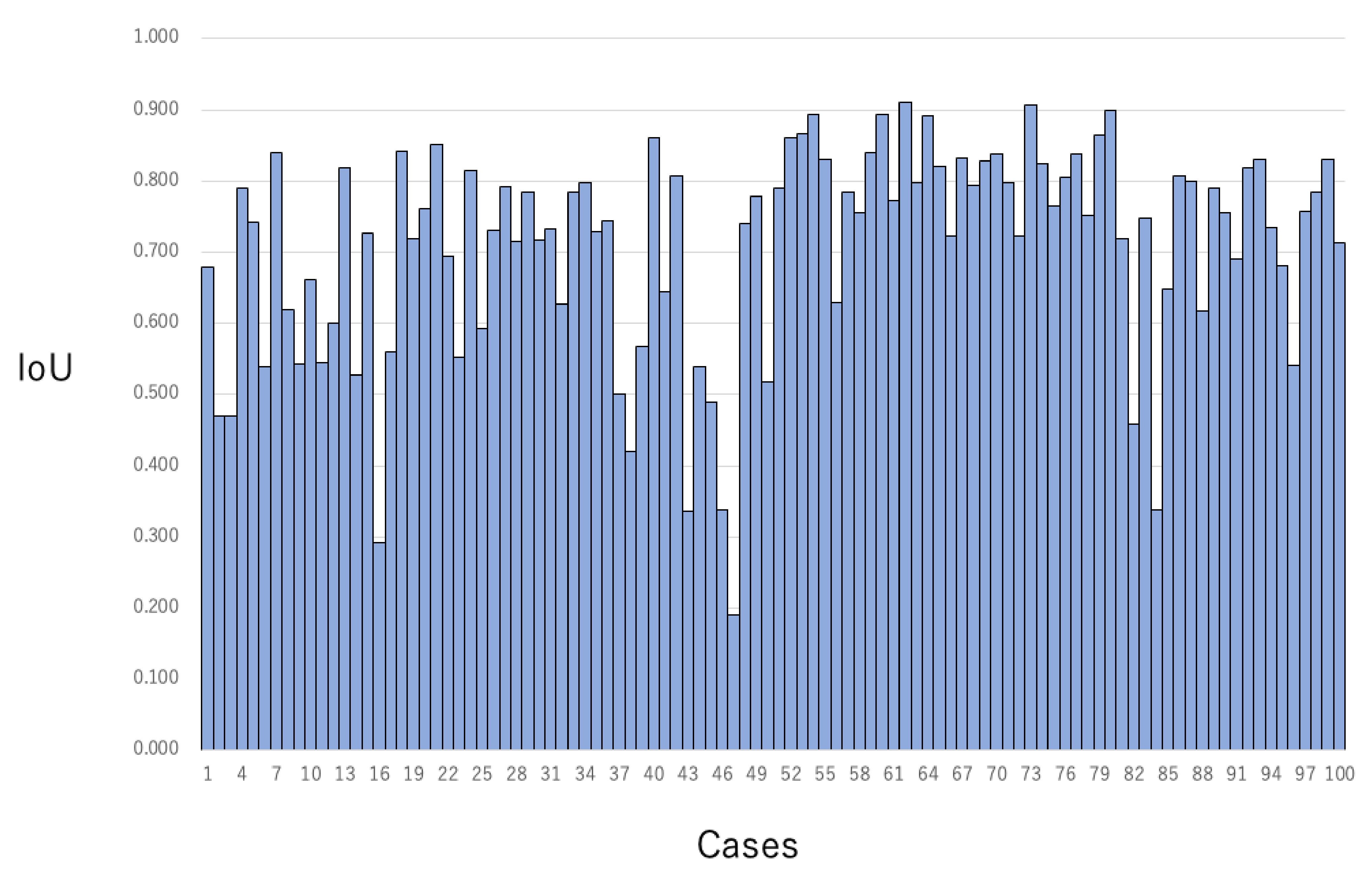
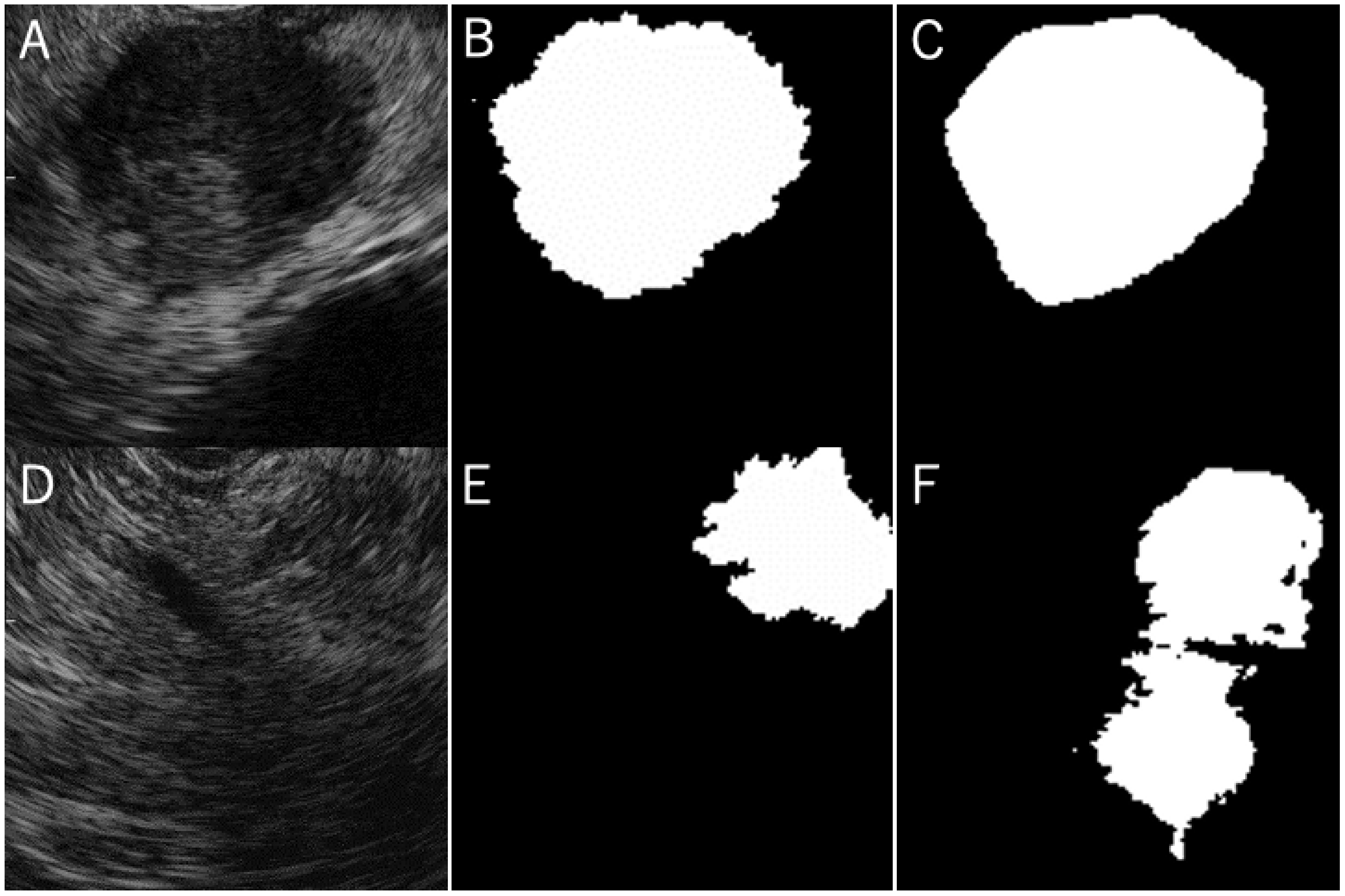
3.2. The Concordance Rate between the Grand Truth Area and Automatic Segmentation Area
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bipat, S.; Phoa, S.S.K.S.; van Delden, O.M.; Bossuyt, P.M.M.; Gouma, D.J.; Lameris, J.S.; Stoker, J. Ultrasonography, computed tomography and magnetic resonance imaging for diagnosis and determining resectability of pancreatic adenocarcinoma: A meta-analysis. J. Comput. Assist. Tomogr. 2005, 29, 438–445. [Google Scholar] [CrossRef]
- Abraham, S.C.; Wilentz, R.E.; Yeo, C.J.; Sohn, T.A.; Cameron, J.L.; Boitnott, J.K.; Hruban, R.H. Pancreaticoduodenectomy (Whipple resections) in patients without malignancy: Are they all ‘chronic pancreatitis’? Am. J. Surg. Pathol. 2003, 27, 110–120. [Google Scholar] [CrossRef]
- Hewitt, M.J.; McPhail, M.J.; Possamai, L.; Dhar, A.; Vlavianos, P.; Monahan, K.J. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: A meta-analysis. Gastrointest. Endosc. 2012, 75, 319–331. [Google Scholar] [CrossRef]
- Yoshinaga, S.; Itoi, T.; Yamao, K.; Yasuda, I.; Irisawa, A.; Imaoka, H.; Tsuchiya, T.; Doi, S.; Yamabe, A.; Murakami, Y.; et al. Safety and efficacy of endoscopic ultrasound-guided fine needle aspiration for pancreatic masses: A prospective multicenter study. Dig. Endosc. 2020, 32, 114–126. [Google Scholar] [CrossRef]
- Tanaka, K.; Hayashi, T.; Utsunomiya, R.; Takigawa, Y.; Kobayashi, Y.; Nagai, K.; Kin, T.; Yane, K.; Takahashi, K.; Shinohara, T.; et al. Endoscopic ultrasound-guided fine needle aspiration for diagnosing pancreatic mass in patients with surgically altered upper gastrointestinal anatomy. Dig. Endosc. 2020, 32, 967–973. [Google Scholar] [CrossRef]
- Kurita, Y.; Kuwahara, T.; Hara, K.; Mizuno, N.; Okuno, N.; Matsumoto, S.; Obata, M.; Koda, H.; Tajika, M.; Shimizu, Y.; et al. Features of chronic pancreatitis by endoscopic ultrasound influence the diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration of small pancreatic lesions. Dig. Endosc. 2020, 32, 399–408. [Google Scholar] [CrossRef]
- Mita, N.; Iwashita, T.; Uemura, S.; Iwasa, Y.; Toda, K.; Mukai, T.; Miyazaki, T.; Yasuda, I.; Shimizu, M. Endoscopic Ultrasound-Guided Fine Needle Biopsy Using 22-Gauge Franseen Needle for the Histological Diagnosis of Solid Lesions: A Multicenter Prospective Pilot Study. Dig. Dis. Sci. 2020, 65, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Leeds, J.S.; Nayar, M.K.; Bekkali, N.L.H.; Wilson, C.H.; Johnson, S.J.; Haugk, B.; Darne, A.; Oppong, K.W. Endoscopic ultrasound-guided fine-needle biopsy is superior to fine-needle aspiration in assessing pancreatic neuroendocrine tumors. Endosc. Int. Open 2019, 7, E1281–E1287. [Google Scholar] [CrossRef]
- Rimbas, M.; Crino, S.F.; Gasbarrini, A.; Costamagna, G.; Scarpa, A.; Larghi, A. EUS-guided fine-needle tissue acquisition for solid pancreatic lesions: Finally moving from fine-needle aspiration to fine-needle biopsy? Endosc. Ultrasound 2018, 7, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Crino, S.F.; Di Mitri, R.; Nguyen, N.Q.; Tarantino, I.; de Nucci, G.; Deprez, P.H.; Carrara, S.; Kitano, M.; Shami, V.M.; Fernandez-Esparrach, G.; et al. Endoscopic Ultrasound-guided Fine-needle Biopsy With or Without Rapid On-site Evaluation for Diagnosis of Solid Pancreatic Lesions: A Randomized Controlled Non-Inferiority Trial. Gastroenterology 2021. [Google Scholar] [CrossRef] [PubMed]
- Crino, S.F.; Ammendola, S.; Meneghetti, A.; Bernardoni, L.; Bellocchi, M.C.C.; Gabbrielli, A.; Landoni, L.; Paiella, S.; Pin, F.; Parisi, A.; et al. Comparison between EUS-guided fine-needle aspiration cytology and EUS-guided fine-needle biopsy histology for the evaluation of pancreatic neuroendocrine tumors. Pancreatology 2021, 21, 443–450. [Google Scholar] [CrossRef]
- Yamashita, Y.; Kitano, M. Benefits and limitations of each type of endoscopic ultrasonography elastography technology for diagnosis of pancreatic diseases. Dig. Endosc. 2021, 33, 554–556. [Google Scholar] [CrossRef] [PubMed]
- Kanno, A.; Yasuda, I.; Irisawa, A.; Hara, K.; Ashida, R.; Iwashita, T.; Takenaka, M.; Katanuma, A.; Takikawa, T.; Kubota, K.; et al. Adverse events of Endoscopic Ultrasound-Guided Fine-Needle Aspiration for Histologic Diagnosis in Japanese Tertiary Centers: A Multicenter Retrospective Study. Dig. Endosc. 2020. [Google Scholar] [CrossRef]
- Yane, K.; Kuwatani, M.; Yoshida, M.; Goto, T.; Matsumoto, R.; Ihara, H.; Okuda, T.; Taya, Y.; Ehira, N.; Kudo, T.; et al. Non-Negligible Rate of Needle Tract Seeding after Endoscopic Ultrasound-Guided Fine Needle Aspiration for Patients Undergoing Distal Pancreatectomy for Pancreatic Cancer. Dig. Endosc. Off. J. Japan Gastroenterol. Endosc. Soc. 2020, 32, 801–811. [Google Scholar] [CrossRef]
- Hatamaru, K.; Kitano, M. Can early diagnosis of EUS-FNA needle tract seeding for pancreatic cancer improve patient prognosis? Dig. Endosc. 2020, 32, 742–744. [Google Scholar] [CrossRef] [PubMed]
- Iwashita, T.; Uemura, S.; Mita, N.; Iwasa, Y.; Ichikawa, H.; Senjyu, A.; Yasuda, I.; Shimizu, M. Utility of endoscopic ultrasound and endoscopic ultrasound-guided fine-needle aspiration for the diagnosis and management of pancreatic cystic lesions: Differences between the guidelines. Dig. Endosc. 2020, 32, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Crino, S.F.; Brandolese, A.; Vieceli, F.; Paiella, S.; Conti Bellocchi, M.C.; Manfrin, E.; Bernardoni, L.; Sina, S.; D’Onofrio, M.; Marchegiani, G.; et al. Endoscopic Ultrasound Features Associated with Malignancy and Aggressiveness of Nonhypovascular Solid Pancreatic Lesions: Results from a Prospective Observational Study. Ultraschall Med. 2021, 42, 167–177. [Google Scholar] [CrossRef]
- Hocke, M.; Dietrich, C.F. Vascularisation pattern of chronic pancreatitis compared with pancreatic carcinoma: Results from contrast-enhanced endoscopic ultrasound. Int. J. Inflamm. 2012, 2012, 420787. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamashita, Y.; Shimokawa, T.; Napoleon, B.; Fusaroli, P.; Gincul, R.; Kudo, M.; Kitano, M. Value of contrast-enhanced harmonic endoscopic ultrasonography with enhancement pattern for diagnosis of pancreatic cancer: A meta-analysis. Dig. Endosc. 2019, 31, 125–133. [Google Scholar] [CrossRef]
- Satoh, T.; Ishiwatari, H.; Sasaki, K. Pancreatic colloid carcinoma diagnosed by contrast-enhanced endoscopic ultrasound-guided fine-needle aspiration. Dig. Endosc. 2020, 32, 150. [Google Scholar] [CrossRef]
- Ishikawa, R.; Kamata, K.; Hara, A.; Tanaka, H.; Okamoto, A.; Yamazaki, T.; Nakai, A.; Omoto, S.; Minaga, K.; Yamao, K.; et al. Utility of contrast-enhanced harmonic endoscopic ultrasonography for predicting the prognosis of pancreatic neuroendocrine neoplasms. Dig. Endosc. 2021, 33, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Omoto, S.; Kitano, M.; Fukasawa, M.; Ashida, R.; Kato, H.; Shiomi, H.; Sugimori, K.; Kanno, A.; Chiba, Y.; Takano, S.; et al. Tissue harmonic versus contrast-enhanced harmonic endoscopic ultrasonography for the diagnosis of pancreatic tumors: A prospective multicenter study. Dig. Endosc. 2021. [Google Scholar] [CrossRef]
- Kitano, M.; Kudo, M.; Yamao, K.; Takagi, T.; Sakamoto, H.; Komaki, T.; Kamata, K.; Imai, H.; Chiba, Y.; Okada, M.; et al. Characterization of small solid tumors in the pancreas: The value of contrast-enhanced harmonic endoscopic ultrasonography. Am. J. Gastroenterol. 2012, 107, 303–310. [Google Scholar] [CrossRef]
- Matsubara, H.; Itoh, A.; Kawashima, H.; Kasugai, T.; Ohno, E.; Ishikawa, T.; Itoh, Y.; Nakamura, Y.; Hiramatsu, T.; Nakamura, M.; et al. Dynamic quantitative evaluation of contrast-enhanced endoscopic ultrasonography in the diagnosis of pancreatic diseases. Pancreas 2011, 40, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Gheonea, D.I.; Streba, C.T.; Ciurea, T.; Saftoiu, A. Quantitative low mechanical index contrast-enhanced endoscopic ultrasound for the differential diagnosis of chronic pseudotumoral pancreatitis and pancreatic cancer. BMC Gastroenterol. 2013, 13, 2. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Shin, H.-C.; Roth, H.R.; Gao, M.; Lu, L.; Xu, Z.; Nogues, I.; Yao, J.; Mollura, D.; Summers, R.M. Deep Convolutional Neural Networks for Computer-Aided Detection: CNN Architectures, Dataset Characteristics and Transfer Learning. IEEE Trans. Med. Imaging 2016, 35, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Minnema, J.; van Eijnatten, M.; Kouw, W.; Diblen, F.; Mendrik, A.; Wolff, J. CT image segmentation of bone for medical additive manufacturing using a convolutional neural network. Comput. Biol. Med. 2018, 103, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. In International Conference on Medical Image Computing and Computer-Assisted Intervention—MICCAI 2015; Springer: Berlin/Heidelberg, Germany, 2015; pp. 234–241. [Google Scholar]
- Anas, E.M.A.; Mousavi, P.; Abolmaesumi, P. A deep learning approach for real time prostate segmentation in freehand ultrasound guided biopsy. Med. Image Anal. 2018, 48, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Guo, Y.; Wang, Y.; Yu, J.; Li, J.; Zhou, S.; Chang, C. Automatic tumor segmentation in breast ultrasound images using a dilated fully convolutional network combined with an active contour model. Med. Phys. 2019, 46, 215–228. [Google Scholar] [CrossRef]
- Li, X.; Guo, Y.; Jiang, F.; Xu, L.; Shen, F.; Jin, Z.; Wang, Y. Multi-Task Refined Boundary-Supervision U-Net (MRBSU-Net) for Gastrointestinal Stromal Tumor Segmentation in Endoscopic Ultrasound (EUS) Images. IEEE Access 2020, 8, 5805–5816. [Google Scholar] [CrossRef]



| Respiratory Movement | |
|---|---|
| RM-A | Respiratory movement of less than 50% of the tumor diameter |
| RM-B | Respiratory movement of 51–99% of the tumor diameter |
| RM-C | Respiratory movement of more than 100% of the tumor diameter |
| Tumor boundary | |
| TB-1 | Tumor boundary of visibility all around |
| TB-2 | Tumor boundary of visibility 50–99% around |
| TB-3 | Tumor boundary of visibility less than 50% around |
| Age, Years, Median (Range) | 70 (29–89) |
|---|---|
| Gender, n, (male/female) | 52/48 |
| Tumor diameter, mm, median (range) | 24 (8–91) |
| Tumor location, n (head/body or tail) | 37/63 |
| Final diagnosis, n | |
| Pancreatic cancer | 67 |
| Neuroendocrine tumor | 10 |
| Autoimmune pancreatitis | 7 |
| Metastatic pancreatic tumor | 6 |
| Chronic pancreatitis | 4 |
| Malignant lymphoma | 2 |
| Solid pseudopapillary neoplasm | 2 |
| Fat necrosis | 1 |
| Mass forming pancreatitis | 1 |
| Degree of respiratory movement, group, n | |
| RM-A | 70 |
| RM-B | 19 |
| RM-C | 11 |
| Degree of tumor boundary, group, n, | |
| TM-1 | 40 |
| TM-2 | 50 |
| TM-3 | 10 |
| N IoU, Median (Range) | TB Group | Total | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| RM group | A | 34 0.80 (0.45–0.92) | 33 0.77 (0.30–0.91) | 3 0.61 (0.32–0.77) | 70 0.78 (0.30–0.91) |
| B | 4 0.80 (0.79–0.81) | 12 0.75 (0.13–0.85) | 3 0.33 (0.32–0.80) | 19 0.79 (0.13–0.85) | |
| C | 2 0.76 (0.72–0.81) | 5 0.79 (0.58–0.84) | 4 0.76 (0.47–0.77) | 11 0.76 (0.46–0.84) | |
| total | 40 0.80 (0.45–0.91) | 50 0.76 (0.13–0.91) | 10 0.68 (0.32–0.80) | 100 0.77 (0.13–0.92) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwasa, Y.; Iwashita, T.; Takeuchi, Y.; Ichikawa, H.; Mita, N.; Uemura, S.; Shimizu, M.; Kuo, Y.-T.; Wang, H.-P.; Hara, T. Automatic Segmentation of Pancreatic Tumors Using Deep Learning on a Video Image of Contrast-Enhanced Endoscopic Ultrasound. J. Clin. Med. 2021, 10, 3589. https://doi.org/10.3390/jcm10163589
Iwasa Y, Iwashita T, Takeuchi Y, Ichikawa H, Mita N, Uemura S, Shimizu M, Kuo Y-T, Wang H-P, Hara T. Automatic Segmentation of Pancreatic Tumors Using Deep Learning on a Video Image of Contrast-Enhanced Endoscopic Ultrasound. Journal of Clinical Medicine. 2021; 10(16):3589. https://doi.org/10.3390/jcm10163589
Chicago/Turabian StyleIwasa, Yuhei, Takuji Iwashita, Yuji Takeuchi, Hironao Ichikawa, Naoki Mita, Shinya Uemura, Masahito Shimizu, Yu-Ting Kuo, Hsiu-Po Wang, and Takeshi Hara. 2021. "Automatic Segmentation of Pancreatic Tumors Using Deep Learning on a Video Image of Contrast-Enhanced Endoscopic Ultrasound" Journal of Clinical Medicine 10, no. 16: 3589. https://doi.org/10.3390/jcm10163589
APA StyleIwasa, Y., Iwashita, T., Takeuchi, Y., Ichikawa, H., Mita, N., Uemura, S., Shimizu, M., Kuo, Y.-T., Wang, H.-P., & Hara, T. (2021). Automatic Segmentation of Pancreatic Tumors Using Deep Learning on a Video Image of Contrast-Enhanced Endoscopic Ultrasound. Journal of Clinical Medicine, 10(16), 3589. https://doi.org/10.3390/jcm10163589








